ABSTRACT
5-Hydroxymethylfurfural (5-HMF) is one of the most important products of maillard reaction. This study sought to elucidate the immunomodulatory effect of 5-HMF by evaluating IFN-γ and IL-4 production in BALB/c mice sensitized with ovalbumin (OVA). Four groups of BALB/c mice (n = 5 for each group) including control, vehicle and two 5-HMF (188 and 750 mg/kg bw) treatment groups were studied. Interleukin-4(IL-4) and interferon gamma (IFN-γ) levels were measured in culture supernatants of spleen cells using enzyme-linked immunosorbent assay. It was found that the IL-4 level was significantly suppressed in 5-HMF treatment groups compared with the vehicle group (P < .05). Moreover, the IFN-γ/IL-4 ratio was significantly increased in a dose-independent manner compared with the vehicle group (P < .05). Oral administration of 5-HMF inhibited IL-4 but not IFN-γ production by splenocytes of mice immunized with OVA. These changes may reflect that 5-HMF could be a novel therapeutic approach for prevention of (Th2) cytokine-dominant disease.
1. Introduction
T-helper 1 (Th1) and T-helper 2 (Th2) cells were known as important regulators of immune response since three decades ago (Mosmann, Cherwinski, Bond, Giedlin, & Coffman, Citation1986). In recent years, numerous studies indicated an imbalance in the Th1/Th2 ratio is associated with many diseases such as allergy (Maggi, Citation1998), asthma (Mazzarella, Bianco, Catena, De Palma, & Abbate, Citation2000), atopic dermatitis (Nuttall, Knight, McAleese, Lamb, & Hill, Citation2002) and systemic lupus erythematosus (SLE) (Chen et al., Citation2000). Thus, regulation of Th1/Th2 balance might be an efficient method in evaluating the therapeutic effect of immunomodulators. Biological functions of Th1 and Th2 cells are mainly associated with cytokine production. Among Th2 cytokines main attention is being on interleukin-4 (IL-4) which IgE production is one of the main function of this cytokine. On the other hand, interferon-γ as a Th1 cytokine promotes cell-mediated immune responses and suppresses Th2 immune responses (Mosmann & Sad, Citation1996). Several drugs including glucocorticoids, cyclosporin A and tacrolimus have been developed for the treatment of diseases through modulation of cytokines. However, clinical application of these drugs is limited due to many side effects (Alexander, Kay, & Barnes, Citation1992; Dubus et al., Citation2001).
5-Hydroxymethylfurfural (5-HMF) is one of the most important products of non-enzymatic maillard reaction where the presence of acidic condition besides high temperature induces a reaction between a reducing sugar and an amino acid (Ramirez-Jimenez, Guerra-Hernández, & García-Villanova, Citation2000; Stadler & Lineback, Citation2008). Variety of foods and beverages are available to provide 5-HMF including cereal products (Garcia-Villanova, Guerra-Hernandez, Martinez-Gomez, & Montilla, Citation1993), instant coffee (del Campo, Berregi, Caracena, & Zuriarrain, Citation2010), dried fruit (Murkovic & Pichler, Citation2006), bread (Ghadimi, Alizadeh, Esfanjani, Hezaveh, & Vayghan, Citation2014), pasta (Resmini, Pellegrino, Pagani, & De Noni, Citation1993), milk (Morales, Romero, & Jimenez-Pérez, Citation1992) and honey (Truzzi et al., Citation2012). However, food-processing conditions such as temperature, duration and water activity affect the amount of 5-HMF content in these foods (Ghadimi et al., Citation2014).
In the recent years, experiments have revealed some health-promoting effects of 5-HMF. Many studies indicated 5-HMF as a novel natural antioxidant with potential applications in cancer chemoprevention (Zhao et al., Citation2013), high glucose-induced oxidative stress reduction (Cao, Cai, Cai, & Tu, Citation2013) and many other beneficial physiologic effects such as neuroprotective effects (Liu et al., Citation2014) and anti-hypoxia (Li et al., Citation2011). An in vitro study indicated 5-HMF as an anti-allergic compound by suppressing IgE-mediated chemical mediator release (Yamada, Nemoto, Shigemori, Yokota, & Isoda, Citation2011).
As mentioned, a recent study illustrated that immune response enhancement must be another profitable effect of 5-HMF. Therefore, the aim of the current study was to examine immunomodulatory effects of 5-HMF in two different doses by evaluating Th1/Th2 cytokine balance in culture supernatants of splenocytes in BALB/c mice sensitized with ovalbumin (OVA).
2. Materials and methods
2.1. Animals and diet
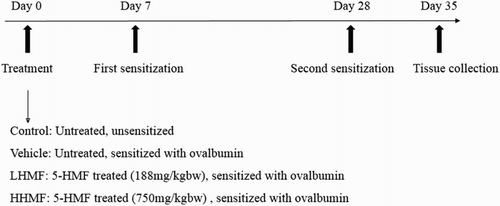
The study protocol was conducted with approval from the research ethics committee of Tabriz University of Medical Sciences, Tabriz, Iran (reference number: TBZMED.REC.1394.8). Eight-week-old female BALB/c mice (30–35 g) were maintained under standard conditions (temperature, 22–23°C; humidity, 50–60%; 12-h light/dark cycle) and specific pathogen-free circumstances. After acclimation for one week, the animals were allocated to four weight-matched groups (five animals per cage). Mice in control and vehicle groups were fed with normal chow-diet () throughout the study and the other two groups were fed by low and high dose of 5-HMF-containing (Matrix Scientific, Pontiac Business Center Drive, Elgin, USA) diets five days per week. In these two groups the dose of 5-HMF was selected to supply a daily intake of 188 (LHMF) and 750 (HHMF) mg/kg of body weight throughout the experiments. The animals were fed pellet diets and water ad libitum.
Table 1. Composition of experimental diet.
2.2. Immunization protocol
Sensitization of the animals to OVA (grade V; Sigma-Aldrich, St. Louis, MO, USA) was performed according to methods described in a previous study (Satter et al., Citation2002). Briefly, mice were immunized intraperitoneally with 100 μg of alum-precipitated OVA on day seven. Three weeks after the first immunization, on day 28, the mice were boosted with the same doses of the antigen. In control group 100 μg of PBS were used ().
2.3. Preparation of spleen cell suspension
One week after the last immunization, the mice were anesthetized using pentobarbital. Spleens were immediately removed and immersed in prepared RPMI-1640 media (Sigma) containing antibiotic-antimycotic (Gibco-BRL Life Technologies, MD, USA). Spleen cell suspensions were prepared following the procedure described previously (Chandra, Citation1991). Briefly, splenocytes were washed by centrifugation in the media, followed by treatment with 0.83% ammonium chloride in Trishydrochloride (pH 7.4) at 37°C for 5 min to remove the red blood cells and three times subsequent washings with the culture media.
2.4. Cytokine production
A suspension of spleen cells (5 × 106 cells/well) was prepared in RPMI-1640 medium containing antibiotic-antimycotic, 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 5 × 105 M 2-mercaptoethanol, and 10% fetal bovine serum and cultured with concanavalin A (Con A) and lipopolysaccharide (LPS) as T cell and B cell mitogens, respectively, under air containing 5% carbon dioxide. After 72 h, the supernatants of cultures from each mouse were collected and pooled for enzyme-linked immunosorbent assay (ELISA) analysis of interferon gamma (IFN-γ) and IL-4.
2.5. Assessment of cytokines in culture supernatants
Levels of IFN-γ and IL-4 in culture supernatants of splenocytes were measured following the instructions from manufacturers using a commercially available kit (Hangzhou Eastbiopharm Co. Ltd.; Hangzhoui, China). Briefly, monoclonal antibody wells pre-coated with mouse cytokines were incubated with serially diluted purified mouse cytokines (standards) or sera and cytokine antibodies labeled with biotin, and then combined with streptavidin-horseradish peroxidase to form an immune complex. Finally, chromogen solutions were added to the plates and after 15 min incubation in the dark, a stop solution was added. Absorbance was measured at 450 nm in a microplate reader (Model 680; Bio-Rad Laboratories, Inc., USA). Limits of detection were 2.53 ng/ml for IL-4 and 2.43 ng/ml for IFN-γ.
3. Statistics
All data were tested for normal distribution and equal variance by the Kolmogorov–Smirnov test. Comparison among groups was performed using one-way ANOVA with Tukey’s correction for multiple comparisons or the Kruskal–Wallis test for nonparametric values. SPSS 13.5 software was used for statistical analysis with P < .05, indicating a statistically significant difference.
4. Results
To determine whether 5-HMF influences Th1 and Th2 cytokine production, we have determined the effects of dietary intake of 5-HMF on secretion of IL-4, a marker for the Th2 response and IFN-γ, and a marker for the Th1 response in the culture supernatant of isolated cells from spleen which is stimulated with Con A and LPS. As shown (), in Con A-stimulated splenocytes, there were significant differences in the mean value of IL-4 between control (265.56 ± 26.90 pg/ml) and vehicle groups (337.28 ± 12.74 pg/ml, P < .001). Treatment with 5-HMF caused a significant decrease in IL-4 production by splenocytes in the LHMF (278.84 ± 2.99 pg/ml) and HHMF (253.82 ± 28.77 pg/ml) groups compared to the vehicle group (P < .01 and P < .001, respectively). On the other hand, as shown in , there was no significant difference in the level of IFN-γ between groups (P > .05). We calculated the ratio between IFN-γ and IL-4 and observed IFN-γ/IL-4 ratio reduction in the vehicle group compared to the control by 52.1% (P < .01). The results showed 5-HMF significantly increased the secretion ratio of IFN-γ/IL-4 in LHMF and HHMF groups by 47.8% (P < .05) and 58.6% (P < .01), respectively, compared to the vehicle group although this ratio was not significantly different between the two 5-HMF groups.
Table 2. The concentration of IL-4 and IFN-γ in the Con A-stimulated supernatant of isolated cells from spleen and their ratios.
As shown (), the results of LPS-stimulated splenocytes cytokine production was approximately similar to Con A. Mean value of IL-4 production in the control group (251.10 ± 17.41 pg/ml) was significantly lower than in the vehicle group (297.44 ± 8.76 pg/ml, P < .001). In LHMF (262.04 ± 8.18 pg/ml) and HHMF (263.02 ± 8.63 pg/ml) groups, a significant reduction of IL-4 production was observed compared to the vehicle group (P < .01). Moreover, IFN-γ level in the control group (190.80 ± 29.54 pg/ml) was significantly more than in the vehicle group (139.62 ± 25.03 pg/ml, P < .05). However, there was no significant difference in the 5-HMF-treated groups compared to the vehicle group. The IFN-γ/IL-4 ratio was similar to Con A-stimulated cells and 5-HMF significantly increased the secretion ratio of IFN-γ/IL-4 in LHMF and HHMF groups by 36.9.0% (P < .05) and 34.7% (P < .05), respectively, compared to the vehicle group. The IFN-γ/IL-4 ratio in the vehicle group decreased compared to the control group by 65.2% (P < .001).
Table 3. The concentration of IL-4 and IFN-γ in the LPS-stimulated supernatant of isolated cells from spleen and their ratios.
5. Discussion
The current study revealed that 5-HMF has a remarkable suppression effect on IL-4 production, a key cytokine which drives Th-2 immune responses. Moreover, a main finding emerged from our results, which indicated that 5-HMF treatment increased the Th1/Th2 ratio and modulated the IFN-γ/IL-4 balance toward the Th1 cytokine. In pathogenesis of many immune-related diseases such as asthma and allergy, the Th1/Th2 ratio was significantly decreased (Kuo, Huang, Yeh, Li, & Hsieh, Citation2001; Rotmagnani, Citation1998). Thus, many approaches were investigated to modulate the Th1/Th2 balance through shifting from the Th2 to the Th1 pole (Kato, Manabe, Tanaka, & Mochizuki, Citation1999; Stirling & Chung, Citation2000). In this regard, several drugs have been developed for the treatment of such diseases through modulation of cytokines. However, clinical application of these drugs is limited due to many side effects (Alexander et al., Citation1992; Dubus et al., Citation2001). Therefore, despite some concerns about safety of 5-HMF-containing foods, it seems that alleviation of Th2-dominant diseases might be considered as a valuable effect of 5-HMF in better management of such diseases.
Several studies demonstrated the potential of antioxidants for the prevention of Th2 cytokines’ production in immune-related diseases (Tripathi, Nair, & Arora, Citation2013; Wu, Turner, & Oliveira, Citation2002). Recently, many studies confirmed antioxidant activity of 5-HMF. An in vitro study by Zhao et al. showed that 5-HMF decreased free radicals. On the other hand, antioxidant enzymes’ activities such as glutation peroxidasa (GPx), superoxide dismutase (SOD), and catalase (CAT) were increased (Zhao et al., Citation2013). Lipid peroxidation and antioxidant defenses’ evaluation in the brain tissue of mice showed that pretreatment with 5-HMF reduced malondialdehyde (MDA) content and augmented the activity of SOD (Ya, Zhang, Zhang, Li, & Li, Citation2012) and protected against the oxidative damage induced by cerebral ischemia in rats (Zhang et al., Citation2015). Yong-Xin et al. suggested functional groups of 5-HMF structure including double bonds, an aldehyde oxygen atom, and oxygen existing in the furan ring by attracting electron atoms may exhibit an antioxidant effect (Li, Li, Qian, Kim, & Kim, Citation2009). It seems that the mechanism of action by which antioxidants such as 5-HMF inhibit IL-4 production is influenced by preventing redox-sensitive transcription factor NF-kB. Inhibition of NF-kB activity prevents production of many Th2 cytokines such as IL-4, IL-5, and IL-13 (Das et al., Citation2001). In line with the observation, an in vitro study by Kim et al. (Citation2011) demonstrated anti-inflammatory properties of 5-HMF through inhibition of NF-κB transcription factor activation. Therefore, the antioxidant activity of 5-HMF might play an important role in the mechanism of Th2/Th1 modulation. The present study was limited to determining the effects of 5-HMF on the production of IFN-γ and IL-4. As there is no similar study to evaluate T cell cytokine responses, the researchers intended to study polyclonal T cell cytokine responses without any identification, separation, and quantification of antigen-specific T cells. This was on the basis of better reflection of polyclonal T cell cytokine response to allergens than antigen-specific T cell clones. However, examining in depth the effect of 5-HMF on Th1/Th2 balance in OVA-immunized mice by analysis of antigen-specific T cells and B-cell proliferation activity is needed, which warrants further studies.
6. Conclusion
In conclusion, the results of the present study suggested for the first time that oral administration of 5-HMF inhibited IL-4 but not IFN-γ production by splenocytes of mice immunized with OVA. Further studies may allow us to use 5-HMF as a novel valuable supplement or medicine for the prevention of (Th2) cytokine-dominant disease. However, more investigation needs to survey precise mechanism of IL-4 suppression and possible complications.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Hamed khodaei is a master student at Faculty of nutrition, Tabriz University of Medical Sciences, Tabriz, Iran. His research interest includes immunoassay development for food safety.
Mohammad alizadeh is associate professor at Nutrition Research Center, Tabriz University of Medical Sciences, Tabriz, Iran. His research interest includes immunoassay development for food safety.
Additional information
Funding
References
- Alexander, A., Kay, A., & Barnes, N. (1992). Trial of cyclosporin in corticosteroid-dependent chronic severe asthma. The Lancet, 339(8789), 324–328. doi: 10.1016/0140-6736(92)91646-P
- del Campo, G., Berregi, I., Caracena, R., & Zuriarrain, J. (2010). Quantitative determination of caffeine, formic acid, trigonelline and 5-(hydroxymethyl) furfural in soluble coffees by 1 H NMR spectrometry. Talanta, 81(1), 367–371. doi: 10.1016/j.talanta.2009.12.010
- Cao, G., Cai, H., Cai, B., & Tu, S. (2013). Effect of 5-hydroxymethylfurfural derived from processed Cornus officinalis on the prevention of high glucose-induced oxidative stress in human umbilical vein endothelial cells and its mechanism. Food Chemistry, 140(1), 273–279. doi: 10.1016/j.foodchem.2012.11.143
- Chandra, R. K. (1991). 1990 McCollum Award Lecture. Nutrition and immunity: Lessons from the past and new insights into the future. American Journal of Clinical Nutrition, 53(1), 1087–1101.
- Chen, S., Hu, D., Shi, X., Shen, N., Gu, Y., & Bao, C. (2000). The relationship between Th1/Th2-type cells and disease activity in patients with systemic lupus erythematosus. Chinese Medical Journal, 113(10), 877–880.
- Das, J., Chen, C.-H., Yang, L., Cohn, L., Ray, P., & Ray, A. (2001). A critical role for NF-κB in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nature Immunology, 2(1), 45–50. doi: 10.1038/83158
- Dubus, J., Marguet, C., Deschildre, A., Mely, L., Le Roux, P., Brouard, J., … Huiart, L. (2001). Local side-effects of inhaled corticosteroids in asthmatic children: Influence of drug, dose, age, and device. Allergy, 56(10), 944–948. doi: 10.1034/j.1398-9995.2001.00100.x
- Garcia-Villanova, B., Guerra-Hernandez, E., Martinez-Gomez, E., & Montilla, J. (1993). Liquid chromatography for the determination of 5-(hydroxymethyl)-2-furaldehyde in breakfast cereals. Journal of Agricultural and Food Chemistry, 41(8), 1254–1255. doi: 10.1021/jf00032a017
- Ghadimi, S. S., Alizadeh, M., Esfanjani, A. T., Hezaveh, S. J. G., & Vayghan, H. J. (2014). Assessment of dietary exposure to 5-hydroxymethylfurfural from traditional Iranian flat breads. Italian Journal of Food Science, 26(2), 169–175.
- Kato, Y., Manabe, T., Tanaka, Y., & Mochizuki, H. (1999). Effect of an orally active Th1/Th2 balance modulator, M50367, on IgE production, eosinophilia, and airway hyperresponsiveness in mice. The Journal of Immunology, 162(12), 7470–7479.
- Kim, H. K., Choi, Y. W., Lee, E. N., Park, J. K., Kim, S. G., Park, D. J., … Yoon, S. (2011). 5-Hydroxymethylfurfural from black garlic extract prevents TNFα-induced monocytic cell adhesion to HUVECs by suppression of vascular cell adhesion molecule-1 expression, reactive oxygen species generation and NF-κB activation. Phytotherapy Research, 25(7), 965–974. doi: 10.1002/ptr.3351
- Kuo, M.-L., Huang, J.-L., Yeh, K.-W., Li, P.-S., & Hsieh, K.-H. (2001). Evaluation of Th1/Th2 ratio and cytokine production profile during acute exacerbation and convalescence in asthmatic children. Annals of Allergy, Asthma & Immunology, 86(3), 272–276. doi: 10.1016/S1081-1206(10)63297-8
- Li, M.-M., Wu, L.-Y., Zhao, T., Xiong, L., Huang, X., Liu, Z.-H., … Ma, Y.-B. (2011). The protective role of 5-HMF against hypoxic injury. Cell Stress and Chaperones, 16(3), 267–273. doi: 10.1007/s12192-010-0238-2
- Li, Y.-X., Li, Y., Qian, Z.-J., Kim, M.-M., & Kim, S.-K. (2009). In vitro antioxidant activity of 5-HMF isolated from marine red alga Laurencia undulata in free-radical-mediated oxidative systems. Journal of Microbiology and Biotechnology, 19(11), 1319–1327.
- Liu, A., Zhao, X., Li, H., Liu, Z., Liu, B., Mao, X., … Jia, Y. (2014). 5-Hydroxymethylfurfural, an antioxidant agent from Alpinia oxyphylla Miq. improves cognitive impairment in Aβ 1–42 mouse model of Alzheimer's disease. International Immunopharmacology, 23(2), 719–725. doi: 10.1016/j.intimp.2014.10.028
- Maggi, E. (1998). The TH1/TH2 paradigm in allergy. Immunotechnology, 3(4), 233–244. doi: 10.1016/S1380-2933(97)10005-7
- Mazzarella, G., Bianco, A., Catena, E., De Palma, R., & Abbate, G. (2000). Th1/Th2 lymphocyte polarization in asthma. Allergy, 55(s61), 6–9. doi: 10.1034/j.1398-9995.2000.00511.x
- Morales, F., Romero, C., & Jimenez-Pérez, S. (1992). An enhanced liquid chromatographic method for 5-hydroxymethylfurfural determination in UHT milk. Chromatographia, 33(1–2), 45–48. doi: 10.1007/BF02276850
- Mosmann, T. R., Cherwinski, H., Bond, M. W., Giedlin, M. A., & Coffman, R. L. (1986). Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. The Journal of Immunology, 136(7), 2348–2357.
- Mosmann, T. R., & Sad, S. (1996). The expanding universe of T-cell subsets: Th1, Th2 and more. Immunology Today, 17(3), 138–146. doi: 10.1016/0167-5699(96)80606-2
- Murkovic, M., & Pichler, N. (2006). Analysis of 5-hydroxymethylfurfual in coffee, dried fruits and urine. Molecular Nutrition & Food Research, 50(9), 842–846. doi: 10.1002/mnfr.200500262
- Nuttall, T., Knight, P., McAleese, S., Lamb, J., & Hill, P. (2002). Expression of Th1, Th2 and immunosuppressive cytokine gene transcripts in canine atopic dermatitis. Clinical & Experimental Allergy, 32(5), 789–795. doi: 10.1046/j.1365-2222.2002.01356.x
- Ramirez-Jimenez, A., Guerra-Hernández, E., & García-Villanova, B. (2000). Browning indicators in bread. Journal of Agricultural and Food Chemistry, 48(9), 4176–4181. doi: 10.1021/jf9907687
- Resmini, P., Pellegrino, L., Pagani, M., & De Noni, I. (1993). Formation of 2-acetyl-3-D-glucopyranosylfuran (glucosylisomaltol) from nonenzymatic browning in pasta drying. Italian Journal of Food Science, 5(4), 341–353.
- Rotmagnani, S. (1998). The Th1/Th2 paradigm and allergic disorders. Allergy, 53(s46), 12–15. doi: 10.1111/j.1398-9995.1998.tb04951.x
- Satter, M. A., Sakai, K., Ahmed, S., Yoshino, K., Yamamoto, S., Shimizu, Y., … Ota, F. (2002). Low-protein diet induces oral tolerance to ovalbumin in mice. Journal of Nutritional Science and Vitaminology, 48(1), 51–58. doi: 10.3177/jnsv.48.51
- Stadler, R. H., & Lineback, D. R. (2008). Process-induced food toxicants: Occurrence, formation, mitigation, and health risks. Hoboken, NJ: John Wiley & Sons.
- Stirling, R., & Chung, K. (2000). New immunological approaches and cytokine targets in asthma and allergy. European Respiratory Journal, 16(6), 1158–1174. doi: 10.1034/j.1399-3003.2000.16f24.x
- Tripathi, P., Nair, S., & Arora, N. (2013). Supplementation of antioxidants glutathione and α-lipoic acid attenuates oxidative stress and Th2 response in allergic airway inflammation. Indian Journal of Allergy, Asthma and Immunology, 27(1), 19–26. doi: 10.4103/0972-6691.116608
- Truzzi, C., Annibaldi, A., Illuminati, S., Finale, C., Rossetti, M., & Scarponi, G. (2012). Determination of very low levels of 5-(Hydroxymethyl)-2-furaldehyde (HMF) in natural honey: Comparison between the HPLC technique and the spectrophotometric white method. Journal of Food Science, 77(7), C784–C790. doi: 10.1111/j.1750-3841.2012.02782.x
- Wu, Z., Turner, D. R., & Oliveira, D. B. (2002). Antioxidants inhibit mercuric chloride-induced early vasculitis. International Immunology, 14(3), 267–273. doi: 10.1093/intimm/14.3.267
- Ya, B., Zhang, L., Zhang, L., Li, Y., & Li, L. (2012). 5-Hydroxymethyl-2-furfural prolongs survival and inhibits oxidative stress in a mouse model of forebrain ischemia. Neural Regeneration Research, 7(22), 1722–1728.
- Yamada, P., Nemoto, M., Shigemori, H., Yokota, S., & Isoda, H. (2011). Isolation of 5-(hydroxymethyl) furfural from Lycium chinense and its inhibitory effect on the chemical mediator release by basophilic cells. Planta Medica-Natural Products and Medicinal Plant Research, 77(5), 434–440.
- Zhang, J.-H., Di, Y., Wu, L.-Y., He, Y.-L., Zhao, T., Huang, X., … Zhu, L.-L. (2015). 5-HMF prevents against oxidative injury via APE/Ref-1. Free Radical Research, 49(1), 86–94. doi: 10.3109/10715762.2014.981260
- Zhao, L., Chen, J., Su, J., Li, L., Hu, S., Li, B., … Chen, T. (2013). In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. Journal of Agricultural and Food Chemistry, 61(44), 10604–10611. doi: 10.1021/jf403098y