ABSTRACT
Pine nut (Pinus koraiensis) meal protein peptides (PNMPP), a kind of Korean pine seed oil by-products, were often considered as waste disposal in food industry. In the present study, we found that 3–10 kDa PNMPP could enhance immunity of ICR mice at 100 mg·(kg·d)−1 both in innate and in adaptive immunity. In innate immunity, PNMPP of 3–10 kDa at 100 mg·(kg·d)−1 could enhance the abdominal macrophage phagocytosis of mice at 30.22% and the phagocytosis index of carbon clearance significantly (P < .05). In addition, we found out that 3–10 kDa PNMPP at low dose could significantly enhance cytokine interferon-γ, interleukin-2, interleukin-4, and interleukin-6 (P < .05). Meanwhile, in adaptive immunity, PNMPP could enhance the median hemolytic concentration of mice. These findings suggested that 100 mg·(kg·d)−1, 3–10 kDa PNMPP could increase the function of immunity.
Abbreviations: ANOVA: analysis of variance; BSA: bull serum albumin; ConA: concanavalin A; CTX: cyclophosphamide; DW: deionized water; FCM: flow cytometry; IFN-γ: interferon-γ; IL-2: interleukin-2; IL-4: interleukin-4; IL-6: interleukin-6; MW: molecular weight; MPM: mouse peritoneal macrophages; OD: optical density; PBS: phosphate buffer solution; PNMPP: pine nut (Pinus koraiensis) meal protein peptides; SA: salicylic acid buffer; SRBC: sheep red blood cells; HC50: median hemolytic concentration
1. Introduction
The seed of Korean pine, Pinus koraiensis, has been used as an edible fruit for a long time because it contains essential nutrients, such as amino acids, fatty acids, and vitamins. Great progress has been made in pine nut oil field. Liu (Liu & Xu, Citation2012) applied steam distillation to extract valuable essential oils, and pine nut oil improves atherosclerotic markers and can be used as a functional ingredient in the food industry to promote cardiovascular health (Kang, Kim, Kim, & Choe, Citation2015). However, pine nut oil by-product has not been fully used. Young (Kim, Kim, Han, Park, & Watanabe, Citation2014) elucidated the pyrolysis characteristics of waste pine nut (P. koraiensis) shell, while pine nut (P. koraiensis) meal protein peptides (PNMPP) were often considered as a waste. To obtain peptides from proteins, the enzymatic hydrolysis of protein has proven to be the commonly used method, which has attracted more attention in the last decade (Wang et al., Citation2014). In a recent study, it was verified that protein peptide could enhance memory (Jia et al., Citation2014) and also had functions such as enhancing the antioxidant (Torres-Fuentes, Contreras, Recio, Alaiz, & Vioque, Citation2015), immunomodulatory (Huang, Chen, Chen, Hong, & Chen, Citation2010), anti-fatigue functions (Li, Liu, Wu, & Chen, Citation2012) and that it has no genetic toxicity. These facts have promoted the production and consumption of protein peptides, which has resulted in an increasing interest in the functional activity of peptides. As a traditional food, the functional development of pine nut is an irresistible trend. Since the immunity of food-borne functional peptides has not been discussed yet, we investigated the immunity of PNMPP in this research.
Currently, immunosuppressive drugs have been widely used to control undesired immune responses, such as autoimmune diseases, allergies, and allograft rejection. FK506, cyclophosphamide (CTX), and prednisone are typical immunosuppressive drugs that have been used in the clinic for many years (Zhang, Qin, & Sun, Citation2006). In the present study, we used CTX as an immunosuppressor to build an immunosuppressor model and focused on T lymphocytes and investigated the immunocompetence effect of PNMPP on mouse T cells both in innate and in adaptive immunity response aspects.
The mouse peritoneal macrophages (MPM) belong to a kind of mononuclear macrophage of mice immune cells, which is the most active biological effect in body cells and has the function of consuming and digesting the pathogens. At present, there are still many researchers using the experiment of red blood cells to evaluate the function of phagocytes (Ichinose, Hara, Sawada, & Maeno, Citation1992). Although this method has been used for years, there are still some limitations. The combining of fluorescent microsphere technique and flow cytometry (FCM) detecting method to measure the function of phagocytes has been used more in many fields (Xiong et al., Citation2013), which have the characteristics of being convenient, fast, and efficient. Fluorescent microsphere technology, combined with FCM detecting methods, was used in this study to evaluate whether PNMPP has an effect on the MPM’s ability of fluorescent microsphere or not.
It is known that cytokine, mainly including lymphocyte cytokine, monocyte cytokine and other cells cytokine, plays an important role in the immune response, such as the regulation of the body’s inflammatory response, cell proliferation, and differentiation. Meanwhile, it is beneficial for anti-infective, anti-tumor effect, and to regulate immunity in vivo. Guan (Guan et al., Citation2011) chose cytokine TNF-α, IL-1β, and interleukin-6 (IL-6) as targets to investigate anti-inflammatory effects on cytokine production by LPS-stimulated RAW 264.7 macrophages in vitro. Thus, in our study, cytokine levels were used as the target to evaluate the immune activities of PNMPP as well. To further investigate the effect of PNMPP on adaptive immunity in vitro (Liu, Wang, & Zhao, Citation2010), we selected the median hemolytic concentration (HC50) of mice as an index (Chingat, Delgadob, Salazara, & Soto, Citation2015) to compare with the carbon clearance index.
As people living standards improving, nutritional and functional food attract wider attention, therefore, development of functional food has become an inevitable trend for the future food industry. Since the efficiency of PNMPP was increased in this study, it could be forecasted that there was a huge practical application and market development value of PNMPP.
2. Materials and methods
2.1. Chemicals
Concanavalin A (ConA), Drabkin’s Reagent, and CTX were purchased from Sigma-Aldrich (St Louis, MO). Interleukin-2 (IL-2), interferon-γ (IFN-γ), interleukin-4 (IL-4), and IL-6 enzyme-linked immunosorbent assay kits were purchased from BioLegend (San Diego, CA). Roswell Park Memorial Institute-1640 (RPMI-1640) medium, fetal bovine sera were obtained from Invitrogen-Gibco (Grand Island, NY).
2.2. Reagents preparation
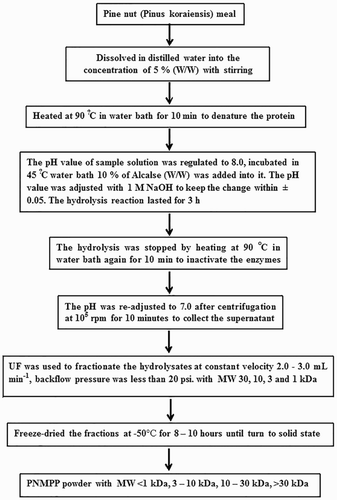
PNMPP was provided by the Laboratory of Nutrition and Functional Food, Jilin University (Changchun, China). The preparation process for PNMPP is shown in (Liu, Luo, & Li, Citation2011). The sheep red blood cells (SRBC) were shaken at a single direction for 10 min to do de-fibering, washed by deionized water (DW) for three times, and then centrifuged at 1500 r/min for 10 min. DW was added to obtain 2% red blood cell, kept at 4°C before use. The blood sample was collected from cavia procellus, the serum was separated and SRBC was added into it (1:5, V/V). After preserving at 4°C for 0.5 h, the supernatant was absorbed and kept at −70°C. The alexin was diluted with salicylic acid buffer (SA; 1:8, V/V) when used. Ten milliliters of bull serum albumin (BSA) was added into 200 mL Hank’s solution to get 5% BSA Hank’s solution; 0.5 g BSA was dissolved in 50 mL phosphate buffer solution (PBS) to obtain 1% BSA solution; 100 μL of fluorescent microspheres was added into 10 mL 1% BSA solution to get regulated liquid fluorescent microspheres and the mixture was kept at 37°C for 30 min and then treated by ultrasonic for 5 min.
2.3. Animal group design
Adult male ICR mice weighing 20 ± 2 g were purchased from Jilin University Experimental Animal Center (Changchun, Jilin, China) and observed under general condition (temperature 22 ± 2°C, relative humidity 30 ± 10%, 12 h light and dark cycle) for five days during the quarantine and acclimation period to confirm that there were no abnormalities. Mice were randomly divided into eight groups (10 animals per group) and were daily gavage-fed different molecular weights (MWs) of PNMPP and DW for 28 days. During intragastric administration, an immunocompromised mouse model was built by CTX. The mice were intraperitoneally injected immunosuppressants on the 22nd and 23rd days (). All of the procedures were carried out in strict accordance with the guidelines from the Care and Use of Laboratory Animals published by the US National Institutes of Health (Lu, Huang, Hu, Wang, & Guan, Citation2014).
Table 1. Animal groups design.
2.4. Carbon clearance test in mice
The mice were weighed and injected the India ink (diluted 3–4 times) via the tail vein (0.01 mL/g body weight). Blood samples were drawn from retro orbital vein at intervals of 2 and 10 min. A 20 μL of blood sample was mixed with 2 mL 0.1% Na2CO3. The optical density (OD) was measured at 600 nm on a microplate reader; Na2CO3 solution was used as the blank control. The mice were sacrificed to obtain the liver and spleen, dry the organ surface with filter paper, and were weighed. The Phagocytic index indicates the ability of mice to carbon clearance. The Phagocytic index is calculated by Equations (1) and (2), where OD1 and OD2 are optical densities of blood sample at 2 and 10 min, t1 is 2 min and t2 is 10 min, respectively.(1)
(2)
2.5. Hemolysis assays
The mice were intraperitoneally injected 2% (V/V) SRBC (0.2 mL). After four days, blood samples were collected from the eyes of the mice, stewing for 1 h until serum dissolved. Serum was then prepared by centrifugation at 1500 r/min for 5 min. A 10 μL serum sample was diluted by 1 mL SA (diluted 5 times). The reaction sample was obtained by 100 μL diluted serum sample added 50 μL SRBC (10%) and 100 μL diluted complement. The blank control sample was obtained by 100 μL SA added 50 μL SRBC (10%) and 100 μL diluted complement. The samples were stored at 37°C for 30 min in the water bath and then were centrifuged at 1500 r/min for 5 min. The supernatant of 50 μL was collected in another 96-well plates (Costar, Washington), mixed with 150 μL Drabkin’s Reagent. Set median hemolytic hole: 12.5 μL SRBC (10%, V/V) + 187.5 μL Drabkin’s Reagent, mixed by micro oscillator and stewed for 10 min, the OD540 was measured on a microplate reader. Compared with the control group, a sample group of HC50 was significantly higher (P < .05) and it could be considered that the experimental results were positive. The HC50 was calculated by Equation (3):(3)
2.6. Macrophage phagocytosis rate of fluorescent microsphere by FCM
Each mouse was injected 0.2 mL 2% SRBC intraperitoneally four days before the experiment. The mice were sacrificed by cervical dislocation. Each mouse was given 3 mL 5% BSA Hank’s solution by intraperitoneal injection. Then, each abdomen of mice was rubbed gently to make the peritoneal macrophages to flow out fully. Then, the abdomen was cut by a slot and the peritoneal macrophages lotion was drawn by syringe. After filtering the lotion, the concentration of the cell number was turned to 4 × 105 to 6 × 105 cells mL−1. Then, the peritoneal macrophages lotion was drawn 1 mL in six-well culture plate and added to regulate liquid fluorescent microspheres (1 × 107 cells·plate−1). The plate was put in a 5% CO2 cell incubator at 37°C for 2 h. After incubation, the cells were washed by PBS twice. A volume of 0.3 mL of PBS was added to each well and the adherent cells were scraped off. The phagocytic ability was reflected by the phagocytic rate (%) as in Equation (4):(4)
If there were significant differences between the experimental group and the control group, the result could be judged as positive.
2.7. Determination of cytokine levels in vivo
In order to obtain the splenocytes suspension of mice, the mouse spleen was removed and placed in 5 mL Hank’s solution containing plate in a sterile environment, the splenocytes suspension was filtered through a 200 mesh screen, and washed in Hank’s solution for three times, and then centrifuged at 1000 r/min for 5 min. Splenocytes collected from the ICR male mice under aseptic conditions were mechanically scraped and plated at a density of 5 × 109 cells mL−1 on 24-well plates containing 2 mL of RPMI 1640 complete medium added ConA (7.5 mg L−1). The plate was incubated at 37°C with 5% CO2 for 36 h. The splenocytes supernatants were centrifuged at 3000 r/min for 10 min until assayed for cytokines. Before determination, the ELISA kit was kept at room temperature for 20 min (Wróblewska et al., Citation2004). The concentrations of cytokine IFN-γ, IL-2, IL-4, IL-6 in the supernatants of splenocyte culture were measured by ELISA using commercially available reagents according to the instructions of the manufacturer (Dingguo Biological Technology Co., Beijing, China), and the OD450 value was measured by a microplate reader. Based on the standard concentration (), draw the standard curve which index was OD450 value. The concentration of the samples was calculated according to the standard curve (Lu et al., Citation2011).
Table 2. Standard curve of cytokine level.
2.8. Statistical analysis
Analysis of variance (ANOVA) was performed with SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). All experiments were performed in triplicate and data are expressed as mean ± standard deviation. Statistical analyses were performed using least significant difference and regression analysis. The significance of the regression coefficients was evaluated using an F-test. The fit quality of the polynomial model equation was expressed by R2. The regression coefficients were used for statistical calculation to generate the regression models. Significant differences were determined at 95% confidence intervals.
3. Results and discussion
3.1. Carbon clearance index
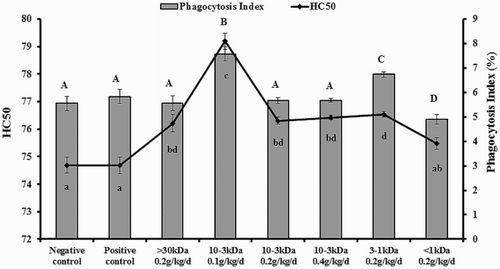
Carbon particles were injected into the blood via the tail vein and were carried to the liver, spleen by blood circulation, and then were removed by macrophage of the organs. Within a certain concentration range, the carbon clearance rate can reflect the phagocytosis of phagocytes, in other words, the body’s immune ability. As shows, compared with the control group, whose value was 5.549 ± 0.29%, the phagocytosis index of carbon clearance was related to the MWs and could be enhanced significantly when administered at the doses of 1–3 kDa and 3–10 kDa on mice (P < .05). The highest activity group was at the dose 3–10 kDa, and the highest value was 7.57 ± 0.28%. In addition, the phagocytosis index of carbon clearance on mice was significantly reduced at a dose of <1 kDa, and the value was 4.897 ± 0.20% (P < .05).
3.2. HC50 index
SRBC, as an antigen, stimulates the mice-secreted specific hemolysin. The amount of antibodies reflects the state of the body’s immune function and the specific immune body level; the level was expressed as HC50. The result of hemolysis is shown in , compared with the negative and positive control groups; after giving the mice PNMPP for four weeks, the content of serum hemolysin was increased significantly (P < .05). The highest mice serum hemolysin was 3–10 kDa low-dose group and the value was 79.19 ± 0.90. Thus, PNMPP could enhance the immunity of mice and 3–10 kDa PNMPP was the optimal composition.
3.3. Macrophage phagocytosis rate of fluorescent microsphere
The results are shown in and . Compared with the negative and positive groups, the macrophage phagocytosis fluorescent microspheres rate of the experimental group was significantly higher, except >30 kDa (P < .05). shows the amount of fluorescent microspheres measured by FCM; x-coordinate expresses the pulse height and the y-coordinate expresses the number of fluorescent microspheres. The number of fluorescent microspheres was the sum of M1 to M5, which could be approximately expressed as M1. The experiment measured 1 × 104 phagocytes marked with fluorescent. The less gated the number was, the larger the macrophage phagocytosis rate was, and the better immune activity was 3–10 kDa PPMNP; 100 mg·(kg·d)−1 had the strongest function that could enhance the abdominal macrophage phagocytosis at a value of 36.01 ± 5.79%.
Figure 3. Macrophage phagocytosis rate of fluorescent microsphere by FCM. Macrophages were stimulated in 6-well flat-bottom plates and cultured for 2 h, collected, and the fluorescent microspheres were calculated by flow cytometry. (a) Negative Control group, (b) Positive Control group, (c) >30 kDa 0.2 g/kg/d group, (d) 3–10 kDa 0.1 g/kg/d group, (e) 3–10 kDa 0.2 g/kg/d group, (f) 3–10 kDa 0.4 g/kg/d group, (g) 1–3 kDa 0.2 g/kg/d group, (h) <1 kDa 0.2 g/kg/d group. These results were from three independent experiments and presented as means ± S.D. Significant differences with control group were designated as P < 0.05.
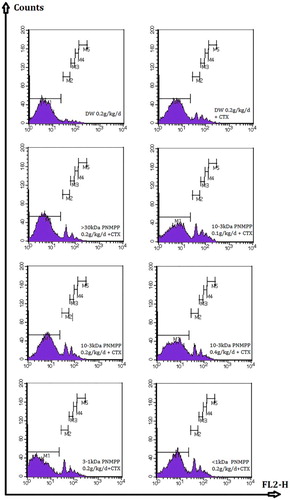
Table 3. The effects of PNMPP on MPM fluorescent microspheres.
3.4. Effect of PNMPP on cytokine secretion in vivo
3.4.1. Standard curve of cytokine level
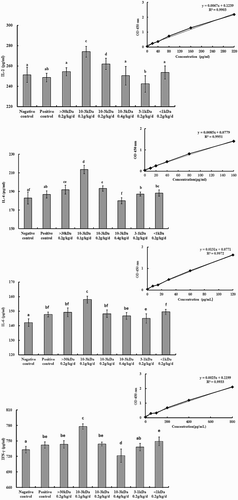
The results of standard curve are shown in . The parameters of the equation were obtained by Excel (Windows Software, USA). The fit of the curve was checked by the coefficient of determination R2, which was calculated to be 0.99. The higher the value of R2 is, the better the curves are adapted to the responses. The following quadratic model explains the standard curve of different cytokine levels (IL-2, IL-4, IL-6, IFN-γ).
3.4.2. Effect of PNMPP on cytokine secretion in vivo
The concentrations of cytokines in the culture supernatant of ConA-induced splenocytes were determined by ELISA. The results are shown in . Compared with the negative and positive groups, different MWs PNMPP had a different reaching influence on cytokine levels, especially on IL-2 and IFN-γ. The cytokine level on IL-2 was higher significantly in 3–10 kDa low-dose PPMNP (P < .05) whose value was 274.048 ± 5.26%, which indicated that the components could enhance the release of IL-2. As for cytokine IL-4, it was found that at the low and moderate dose in the MWs of 3–10 kDa, the concentrations of cytokines were significantly higher compared to the control groups as well (P < .05), and the value was 213.588 ± 4.49%. When it came to IL-6, IFN-γ, the cytokines levels were enhanced significantly at 3–10 kDa low dose (P < .05), and the values were 158.025 ± 2.4% and 777.95 ± 5.18%. The results indicated that PNMPP could enhance the cytokine levels of IL-2, IL-4, IL-6, and IFN-γ and the cytokine secretion was related to the MWs, and the most significant (P < .05) dose was in the MWs of 3–10 kDa at 100 mg·(kg·d)−1.
4. Conclusions
In this paper, the effect of PNMPP on innate and adaptive immunity response was observed, and the optimal composition was selected. PNMPP could enhance immunity in ICR mice at 100 mg·(kg·d)−1 both in innate and in adaptive immunity. In the innate immunity aspect, PNMPP of 3–10 kDa could enhance the phagocytosis index of carbon clearance significantly (P < .05). Moreover, 3–10 kDa PPMNP low dose had the strongest function to enhance the abdominal macrophage phagocytosis of mice and cytokine release. In adaptive immunity, 3–10 kDa PNMPP at low dose could enhance the HC50 of mice. These findings suggested that a low dose 3–10 kDa PNMPP could increase the function of immunity. This paper provided a reasonable solution for the development of pine nut by-products, and it is meaningful to further explore its action mechanism.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Songyi Lin is a Professor of College of Food Science and Engineering at Jilin University, China. She has a Ph.D. in Agricultural Mechanization Engineering from Jilin University, and her research interests are focused on food processing quality control methods and functional food quality control principles. Her work has been published in a number of different journals.
Xuanting Liu is a Master student of College of Food Science and Engineering at Jilin University, China. Her research interests are focused on functional food quality control techniques and principles, and her work has been published in a number of different journals.
Bolong Liu is an inspector of the food safety risk inspection and monitoring assessment center of Jilin province, China. He has a M.E. in Food Engineering from Jilin University, and his research interests are focused on food processing quality control methods. His work has been published in a number of different journals.
Yali Yu is an Associate Professor of College of Food Science and Engineering at Jilin University, China. She has a Ph.D. in Agricultural Mechanization Engineering from Jilin University, and her research interests are focused on the application of functional factor extracting from natural plant in food science and metabolic regulation of human, and her work has been published in a number of different journals.
References
- Chingat, S., Delgadob, G., Salazara, L. M., & Soto, C. Y. (2015). The ATPase activity of the mycobacterial plasma membrane is inhibited by the LL37-analogous peptide LLAP. Peptides, 71, 222–228. doi:10.1016/j.peptides.2015.07.021
- Guan, S., Feng, H. H., Song, B. C., Guo, W. X., Xiong, Y., Huang, G. R., … Deng, X. (2011). Salidroside attenuates LPS-induced pro-inflammatory cytokine responses and improves survival in murine endotoxemia. International Immunopharmacology, 11, 2194–2199. doi:10.1016/j.intimp.2011.09.018
- Huang, S. M., Chen, K. N., Chen, Y. P., Hong, W. S., & Chen, M. J. (2010). Immunomodulatory properties of the milk whey products obtained by enzymatic and microbial hydrolysis. International Journal of Food Science and Technology, 45, 1061–1067. doi:10.1111/j.1365-2621.2010.02239.x
- Ichinose, M., Hara, N., Sawada, M., & Maeno, T. (1992). The neuropeptide, neuromedin C, activates a potassium current in mouse macrophages. Cellular Immunology, 156, 508–518. doi:10.1016/0014-5793(92)81526-R
- Jia, Y. P., Liu, X. T., Lin, S. Y., Wang, K., Wang, S., Liu, H. R., & Jiang, Y. (2014). Effects of pine nut (Pinus koraiensis) meal protein peptides on memory function of mice. Advanced Materials Research, 881–883, 815–818. doi:10.4028/www.scientific.net/AMR.881-883.815
- Kang, Y. H., Kim, K. K., Kim, T. W., & Choe, M. (2015). Anti-atherosclerosis effect of pine nut oil in high-cholesterol and high-fat diet fed rats and its mechanism studies in human umbilical vein endothelial cells. Food Science and Biotechnology, 24(1), 323–332. doi:10.1007/s10068-015-0043-x
- Kim, Y. M., Kim, S., Han, T. U., Park, Y. K., & Watanabe, C. (2014). Pyrolysis reaction characteristics of Korean pine (Pinus Koraiensis) nut shellYoung. Journal of Analytical and Applied Pyrolysis, 110, 435–441. doi:10.1016/j.jaap.2014.10.013
- Li, H., Liu, E. Q., Wu, Y. H., & Chen, S. L. (2012). The anti-fatigue effect of black soybean peptide in mice. Advanced Materials Research, 554–556, 1475–1482. doi:10.4028/www.scientific.net/AMR.554-556.1475
- Liu, R., & Xu, B. J. (2012). Characterization of essential oil in pine nut shells from commodity waste in China by steam distillation and GC-MS. Food Analytical Methods, 5(3), 435–440. doi:10.1007/s12161-011-9264-7
- Liu, T. X., Wang, J., & Zhao, M. M. (2010). In vitro haem solubility of red cell fraction of porcine blood under various treatments. International Journal of Food Science and Technology, 45, 719–725. doi:10.1111/j.1365-2621.2010.02195.x
- Liu, X. Y., Luo, Y. K., & Li, Z. (2011). Effects of pH, temperature, enzyme-to-substrate ratio and reaction time on the antigenicity of casein hydrolysates prepared by papain. Food and Agricultural Immunology, 23(1), 69–82. doi:10.1080/09540105.2011.604770
- Lu, J., Guan, S., Shen, X., Qian, W. H., Huang, G. R., Deng, X. M., & Xie, G. H. (2011). Immunosuppressive activity of 8-gingerol on the immune responses in mice. Molecules, 16, 2636–2645. doi:10.3390/molecules16032636
- Lu, J., Huang, G. R., Hu, S. Z., Wang, Z. N., & Guan, S. (2014). 1,3-Dichloro-2-propanol induced hyperlipidemia in C57BL/6J mice via AMPK signaling pathway. Food and Chemical Toxicology, 64, 403–409. doi:10.1016/j.fct.2013.11.049
- Torres-Fuentes, C., Contreras, M. D. M., Recio, I., Alaiz, M., & Vioque, J. (2015). Identification and characterization of antioxidant peptides from chickpea protein hydrolysates. Food Chemistry, 180, 194–202. doi:10.1016/j.foodchem.2015.02.046
- Wang, K., Wang, Y., Lin, S. Y., Liu, X. Y., Yang, S. L., & Jones, G. S. (2014). Analysis of DPPH inhibition and structure change of corn peptides treated by pulsed electric field technology. Journal of Food Science and Technology, 52, 4351–4359. doi:10.1007/s13197-014-1450-3
- Wróblewska, B., Karamać, M., Amarowicz, R., Szymkiewicz, A., Troszynska, A., & Kubicka, E. (2004). Immunoreactive properties of peptide fractions of cow whey milk proteins after enzymatic hydrolysis. International Journal of Food Science and Technology, 39(8), 839–850. doi:10.1111/j.1365-2621.2004.00857.x
- Xiong, Y., Zhang, S., Lu, J., Sun, S. C., Song, B. C., Xu, L. L., … Guan, S. (2013). Investigation of effects of farrerol on suppression of murine T lymphocyte activation in vitro and in vivo. International Immunopharmacology, 16, 313–321. doi:10.1016/j.intimp.2013.04.010
- Zhang, Y. B., Qin, F., & Sun, H. X. (2006). Immunosuppressive activity of Semen Persicae ethanol extract on specific antibody and cellular response to ovalbumin in mice. Chemistry and Biodiversity, 3, 967–974. doi:10.1002/cbdv.200690105