ABSTRACT
Licorice has immunomodulatory and anti-asthmatic properties. This study aims to test the effect of licorice on pulmonary fibrosis (PF). PF was induced in mice through bleomycin instillation. One group of the mice received bleomycin (positive control), another one received phosphate-buffered saline and the other two received 300 mg/kg of the extracts for 20 days after the bleomycin instillation. Hematoxylin-eosin and Masson’s staining were used in the lung tissues. The expression of α-SMA, TGF-β1, IL-1β, and TNF-α were evaluated using reverse-transcriptase-polymerase-chain-reaction. Bleomycin led to tissue-degenerative changes, collagen deposition, and overexpression of the genes in the lung tissues. In the mice receiving the aqueous extract, the weight gain was higher (p < .004), and tissue-degenerative changes (p < .001) and collagen deposition were lower than the positive control. The expressions of the genes were lower than the positive control. These results were not observed in the hydroalcoholic extract. Aqueous extract of licorice alleviated the severity of PF.
Introduction
Millions of people around the world, for unknown reasons, are affected by the idiopathic pulmonary fibrosis. So far, no specific treatment has been found for this condition (Nalysnyk, Cid-Ruzafa, Rotella, & Esser, Citation2012). Since pulmonary fibrosis (PF) is one of the side effects of the chemotherapy agent, bleomycin, the animal model of intratracheal instillation of bleomycin is employed for the studies (Mouratis & Aidinis, Citation2011). Various factors (including bleomycin, which injures the pulmonary epithelial cells) lead to the activation of innate immunity response as well as the secretion of pro-inflammatory cytokines such as IL-1β and TNF-α (Luzina, Todd, Sundararajan, & Atamas, Citation2014; Wynn, Citation2011). Immune responses lead to the influx of leukocytes into the inflamed tissues. These activated cells result in the release of reactive oxygen species and proteases, which then aggravates the tissue injury (Todd, Luzina, & Atamas, Citation2012). TGF-β1 is an important cytokine in the inflammatory process leading to tissue fibrosis, which along with TNF-α and IL-6 result in fibroblasts proliferation, and finally induces the differentiation of these cells into myofibroblasts (O’Donoghue et al., Citation2012; Xiao et al., Citation2012). Expression of α-SMA is one of the characteristics of myofibroblasts. Myofibroblasts cause the increased secretion of extracellular matrix, the production of hydroxyproline as well as the collagen deposition in the lung tissues (Scotton & Chambers, Citation2007; Waisberg, Parra, Barbas-Filho, Fernezlian, & Capelozzi, Citation2012). This process leads to reduced alveolar space, abnormal accumulation of connective tissue cells and collagen deposition in the lung tissues (Fernandez & Eickelberg, Citation2012; Wynn, Citation2008).
Unfortunately, current treatments (including the anti-inflammatory and immunosuppressive ones) do not show favorable results in fibrosis prevention; thus, many studies are carried out to develop new anti-fibrotic medications (Meyer, Citation2014). Over the recent years, the effects of herbal extracts and their components on fibrotic process have been studied and their positive effects have been frequently reported. Huajie et al. demonstrated that Tanshinone IIA can reduce the accumulation of the inflammatory cells, expression of TGF-β1, and collagen deposition, which result in the alleviation of bleomycin-induced PF (Wu et al., Citation2014). It has been shown that Boswellic acids extract decreases the expression of TGF-β1 and TNF-α, and the accumulation of neutrophils, eosinophils and fibroblasts in the lung tissues of the rats afflicted with PF (Ali & Mansour, Citation2011). Tu Ji et al. reported that Paeoniflorin can alleviate the bleomycin-induced PF through the reduced levels of TGF-β1 and collagen type I, the collagen deposition and the accumulation of inflammatory cells in the lung tissues (Ji et al., Citation2013).
Because of its anti-inflammatory, anti-asthmatic and antioxidant properties, the licorice root might be effective against PF (Aly, Al-Alousi, & Salem, Citation2005; Asl & Hosseinzadeh, Citation2008; Chin et al., Citation2007). Lin et al. reported that the simultaneous use of Glycyrrhiza glabra, Salvia miltiorrhiza and Ligusticum chuanxiong can effectively attenuate the mRNA expression of COL1A, α-SMA and TGF-β1, and also reduce the collagen deposition and the accumulation of inflammatory cells in the fibrotic hepatic tissues of the rats (Lin, Hsu, Chiu, & Huang, Citation2008). Ram et al. investigated the effect of glycyrrhizin, a component of licorice, on inflammatory factors in the asthma animal model. They demonstrated that glycyrrhizin could reduce the levels of IL-4, IL-5 and IFN-γ in BALB-C mice. Therefore, glycyrrhizin can mitigate the severity of asthma inflammation (Ram et al., Citation2006). Furthermore, licorice contains flavonoids (including glabridin) that has anti-inflammatory and antioxidant properties (Kang et al., Citation2005; Kim et al., Citation2013). As licorice extract contains all these components, it seems effective against fibrosis. This study aims to investigate the effects of hydroalcoholic and aqueous extracts of licorice root on the bleomycin-induced PF in C57BL/6 mice.
Materials and methods
Preparation of aqueous and ethanolic extracts of licorice root
To prepare the extracts of licorice, its root was collected and verified by our experts. To prepare the ethanolic extract (hydroalcoholic), 10 grams of the licorice root was ground; then it was macerated in 100 ml of ethanol 80% (v/v) (Merck, Germany), and incubated for 8 hours at the ambient temperature in darkness. The extract, then, was filtered and dried in a shaker incubator at 40°C. Subsequently, the extract was obtained and stored at –20°C until use. To prepare the aqueous extract, 0.1 gram of the licorice powder was dissolved in 1 ml distilled water, and then filtered. The mice were gavaged with hydroalcoholic or aqueous extracts of licorice in two independent groups with a final concentration of 300 mg/kg (Huo, Wang, Liang, Bao, & Gu, Citation2011). The yield of ethanol extraction method is 15% of the dry powder weight.
Animals
C57BL/6 male mice aged 6–8 weeks weighing 20–30 grams were purchased from the Pasteur Institute of Iran, Tehran. They were kept in the animal house of Kurdistan University of Medical Sciences with a 12-hour light/dark photoperiod at 22–24°C. The mice were fed with pellets and had access to enough amounts of water and feed. The experiments were conducted in accordance with the Declaration of Helsinki.
Induction of PF
The mice were anesthetized using ketamine (Rotexmedica, Germany) and xylazine (Sigma, USA). Then, an incision was made on the anterior side of the neck, and after fixing the trachea, 2 mg/kg of bleomycin sulfate (Nippon Kayaku, Japan), 50 µL, was instilled in the trachea (the positive control). For the negative control, only the solvent of bleomycin phosphate-buffered saline (PBS) was instilled. For the other two, 24 hours after the bleomycin instillation, the aqueous and hydroalcoholic extracts of licorice root were administered daily for 20 days. On day 21, mice were euthanized and their lungs were removed for pathological analysis and gene expression examination (Gharaee-Kermani, Ullenbruch, & Phan, Citation2005).
Weighing the mice
The mice were weighed every three days using a digital scale, and the weight changes were recorded.
Histopathology
After removing the superior lobe of the right lung, the tissues were placed in the Bouin’s solution for 24 hours, and then the paraffin blocks were prepared. Finally, 5 µm sections were cut, then hematoxylin-eosin as well as Masson’s trichrome stainings were utilized to evaluate the histological changes and collagen deposition, respectively. In order to assess the changes of lung more accurately, the percentage of alveolar spaces and pulmonary connective tissues were calculated using morphometric analysis and Graticule checkerboard 18×kpl-w12.5.
Reverse-transcriptase-polymerase-chain-reaction
The small lobe of the right lung was homogenized in liquid nitrogen. The RNA of this tissue was extracted according to the manufacturer’s protocol (Pars-Tous, Iran). Its optimal density was measured at 260 nm and then the RNA concentration was calculated. Afterwards, 1 µg of RNA was used for cDNA synthesis using the kit (Pars-Tous, Iran). The cDNA was used for PCR reaction. In the PCR, Taq polymerase, dNTP and MgCl2 were used with the concentration of 2.5 unit/reaction, 0.1 mM, and 1.5 mM, respectively. Expression of α-SMA, TGF-β1, IL-1β and TNF-α were investigated using designed specific primers. The β-actin gene was used as the internal control (). PCR profile was 94°C 30 seconds, 56°C 30 seconds and 72°C 45 seconds, which was repeated for 40 cycles.
Table 1. Sequences of the primers designed to determine the expression of α-SMA, TGF-β1, IL-1β, TNF-α and β-actin in total mRNA extracted from the lung tissues of the C57BL/6 mice.
Statistical analysis
Using the Kruskal–Wallis, differences in bodyweight changes of the mice, alveolar space and connective tissue were compared by SPSS software, version 12.
Results
Bodyweight changes in the mice
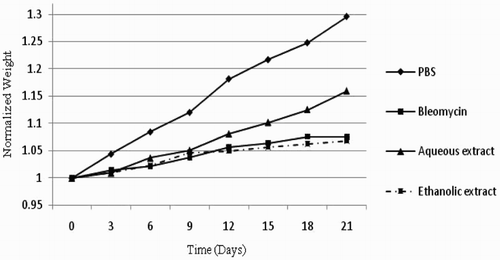
In the group receiving PBS (the negative control), the increase in bodyweight was higher than that of other groups (p < .05). The mice receiving bleomycin (the positive control) showed lower increase in bodyweight compared to the group receiving PBS (p = .002). The bodyweight of the mice receiving aqueous extract of licorice and bleomycin showed significant increase compared to the positive control (p = .004). However, the bodyweight of the mice receiving bleomycin plus hydroalcoholic licorice extract did not show significant increase compared to the positive control (p = .06) ().
Figure 1. Changes in the bodyweight of the C57BL/6 mice during the 21-day period. Weight gain of the mice receiving bleomycin plus fresh aqueous extract of licorice was significantly higher than those receiving only bleomycin (p = .004). The data were normalized in all groups: PBS (the negative control), Bleomycin (the positive control), Ethanolic extract (Bleomycin+ethanolic extract of licorice) and Aqueous extract (Bleomycin+aqueous extract of licorice).
Histopathologic results
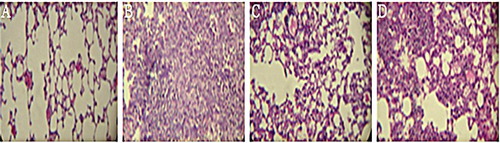
For the mice receiving only bleomycin, the alveolar space decreased significantly and the connective tissue increased significantly compared to the mice receiving PBS (p < .001). Alveolar space in the group receiving aqueous extract increased significantly compared to the one receiving only bleomycin (p < .001), while the connective tissue showed a significant decrease (p < .001). Nevertheless, no significant changes were observed in the lung tissues in the mice receiving the hydroalcoholic extract ().
Figure 2. Hematoxylin-eosin staining of the lung tissues sections. (A) Sections of the group receiving PBS (negative control), which shows normal lung tissues. (B) Sections of the group receiving bleomycin (positive control): a reduction in alveolar space and an increase in the accumulation of connective tissue can be seen. (C) Sections of the group receiving aqueous extract of licorice for 20 days after the administration of bleomycin. In this group, the alveolar space increased and the connective tissue decreased compared to the positive control. (D) The group receiving hydroalcoholic extract of licorice for 20 days following bleomycin instillation had no significant changes compared to the bleomycin group (the positive control). Magnification 400×.
Collagen deposition
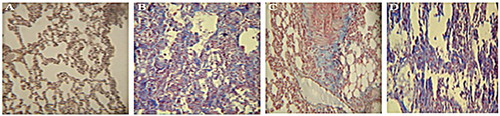
Collagen deposition in the group receiving bleomycin was higher compared to the group receiving PBS. It showed a decrease in the recipient of the aqueous extract compared to the group receiving bleomycin. However, in the group receiving the hydroalcoholic extract, the collagen deposition showed no difference compared to the group receiving bleomycin ().
Figure 3. Masson’s trichrome staining in the lung tissue sections. (A) Sections in the group receiving PBS (the negative control) in which collagen fibers (blue) are not seen. (B) Sections for the group receiving bleomycin in which collagen deposition is seen. (C) Sections for the group receiving the aqueous extract of licorice in which collagen deposition decreased compared to the group receiving bleomycin. (D) Sections for the group receiving hydroalcoholic extract of licorice in which an abundance of the collagen deposition is seen. Magnification 400×.
Gene expression
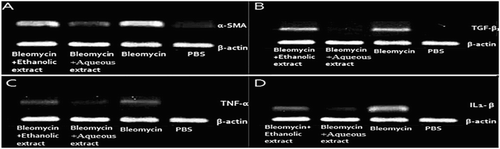
The expression of α-SMA, TGF-β1, IL-1β and TNF-α in the group receiving bleomycin showed an increase compared to the group receiving PBS. The expression of these genes in the group receiving aqueous extract showed a decrease compared to the group receiving bleomycin, whereas such difference was not observed in the group receiving hydroalcoholic extract ().
Figure 4. Amplification products were electrophoresed on 2% agarose gel: (A) 21 days after bleomycin instillation, the expression of α-SMA was increased in the lung tissues. However, aqueous extract of licorice reduced the expression of this gene. (B) TGF-β1 expression in the lung tissues receiving aqueous extract decreased compared to the group receiving bleomycin. (C) TNF-α expression in the lung tissues receiving bleomycin increased and the expression of those receiving aqueous extract decreased compared to the group receiving bleomycin. (D) IL-1β expression in the lung tissues receiving aqueous extract was lower than the group receiving bleomycin. PBS (negative control), Bleomycin (positive control), Ethanolic extract (Bleomycin+ethanolic extract of licorice) and Aqueous extract (Bleomycin+aqueous extract of licorice).
Discussion
After bleomycin injury to epithelial cells of the lung, the innate immunity cells will activate and pro-inflammatory cytokines such as IL-1 and TNF-α cause a broad migration of immune cells to the tissue, which result in the aggravation of tissue injury. Consequently, the presence of some growth factors such as TGF-β1 causes collagen deposition, pathological structural changes in bronchioles and finally lung tissue fibrosis (Fernandez & Eickelberg, Citation2012; Wynn, Citation2008). In the present study, we demonstrate that licorice extract decreases the expression of some genes associated with fibrosis, and it alleviates some degenerative changes in the lung tissues. This has been the first report on the effect of licorice extract on the animal model of PF.
In this study, we observed that licorice extract reduced the expression of IL-1β and TNF-α. It also reduced collagen deposition in the lung tissues. Lin et al. demonstrated that the combination of G. glabra, S. miltiorrhiza and L. chuanxiong extracts significantly reduced the severity of the hepatic fibrosis. These extracts reduced the expression of NF-κB, α-SMA, TGF-β1, COLA2, iNOS and ICAM, and they also diminished the collagen content in the liver tissue of the rats studied (Lin et al., Citation2008). Kim KR et al. investigated the effect of licorice extracts in the acute inflammation model of the middle ear. In this model, licorice extract caused reduced levels of IL-1β and TNF-α in the serum. In addition, the reduction of these cytokines was observed in collagen-induced arthritis after oral administration of licorice extracts (Kim et al., Citation2010).
IL-1β and TNF-α were the initiators of the inflammation, and TNF-α is the growth factor as well as being a stimulator of fibroblasts causing collagen secretion (Keane, Citation2008; Wynn, Citation2011). The inhibitor of IL-1β in the rats caused reduced inflammation and reduced the severity of PF (Guo et al., Citation2013). Since one of the properties of fibrosis is increasing the collagen secreted by fibroblasts and myofibroblasts in connective tissue of the lungs (Kendall & Feghali-Bostwick, Citation2014), the reduction in the cytokines, IL-1β and TNF-α can result in reduced severity of fibrosis. Histopathological investigations of the lung tissues in the present study showed a reduction in the collagen deposition.
In addition, we observed that the aqueous extract reduced the expression of TGF-β1. TGF-β1 has an important role in determining the direction of inflammation in fibrosis (Han, Li, Singh, Wolf, & Wang, Citation2012; Li & Flavell, Citation2008). In chronic inflammations, TGF-β1 increases the accumulation and proliferation of fibroblasts, and differentiates fibroblasts into myofibroblasts, which leads to collagen secretion and deposition (Usuki, Matsuda, Azuma, Kudoh, & Gemma, Citation2012; Xiao et al., Citation2012). The use of TGF-β1 soluble receptor led to reduced severity of PF (Wang, Hyde, Gotwals, & Giri, Citation2002). In addition, interrupting the signaling of TGF-β1 by knocking out smad-3 can prevent PF (Bonniaud et al., Citation2004). In the present study, licorice extract decreased collagen deposition in the lung tissues through the reduction of TGF-β1expression.
As we observed, aqueous extract reduced the expression of α-SMA of the lung tissues. The increased expression of α-SMA was an indicator of the stimulation of fibroblast cells, their differentiation into myofibroblasts and the production of collagen (Scotton & Chambers, Citation2007), while the reduction in α-SMA indicates a decrease in PF (Ju et al., Citation2012). In their study, Lin et al. demonstrated that the combination of G. glabra, S. miltiorrhiza and L. chuanxiong extracts reduced the expression of α-SMA in the hepatic fibrosis (Lin et al., Citation2008). According to Ying et al., one of the derivatives of licorice called 18α-glycyrrhizin decreased the gene expression and protein production of α-SMA in the liver cells (Qu, Chen, Zong, Xu, & Lu, Citation2012).
Glycyrrhizin, a component of licorice root, can reduce the expression of NF-κB as well as the expression of the inflammatory cytokines such as TNF-α and IL-1β. Consequently, it can be considered as an anti-inflammatory agent (Kim et al., Citation2006; Thiyagarajan, Chandrasekaran, Deepak, & Agarwal, Citation2011). Glycyrrhizin modulates the acute pulmonary injury caused by lipopolysaccharide via the reduction of IL-1, TNF-α and other inflammatory mediators (Ni et al., Citation2011). In addition, glycyrrhizin decreases the production of IL-4, IL-5 and IL-13, and it reduced the infiltration of inflammatory cells as well as reducing the inflammation and spasm in bronchioles, which resulted in reduced severity of asthma (Ma et al., Citation2013). Having a flavonoid such as glabridin, which has a modulating effect in oxidative stress caused by tissue injuries, licorice can prevent inflammation (Kim et al., Citation2013). Besides, due to reduced AP-1, NF-κB and MAP kinase as well as having phytoestrogenic properties, glabridin can be considered as an anti-inflammatory agent (Kang et al., Citation2005; Kwon, Oh, & Kim, Citation2008).
In this study, unlike the fresh aqueous extract, the hydroalcoholic extract did not reduce the severity of PF in the mice. Tian, Yan, and Row (Citation2008) showed that glycyrrhizin and glabridin exist in aqueous and hydroalcoholic extracts of licorice. However, glycyrrhizin content reduced significantly due to an increased ratio of ethanol to water. The reduced effects of the hydroalcoholic extract, compared to the aqueous extract, on PF can be a result of reduced glycyrrhizin in hydroalcoholic extract. Glabridin content, however, in both extracts was not significantly different (Tian et al., Citation2008).
Finally, the results show that the licorice extract causes reduced expression of important genes in PF, collagen deposition, connective tissue and increased alveolar space, thereby reduced severity of PF. Moreover, fresh aqueous extract was more effective than hydroalcoholic extract.
Acknowledgements
This paper was the result of the final thesis funded by the Kurdistan University of Medical Sciences. The authors gratefully appreciate the kind help of Dr Anjamrooz SH, Department of Anatomy, in the development of pulmonary fibrosis model.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Mehri Ghorashi is MSc of immunology and her research interests include pulmonary fibrosis and immunopharmacology.
Mohammad Ali Rezaee is MSc of immunology at the Kurdistan University of Medical Sciences. His research interests include pulmonary fibrosis and cell therapy.
Mohammad Jafar Rezaie is an associate professor of anatomy at the Kurdistan University of Medical Sciences. His research interests include pulmonary fibrosis and cancer.
Mehdi Mohammadi is MSc of immunology at the Kurdistan University of Medical Sciences. His research interests include pulmonary fibrosis, immunomodulation and cell therapy.
Ali Jalili is an associate professor of immunology at the Kurdistan University of Medical Sciences. His research interests include pulmonary fibrosis and cancer.
Mohammad Reza Rahmani is an associate professor of immunology at the Kurdistan University of Medical Sciences. His research interests include pulmonary fibrosis and cell therapy.
Additional information
Funding
References
- Ali, E. N., & Mansour, S. Z. (2011). Boswellic acids extract attenuates pulmonary fibrosis induced by bleomycin and oxidative stress from gamma irradiation in rats. Chinese Medicine, 6, 36. doi:10.1186/1749-8546-6-36
- Aly, A. M., Al-Alousi, L., & Salem, H. A. (2005). Licorice: a possible anti-inflammatory and anti-ulcer drug. AAPS PharmSciTech, 6(1), E74–E82. doi:10.1208/pt060113
- Asl, M. N., & Hosseinzadeh, H. (2008). Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytotherapy Research, 22(6), 709–724. doi:10.1002/ptr.2362
- Bonniaud, P., Kolb, M., Galt, T., Robertson, J., Robbins, C., Stampfli, M., … Gauldie, J. (2004). Smad3 null mice develop airspace enlargement and are resistant to TGF-beta-mediated pulmonary fibrosis. The Journal of Immunology, 173(3), 2099–2108. doi: 10.4049/jimmunol.173.3.2099
- Chin, Y. W., Jung, H. A., Liu, Y., Su, B. N., Castoro, J. A., Keller, W. J., … Kinghorn, A. D. (2007). Anti-oxidant constituents of the roots and stolons of licorice (Glycyrrhiza glabra). Journal of Agricultural and Food Chemistry, 55(12), 4691–4697. doi:10.1021/jf0703553
- Fernandez, I. E., & Eickelberg, O. (2012). New cellular and molecular mechanisms of lung injury and fibrosis in idiopathic pulmonary fibrosis. The Lancet, 380(9842), 680–688. doi:10.1016/s0140-6736(12)61144-1
- Gharaee-Kermani, M., Ullenbruch, M., & Phan, S. H. (2005). Animal models of pulmonary fibrosis. Methods in Molecular Medicine, 117, 251–259. doi:10.1385/1-59259-940-0:251
- Guo, J., Gu, N., Chen, J., Shi, T., Zhou, Y., Rong, Y., … Chen, W. (2013). Neutralization of interleukin-1 beta attenuates silica-induced lung inflammation and fibrosis in C57BL/6 mice. Archives of Toxicology, 87(11), 1963–1973. doi:10.1007/s00204-013-1063-z
- Han, G., Li, F., Singh, T. P., Wolf, P., & Wang, X. J. (2012). The pro-inflammatory role of TGFbeta1: a paradox? International Journal of Biological Sciences, 8(2), 228–235. doi: 10.7150/ijbs.8.228
- Huo, H. Z., Wang, B., Liang, Y. K., Bao, Y. Y., & Gu, Y. (2011). Hepatoprotective and antioxidant effects of licorice extract against CCl(4)-induced oxidative damage in rats. International Journal of Molecular Sciences, 12(10), 6529–6543. doi:10.3390/ijms12106529
- Ji, Y., Wang, T., Wei, Z. F., Lu, G. X., Jiang, S. D., Xia, Y. F., & Dai, Y. (2013). Paeoniflorin, the main active constituent of Paeonia lactiflora roots, attenuates bleomycin-induced pulmonary fibrosis in mice by suppressing the synthesis of type I collagen. Journal of Ethnopharmacology, 149(3), 825–832. doi:10.1016/j.jep.2013.08.017
- Ju, W., Zhihong, Y., Zhiyou, Z., Qin, H., Dingding, W., Li, S., … An, H. (2012). Inhibition of alpha-SMA by the ectodomain of FGFR2c attenuates lung fibrosis. Molecular Medicine, 18, 992–1002. doi:10.2119/molmed.2011.00425
- Kang, J. S., Yoon, Y. D., Cho, I. J., Han, M. H., Lee, C. W., Park, S. K., & Kim, H. M. (2005). Glabridin, an isoflavan from licorice root, inhibits inducible nitric-oxide synthase expression and improves survival of mice in experimental model of septic shock. Journal of Pharmacology and Experimental Therapeutics, 312(3), 1187–1194. doi:10.1124/jpet.104.077107
- Keane, M. P. (2008). The role of chemokines and cytokines in lung fibrosis. European Respiratory Review, 17(109), 151–156. doi:10.1183/09059180.00010908
- Kendall, R. T., & Feghali-Bostwick, C. A. (2014). Fibroblasts in fibrosis: Novel roles and mediators. Frontiers in Pharmacology, 5, 33483. doi:10.3389/fphar.2014.00123
- Kim, H. S., Suh, K. S., Ko, A., Sul, D., Choi, D., Lee, S. K., & Jung, W. W. (2013). The flavonoid glabridin attenuates 2-deoxy-D-ribose-induced oxidative damage and cellular dysfunction in MC3T3-E1 osteoblastic cells. International Journal of Molecular Medicine, 31(1), 243–251. doi:10.3892/ijmm.2012.1172
- Kim, J. K., Oh, S. M., Kwon, H. S., Oh, Y. S., Lim, S. S., & Shin, H. K. (2006). Anti-inflammatory effect of roasted licorice extracts on lipopolysaccharide-induced inflammatory responses in murine macrophages. Biochemical and Biophysical Research Communications, 345(3), 1215–1223. doi:10.1016/j.bbrc.2006.05.035
- Kim, K. R., Jeong, C. K., Park, K. K., Choi, J. H., Park, J. H., Lim, S. S., & Chung, W. Y. (2010). Anti-inflammatory effects of licorice and roasted licorice extracts on TPA-induced acute inflammation and collagen-induced arthritis in mice. Journal of Biomedicine and Biotechnology, 709378. doi:10.1155/2010/709378
- Kwon, H. S., Oh, S. M., & Kim, J. K. (2008). Glabridin, a functional compound of liquorice, attenuates colonic inflammation in mice with dextran sulphate sodium-induced colitis. Clinical & Experimental Immunology, 151(1), 165–173. doi:10.1111/j.1365-2249.2007.03539.x
- Li, M. O., & Flavell, R. A. (2008). Contextual regulation of inflammation: A duet by transforming growth factor-beta and interleukin-10. Immunity, 28(4), 468–476. doi:10.1016/j.immuni.2008.03.003
- Lin, Y. L., Hsu, Y. C., Chiu, Y. T., & Huang, Y. T. (2008). Antifibrotic effects of a herbal combination regimen on hepatic fibrotic rats. Phytotherapy Research, 22(1), 69–76. doi:10.1002/ptr.2265
- Luzina, I. G., Todd, N. W., Sundararajan, S., & Atamas, S. P. (2014). The cytokines of pulmonary fibrosis: Much learned, much more to learn. Cytokine. doi:10.1016/j.cyto.2014.11.008
- Ma, C., Ma, Z., Liao, X. L., Liu, J., Fu, Q., & Ma, S. (2013). Immunoregulatory effects of glycyrrhizic acid exerts anti-asthmatic effects via modulation of Th1/Th2 cytokines and enhancement of CD4(+)CD25(+)Foxp3+ regulatory T cells in ovalbumin-sensitized mice. Journal of Ethnopharmacology, 148(3), 755–762. doi:10.1016/j.jep.2013.04.021
- Meyer, K. C. (2014). Diagnosis and management of interstitial lung disease. Translational Respiratory Medicine, 2, 4. doi:10.1186/2213-0802-2-4
- Mouratis, M. A., & Aidinis, V. (2011). Modeling pulmonary fibrosis with bleomycin. Current Opinion in Pulmonary Medicine, 17(5), 355–361. doi:10.1097/MCP.0b013e328349ac2b
- Nalysnyk, L., Cid-Ruzafa, J., Rotella, P., & Esser, D. (2012). Incidence and prevalence of idiopathic pulmonary fibrosis: Review of the literature. European Respiratory Review, 21(126), 355–361. doi:10.1183/09059180.00002512
- Ni, Y. F., Kuai, J. K., Lu, Z. F., Yang, G. D., Fu, H. Y., Wang, J., … Jiang, T. (2011). Glycyrrhizin treatment is associated with attenuation of lipopolysaccharide-induced acute lung injury by inhibiting cyclooxygenase-2 and inducible nitric oxide synthase expression. Journal of Surgical Research, 165(1), e29–35. doi:10.1016/j.jss.2010.10.004
- O’Donoghue, R. J., Knight, D. A., Richards, C. D., Prele, C. M., Lau, H. L., Jarnicki, A. G., … Mutsaers, S. E. (2012). Genetic partitioning of interleukin-6 signalling in mice dissociates Stat3 from smad3-mediated lung fibrosis. EMBO Molecular Medicine, 4(9), 939–951. doi:10.1002/emmm.201100604
- Qu, Y., Chen, W. H., Zong, L., Xu, M. Y., & Lu, L. G. (2012). 18alpha-Glycyrrhizin induces apoptosis and suppresses activation of rat hepatic stellate cells. Medical Science Monitor, 18(1), Br24–Br32. doi: 10.12659/MSM.882196
- Ram, A., Mabalirajan, U., Das, M., Bhattacharya, I., Dinda, A. K., Gangal, S. V., & Ghosh, B. (2006). Glycyrrhizin alleviates experimental allergic asthma in mice. International Immunopharmacology, 6(9), 1468–1477. doi:10.1016/j.intimp.2006.04.020
- Scotton, C. J., & Chambers, R. C. (2007). Molecular targets in pulmonary fibrosis: The myofibroblast in focus. Chest, 132(4), 1311–1321. doi:10.1378/chest.06-2568
- Thiyagarajan, P., Chandrasekaran, C. V., Deepak, H. B., & Agarwal, A. (2011). Modulation of lipopolysaccharide-induced pro-inflammatory mediators by an extract of Glycyrrhiza glabra and its phytoconstituents. Inflammopharmacology, 19(4), 235–241. doi:10.1007/s10787-011-0080-x
- Tian, M., Yan, H., & Row, K. H. (2008). Extraction of glycyrrhizic acid and glabridin from licorice. International Journal of Molecular Sciences, 9(4), 571–577. doi: 10.3390/ijms9040571
- Todd, N. W., Luzina, I. G., & Atamas, S. P. (2012). Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair, 5(1), 11. doi:10.1186/1755-1536-5-11
- Usuki, J., Matsuda, K., Azuma, A., Kudoh, S., & Gemma, A. (2012). Sequential analysis of myofibroblast differentiation and transforming growth factor-beta1/smad pathway activation in murine pulmonary fibrosis. Journal of Nippon Medical School, 79(1), 46–59. doi: 10.1272/jnms.79.46
- Waisberg, D. R., Parra, E. R., Barbas-Filho, J. V., Fernezlian, S., & Capelozzi, V. L. (2012). Increased fibroblast telomerase expression precedes myofibroblast alpha-smooth muscle actin expression in idiopathic pulmonary fibrosis. Clinics (Sao Paulo), 67(9), 1039–1046. doi: 10.6061/clinics/2012(09)10
- Wang, Q., Hyde, D. M., Gotwals, P. J., & Giri, S. N. (2002). Effects of delayed treatment with transforming growth factor-beta soluble receptor in a three-dose bleomycin model of lung fibrosis in hamsters. Experimental Lung Research, 28(6), 405–417. doi:10.1080/01902140290096700
- Wu, H., Li, Y., Wang, Y., Xu, D., Li, C., Liu, M., … Li, Z. (2014). Tanshinone IIA attenuates bleomycin-induced pulmonary fibrosis via modulating angiotensin-converting enzyme 2/ angiotensin-(1–7) axis in rats. International Journal of Medical Sciences, 11(6), 578–586. doi:10.7150/ijms.8365
- Wynn, T. A. (2008). Cellular and molecular mechanisms of fibrosis. The Journal of Pathology, 214(2), 199–210. doi:10.1002/path.2277
- Wynn, T. A. (2011). Integrating mechanisms of pulmonary fibrosis. The Journal of Experimental Medicine, 208(7), 1339–1350. doi:10.1084/jem.20110551
- Xiao, L., Du, Y., Shen, Y., He, Y., Zhao, H., & Li, Z. (2012). TGF-beta 1 induced fibroblast proliferation is mediated by the FGF-2/ERK pathway. Frontiers in Bioscience, 17, 2667–2674. doi: 10.2741/4077
