ABSTRACT
The aim of this study was to determine if extracts from selected spices (caraway, ginger, chili, sweet peppers, anise, sesame, nutmeg and black pepper) might be harmful to people suffering from celiac disease, wheat allergy or non-celiac gluten sensitivity. All of these spice extracts exhibited some reaction to antibodies found in sera from two celiac patients and to sera from rabbits that had been sensitized with the specific peptides, QQQPP, PQQQ and QQQP. These peptides had sequences that might be included in active epitopes for celiac disease and wheat allergy. Methodology followed in this study included ELISA, SDS-PAGE and immunoblotting. The observed reactivities suggest that spice proteins might produce adverse reactions in celiac patients, patients with various wheat allergies or with non-celiac gluten sensitivity. However, further work would be needed to elucidate this possibility.
Introduction
Celiac disease (also known as celiac sprue or gluten-sensitive enteropathy) is an immune-mediated inflammatory disease of the small intestine, caused by intolerance to gluten, that occurs in genetically predisposed individuals (Haraszi, Chassaigne, Maquet, & Ulberth, Citation2011; Shan et al., Citation2002). The disease affects around 1% of the world population (Rewers, Citation2005), with at least 30,000 sufferers in Poland (Cielecka, Dereń, & Grzegorczyk, Citation2010). Celiac disease is a multifactorial disorder caused by a combination of many genetic (HLA and non-HLA genes) and environmental (gluten) factors. The genetic predisposition to celiac disease is strongly associated with particular gene variants within the human leukocyte antigen (HLA) complex, particularly HLA-DQ2 in the majority of patients and HLA-DQ8 in the minority of patients. These HLA molecules bind and present gluten peptides to specific T cells in the intestines of celiac patients. Abnormal immune responses to gluten lead to chronic inflammation, which results in villus atrophy, and disruption of intestinal digestion and absorption (Sollid, Citation2000, Citation2002). Classical symptoms of the disease include weight loss, anemia, osteoporosis, arthralgia, fatigue, abdominal discomfort, diarrhea and malabsorption of many nutrients, including folic acid, fat, soluble vitamins, iron and calcium (Sollid & Lundin, Citation2009).
Besides having a role in the pathogenesis of celiac disease, gluten is also an etiologic factor in two other disease entities: IgE-dependent wheat allergy and non-celiac gluten sensitivity (Haraszi et al., Citation2011).
Wheat allergy is IgE-mediated immediate immunologic reaction to wheat proteins. Wheat allergens include seeds storage proteins of wheat, that are, besides gluten proteins, non-gluten proteins such as i.a. albumins, globulins, α-amylase/trypsin inhibitors and LTP. Cross-linking of IgE antibodies by allergen leads to release of inflammatory mediators from basophils and mast cells. Allergic reactions to wheat or its products may occur via ingestion, inhalation or skin contact and include symptoms ranging from mild local reactions to anaphylaxis (Battais, Richard, Jacquenet, Denery-Papini, & Moneret-Vautrin, Citation2008). The most commonly reported ones include urticaria, atopic dermatitis (AD) and wheat-dependent exercise-induced anaphylaxia (WDEIA), while AD occurs mainly in children, urticaria and WDEIA concern adults (Räsänen, Lehto, Turjanmaa, Savolainen, & Reunala, Citation1994; Varjonen, Petman, & Makinen-Kiljunen, Citation2000; Varjonen, Vainio, & Kalimo, Citation1997).
Non-celiac gluten sensitivity (NCGS) is defined as a non-allergic and non-autoimmune condition in which the consumption of gluten can lead to symptoms similar to those seen in celiac disease (Catassi et al., Citation2013). Characteristic for this condition is the occurrence of clinical symptoms from gastrointestinal tract (diarrhea, bloating and abdominal pain) and other systems (easy fatigue and headache), which disappear when we exclude cereal grains from the diet. In this group of patients, diagnosis of wheat allergy or celiac disease proved to be negative. Pathomechanism of reaction is not explained (Bartuzi, Citation2014).
The gluten fractions responsible for gluten-related disorders are prolamins of wheat (gliadin), barley (hordein) and rye (secalin), which together make up around 50% of gluten proteins (Kagnoff, Citation2005). In this study, we focus on anti-gliadin antibodies, since gliadin (a monomeric protein fraction soluble in 70% ethanol) is the main protein responsible for allergic reactions to gluten, and wheat contains the most sequences toxic to celiac sufferers. As well as containing gliadin, wheat gluten also contains glutenin polymers (soluble in dilute acid or sodium hydroxide solutions). Both are plant storage proteins and constitute about 75% of the total protein in wheat grain (Belderok, Mesdag, & Donner, Citation2000; Khan et al., Citation2010; Tsuji, Kimoto, & Natori, Citation2001).
Analysis of the amino acid composition of gluten proteins reveals that they contain large proportions of proline (P) and glutamine (Q) – around 15% and 35%, respectively (Dewar, Pereira, & Ciclitira, Citation2004). The chemical structures of proline and glutamine cause the peptide bonds in their polypeptide chains to be resistant to the proteolytic enzymes found in the human gastrointestinal tract (Hausch, Shan, Santiago, Gray, & Khosla, Citation2002). Several studies have detected gluten peptides involved in pathogenesis of celiac disease and wheat allergy. The QQQPP motif has been identified as the main IgE-binding epitope in LMW glutenin for patients with atopic dermatitis (Tanabe et al., Citation1996). Cornell and De Ritis have identified the amino acid sequence motifs responsible for celiac disease as: QQQP, QQPY, PSQQ and QPYP. These tetrapeptides are present in 134 protein sequences of wheat, in 10 of barley, and in 9 oat protein sequences (Cornell, Citation1996; De Ritis et al., Citation1988).
Current treatment of gluten-related disorders is life-long elimination of this protein from the diet. European Commission Regulation 2003/89/EC requires mandatory labeling for cereal products that contain ingredients with gluten. According to the Codex Alimentarius, products that contain less than 20 mg/kg of gluten can be classified as gluten-free, while those with 100 mg/kg of gluten may be packaged as foods specially processed to reduce gluten content (Anonymus Codex Alimentarius, Citation1998). Corn, rice, soybean, millet, buckwheat, tapioca, amaranth, cassava, lentils, sorghum, sago and teff are categorized as gluten-free (Weber, Cléroux, & Godefroy, Citation2009).
Proteins from other plants, including spices used for seasoning, have yet to be tested for their toxicity to patients with celiac disease. However, spices sometimes cause allergic reactions, most often as a result of cross-reactions with other plant allergens. The consumption of certain spices may therefore represent a health risk to celiac and wheat-allergy sufferers of which consumers, food regulators and manufacturers are unaware. This article attempts to determine whether spice proteins are able to cross-react with cereal proteins, causing an adverse reaction in sufferers of celiac disease and wheat allergy.
Materials and methods
Preparation of samples
The materials used in the study were oilseeds such as sesame (Sesamum indicum) and spices commonly used in the confectionery and bakery industries, such as caraway (Carum carvi), ginger (Zingiber officinale), chili and sweet peppers (Capsicum annum), anise (Pimpinella anisum), nutmeg (Myristica fragrans) and black pepper (Piper nigrum). All of these products were bought in specialist stores (chemist’s or spice shops) and milled to a fine powder (particle size 700 μm) using a laboratory mill (Bionovo, Legnica, PL). The samples were then extracted twice using Tris-glycine (T-G, pH 8.3) with 0.05 M Tris and 0.33 M glycine. One gram of each specimen was mixed with 5 ml of the T-G buffer and incubated on a laboratory shaker (TTS 2, Yellow Line, IKA-Werke GmbH & Co. KG, Staufen, Germany) for 1 hour. The samples were centrifuged (10,000 g, 10 min) using a Sigma 2-16P centrifuge (Polygen, Wroclaw, PL) and the supernatants collected in tubes. The precipitates were separated, mixed again with 5 ml of T-G buffer and incubated on the shaker for a further 1 hour. All samples were then centrifuged again, after which the supernatants were collected and dialyzed against T-G buffer (pH 8.3) containing 3 mmol/1 sodium azide using a molecular porous dialysis membrane (Spectra/Por 1, cutoff: 6–8 kDa; Spectrum Laboratories Inc., Florida, USA). The dialysates were freeze-dried and stored at −20°C prior to analysis. Protein concentrations were determined using the Pierce method and the BCA Protein Assay Kit (Thermoscientific, Rockford, USA) with BSA as a standard.
Estimation of IgG binding to spice proteins using indirect ELISA assay
The spice samples were diluted in carbonate coating buffer. These diluted solutions were coated onto the wells (2–5 μg/ml protein concentration for each well) of 96-microwell ELISA plate (Immunoplate Maxisorp F96 certified; Nunc, Rosklide, Denmark) overnight at 4°C. The plate was then washed four times with washing buffer – PBST (pH = 7.4, PBS, 0.1% Tween 20) on an ELISA microplate washer (ThermoLabsystems, type Wellwash 4). After that the plate was blocked for 2 hours at room temperature (RT) with 3% skimmed milk and washed four times in wash buffer. An estimated 100 µl of solution of anti-gliadin antibodies present in sera of patients with celiac disease diluted at a ratio of 1:100 in PBST buffer and rabbit antibodies against peptides (QQQPP, PQQQ and QQQP) diluted 1:100 were added per well and incubated for 1 hour at RT, then washed four times as described above. The sera of patients with celiac disease (no. 625: IgG 48.5 u/ml; no. 813: IgG 58.2 u/ml) were obtained from the Polish Mother’s Memorial Hospital Research Institute. Rabbit sera were purchased from Polgen. A total of 100 µl of monoclonal anti-human IgG (diluted 1:5000, c = 4 mg/ml) (Sigma-Aldrich, St. Louis, MO, USA) or anti-rabbit antibodies (diluted 1:5000, c = 3.7 mg/ml) (Sigma-Aldrich, St. Louis, MO, USA) conjugated with alkaline phosphatase was added to each well. The antibodies were incubated for 1 hour at RT. Wells were then washed four times with washing buffer. To each well, 100 µl of substrate solution (pNPP, P7998, Sigma-Aldrich) was then added and the plate was incubated for 30 minutes at RT. Then, 100 µl of stop solution (3 M NaOH) was added and the colorimetric reaction was read at 405 nm. Background was determined as the optical density (OD) of control wells without primary antibodies. The results were expressed as immunoreactivity values per gram of sample. Immunoreactivity level was calculated according to the following equation:where
is the average absorbance of test sample,
is the average absorbance of control sample, D is the dilution value of samples that were made for coating the plate in the first step of ELISA assay and
is the volume of extract.
SDS-PAGE and immunoblotting
SDS-PAGE electrophoresis (15% separating gel, 4% stacking gel) was performed according to the Laemmli method (Citation1970) in Tris-glycine running buffer (pH 8.3, 192 mM glycine, 25 mM Tris, 0.1% SDS). Separation was conducted using the Blue Vertical Mini Slab Gel System (BV102) (Serva, Heidelberg, Germany). The extracts were mixed with sample buffer (ratio: 1:1) containing 125 mM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 5% β-mercaptoethanol and 0.002% bromophenol blue. The samples were then denatured by heating to 95°C for 5 minutes and deposited on gel (20 µg protein per well). Prestained Protein Molecular Weight Marker (Thermoscientific, Rockford, USA) with a molecular weight range of 20 to 120 kDa or Unstained Protein Molecular Weight Marker (Fermentas UAB, Vilnius, LTU) with a mass between 14.4 and 116 kDa was used as a molecular weight standard. Electrophoresis was performed at a constant 90 V as the samples entered the stacking gel, and the voltage increased to 135 V when the proteins began to migrate into the separating gel (power supply Desatronic type 3000/200, Desaga, Heidelberg, GE).
Electrophoretic transfer of the separated proteins from the gel onto a nitrocellulose membrane was carried out overnight using a Minitrans apparatus (Kucharczyk, Warsaw, PL) in Tris-glycine buffer (pH 8.3; 25 mM Tris, 192 mM glycine, 20% methanol) at 20 V. After that the membranes were analyzed by Western Blot and submitted to reaction with sera with incubation time identical like in ELISA procedure described previously. Dilution of sera from patients with celiac disease (no. 625 and 813) and rabbit sera against peptides (QQQPP, PQQQ and QQQP) was 1:100. The secondary anti-human and anti-rabbit antibodies were diluted to a ratio of 1:5000. After each incubation, the membranes were rinsed with washing buffer PBS-T (3 times for 5 minutes). The color developed in a solution of alkaline phosphatase substrate BCIP/NBT (Sigma-Aldrich, St. Louis, MO, USA) until bands with sufficient intensity formed (15 minutes). Gliadin from wheat (Sigma-Aldrich, St. Louis, MO, USA) was used as a positive control (5 µg per well). In the case of human sera, sera of healthy people with no history of celiac disease or wheat allergies were used as a negative control. In the case of rabbit sera, the negative control contained no primary antibody. The blots obtained were analyzed using the software program Gelscan.
Results and discussion
Spices including caraway, ginger, chili and sweet peppers, anise, sesame, nutmeg and black pepper were tested in an ELISA and immunoblot analysis using rabbit sera against specific peptides and human sera of patients with celiac disease. The experiment was designed to discover whether antibodies that recognize allergenic or toxic gluten proteins can cross-react with spice proteins, which may therefore be harmful to people with gluten-related disorders. The antibodies chosen were able to identify the specific peptides and proteins involved in the pathogenesis of hypersensitivity (intolerance and allergy) to gluten. The human sera of patients with celiac disease contained antibodies against gliadin, the main protein responsible for allergic and toxic reactions to gluten. The rabbit antibodies were directed against the following specific peptide sequences. QQQPP peptide is the shortest peptide in gliadin, a major allergen in wheat flour, and is capable of reacting with IgE antibodies (Tanabe et al., Citation1996). PQQQ is a common allergenic peptide fragment that causes wheat allergies (IgE), while QQQP peptide is one of the α-gliadin tetrapeptides with proven in vivo activity in the pathogenesis of celiac disease (Ensari et al., Citation1998).
The antibodies chosen were capable of binding with proteins in all the tested spices, although within different molecular weight ranges. Thus, rabbit anti-QQQPP and anti-PQQQ antibodies can identify proteins from spices that contain specific peptides responsible for wheat allergy, while human anti-gliadin and rabbit anti-QQQP antibodies recognize protein fractions that are toxic for celiac sufferers.
The immunoreactivity values of spices towards anti-QQQPP antibodies ranged from 1356 ± 16 to 434 ± 15 in caraway and chili pepper, respectively. Referring to the results obtained with anti-PQQQ antibodies, anise and sesame showed the highest immunoreactivity values at 1926 ± 57 and 1825 ± 144, respectively. The lowest reactivity was observed with ginger (655 ± 21). The highest level of immunoreactivity to anti-QQQP antibodies was reported in sweet (1054 ± 22) and chili pepper (698 ± 9), while the lowest in ginger (71 ± 11) and sesame (76 ± 4) ((a)). Taking into account the results of ELISA test with human sera ((b)) the immunoreactivity values of spices towards patient serum no. 625 were in a range from 138 ± 4 to 44 ± 2 in chili pepper and ginger, respectively. In the case of serum no. 813, the results were found to vary from 1096 ± 5 in black pepper to 600 ± 10 in nutmeg.
Figure 1. Immunoreactivity level for tested spices determined with (a) anti-QQQPP, anti-PQQQ (no. 776) and anti-QQQP (no. 779) rabbit antibodies and (b) anti-gliadin human sera (no. 625 and 813).
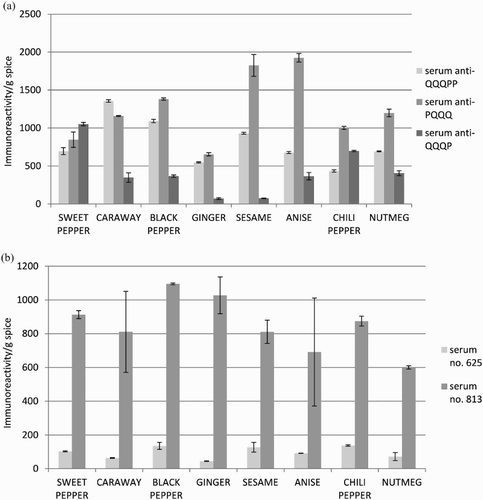
The results of ELISA assays ((a) and (b)) showed that spice proteins contain peptides and epitopes that might be harmful for people with celiac disease and wheat allergy. The differences in the results obtained with human and rabbit sera are due to the fact that human serum recognizes more than one epitope compared to rabbit serum focused only on QQQPP, PQQQ and QQQP peptides.
The molecular masses of major proteins that bind human anti-gliadin IgG were in the range of 94.1 kDa in chili pepper to 7.6 kDa in caraway ((d) and (e); ).
Figure 2. Immunoblot analysis of spice proteins with: (a) anti-QQQPP rabbit serum; (b) anti-PQQQ rabbit serum; (c) anti-QQQP rabbit serum; (d) anti-gliadin human serum no. 625; (e) anti-gliadin human serum no. 813 (M: molecular weight marker, kDa; 1: sweet pepper; 2: caraway; 3: black pepper; 4: ginger; 5: sesame; 6: anise; 7: chili pepper; 8: nutmeg; 9: negative control; 10: positive control).
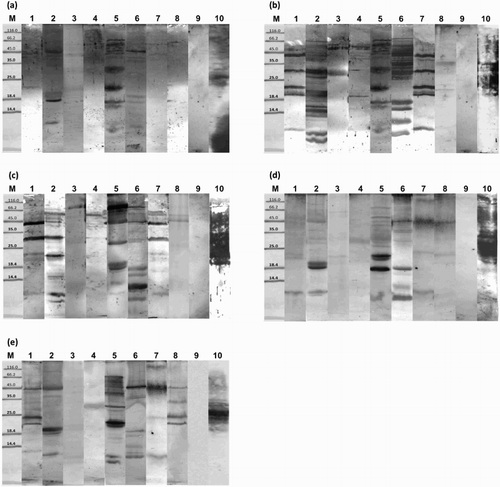
Table 1. Molecular masses of spice proteins showing cross-reactivity with rabbit and human sera.
Anti-QQQPP rabbit antibodies were found to recognize spice proteins with molecular weights in a range from 91 kDa in anise to 11.4 kDa in sesame ((a)). The molecular weights of the major proteins which bind anti-PQQQ rabbit IgG vary from 81.5 kDa in sesame to 10 kDa in caraway ((b)). Anti-QQQP rabbit antibodies react with spice proteins in molecular weight ranges from 78.4 kDa in anise to 9.3 kDa in caraway ((c)).
OD analysis of individual bands on the blots identified spice proteins that provoke the most intense response from the tested antibodies (). Sera from patients with celiac disease (serum number 813 and 625) showed the strongest binding to sesame (22.5, 26.7, 29.3, 41.5 and 52.8 kDa), caraway (24.8, 22.9, 24.1 and 60.2 kDa), sweet pepper (30.6 kDa), anise (61.8, 12.9, 60.4 and 23.2 kDa), chili pepper (60.1 kDa) and nutmeg (30.4, 48.6 and 58 kDa) proteins.
The most intense reactions in anti-QQQPP rabbit serum occurred with anise (61.8 kDa) protein and caraway (58.7, 53.9 and 21 kDa) proteins. The largest number of proteins that reacted with anti-QQQPP antibodies within a single group was among sesame proteins. The most intense bands were obtained in the case of sesame proteins with the following molecular weights: 41.9, 27.9, 70.2, 20.7, 77.2, 19.1 and 62 kDa. Rabbit anti-PQQQ serum showed the strongest reaction with sesame protein with a molecular weight of 37.1 kDa. Intense reactions were also observed with sesame (23.6, 60.5 and 12.4 kDa), black pepper (38.7 kDa), sweet pepper (57.3, 41.6, 29.7 and 25.3 kDa) and chili pepper (57.1, 41, 29.6, 25.1 and 12.3 kDa) proteins. A large number of caraway and anise proteins gave a visible reaction color. The most intense binding between rabbit anti-QQQP antibodies and spice proteins was with anise (13.5, 56.6, 10.4, 20 and 78.4 kDa), sweet pepper (54.9 and 39.4 kDa), ginger (66.7 kDa), chili pepper (55.9, 40.3 and 11.7 kDa) and caraway (40, 11, 26.4 and 68.2 kDa) proteins. The most strongly bound sesame protein was a molecule with a weight of 76.4 kDa.
Analysis of the results shows that most spice proteins recognized by anti-gliadin antibodies also react with both anti-QQQPP antibodies and anti-QQQP antibodies. This is to be expected, since QQQPP peptide and QQQP peptide are fragments of gliadin. Blots analysis using two different human sera from celiac patients demonstrated that their reactivity towards gliadin was similar, with minor differences. In many cases, spice proteins that react with anti-QQQP antibodies also reacted with anti-PQQQ antibodies. The most recognizable protein to human IgG was ginger in a range from 39.8 to 39.9 kDa. Human antibodies (serum number 625) and rabbit anti-QQQPP reacted most strongly to anise protein with a molecular weight of 61.8 kDa. Both rabbit anti-QQQP antibodies and human antibodies (serum number 625 and 813) reacted with anise proteins with molecular weights between 12.9 and 13.5 kDa. Sesame protein with molecular weight in a range of 12.2 to 12.4 kDa was recognized by both human sera and rabbit anti-PQQQ antibodies.
Sesame proteins with molecular weight ranges from 77.2 to 76.4, 20.7 to 21.8 and 19.1 to 20.1 kDa, which were recognized by anti-QQQPP antibodies also reacted with anti-QQQP antibodies. It is important to note that sesame proteins with molecular weights between 37.1 and 36 kDa bonded most strongly to anti-PQQQ antibodies (relevant in IgE-mediated reactions to gluten), and may therefore be homologs of peroxidase – a wheat allergen with a molecular mass of 36 kDa (Sánchez-Monge et al., Citation1992). Similarly, two caraway proteins with masses of 27.7 kDa and 17.2 kDa, and anise protein with a mass of 46.6 kDa may be homologous to wheat allergens with masses of 27 kDa (Kimoto et al., Citation1998), 17 kDa (Amano et al., Citation1998; Fränken, Stephan, Meyer, & König, Citation1994; Kimoto et al., Citation1998) and 47 kDa (James, Sixbey, Helm, Bannon, & Burks, Citation1997), respectively.
To summarize, our studies show that anti-gliadin antibodies can recognize certain spice proteins, which may therefore cross-react with gluten proteins and have toxic effects on the intestinal mucosa of patients with celiac disease. It is confirmed by reaction of spice proteins with rabbit antibodies that recognize QQQP peptide, which is one of the toxic peptides of the α-gliadin. Thus, one cannot exclude the possibility that even small quantities of spices in the diets of celiac sufferers could have an adverse effect on patients’ health. This may explain the treatment failure in celiac disease despite use of a gluten-free diet. The risk of adverse reaction to spices increases because patients with celiac disease have increased susceptible to development of food sensitivities (Adams, Citation2004; Ciacci et al., Citation2004).
Many polypeptide chains in dietary proteins containing prolamine fractions (e.g. maize), and even some that contain no fractions at all, may contain potentially toxic sequences including tetrapeptides PSQQ, QQQP, QQPY or QPYP. However, these proteins are not etiologic factors in celiac disease. This suggests that the environment, such as presence of additional specific amino acid residues, determines the toxicity of these structural motifs (Cornell & Mothes, Citation1993). Likewise, spice proteins with peptide sequences homologous with the proteins responsible for celiac disease were recognized in our study by both rabbit (anti-QQQP) and human antibodies. However, the presence of these specific peptide sequences does not necessarily mean that the spices which contain them will be harmful to patients with celiac disease or wheat allergy. This is because the structural environment of the peptides has a critical influence. The amino acid composition determines whether the gluten protein epitopes are active or inactive, enabling spice proteins to be used in a gluten-free diet (Haraszi et al., Citation2011). Nonetheless, the possibility that these peptides may have adverse effects on the intestinal mucosa of patients with celiac disease cannot be excluded. Further research is therefore required to determine the sequences of these cross-reacting proteins, with particular regard to the homology between spice proteins and 33mer peptide, which is toxic to patients with celiac disease (Arentz-Hansen et al., Citation2000; Shan et al., Citation2002).
It cannot be excluded that the reaction of spice proteins with sera from patients with celiac disease may be the result of binding by antibodies directed to non-gluten proteins. It is likely especially that spices contain many non-gluten proteins, which are common cross-reacting allergens such as 2S albumins, globulins and germin-like proteins (Schöll & Jensen-Jarolim, Citation2004). This possibility confirmed study of Huebener et al. (Citation2015), who have discovered that humoral response to wheat in celiac patients is not limited to gluten antigens but is directed against specific non-gluten proteins of wheat. From this point of view, reaction with antibodies against non-gluten proteins, that are considered as not relevant in the pathogenesis of celiac disease, let us think that spice proteins are not toxic for celiac patients. However it is possible that antibodies to non-gluten proteins would themselves contribute to the celiac disease-associated pathways in the gut. Then the presence of such proteins in spices makes them dangerous for celiac sufferers. This point needs further investigation.
Conclusions
Cross-reactivity between anti-gliadin antibodies and spice proteins indicates that patients with celiac disease or wheat allergies may also have an intolerance to many spices. The examined spices contain proteins with a peptide sequence homologous to the toxic and allergenic gluten proteins of cereals. It may be translated into harmful effects of spices on intestinal mucosa of patients with celiac disease and allergenic potential of spices for people with wheat allergy.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Marta Słowianek, PhD is research assistant at Institute of General Food Chemistry, and the member of the Food and Environment Analytics group. Her research activity and interests include: analysis of quality and health safety of food and its bioactive components, food chemistry, food allergy: pollen-food syndrome, cross-reactive allergens, methods of reduction of food allergenicity, new therapeutic strategies in the treatment of allergies, and impact of stress on allergenicity of plant, identification, and characterization of allergens in foodstuffs.
Dorota Mańkowska, PhD, is adjunct at Institute of General Food Chemistry, the member of the Food and Environment and Analytics group. Her research activity and interests include: application of fluorescense spectroscopy in pharmaceuticals and food analysis, food allergens, analysis of biologically active herbal plants, and quality analysis of organic vs. conventional foods.
Joanna Leszczyńska, PhD, DSc., is associate professor at Institute of General Food Chemistry, the leader of the Food and Environment Analytics group. Her research activity and interests include: the analysis of food allergens, biologically active and toxic compounds in food and the allergic components of foodstuffs with the use of spectroscopic analytical methods (UV-VIS, AAS), chromatographic techniques (GC, HPLC) as well as the electrochemical and enzymatic methods. The other area of research is the environment. The main interests focus on the investigation of the abiotic stress and allergenicity of edible plants and on the phytoremediation of soil and water.
References
- Adams, S. (2004). Atopic dermatitis is common in people with celiac disease. Journal of Allergy and Clinical Immunology, 113, 1159–1203. doi: 10.1016/j.jaci.2004.01.002
- Amano, M., Ogawa, H., Kojima, K., Kamidaira, T., Suetsugu, S., Yoshihama, M., … Matsumoto, I. (1998). Identification of the major allergens in wheat flour responsible for baker’s asthma. Biochemical Journal, 330, 1229–1234. doi: 10.1042/bj3301229
- Anonymus Codex Alimentarius. (1998). CODEX STAN 118. ALINORM 08/31/26, Appendix III.
- Arentz-Hansen, H., Körner, R., Molberg, Ø., Quarsten, H., Vader, W., Kooy, Y. M., … McAdam, S. N. (2000). The intestinal T cell response to α-gliadin in adult celiac disease in focused on a single deamidated glutamine targeted by tissue transglutaminase. The Journal of Experimental Medicine, 191(4), 603–612. doi: 10.1084/jem.191.4.603
- Bartuzi, Z. (2014). Food allergy to wheat and celiac disease. Alergia, 2, 4–10.
- Battais, F., Richard, C., Jacquenet, S., Denery-Papini, S., & Moneret-Vautrin, D. A. (2008). Wheat grain allergies: An update on wheat allergens. European Annals of Allergy and Clinical Immunology, 40(3), 67–76.
- Belderok, B., Mesdag, J., & Donner, D. A. (2000). 4: A brief survey of gluten proteins and wheat starches. Plant Foods for Human Nutrition, 55(1), 30–39. doi: 10.1023/A:1008199314267
- Catassi, C., Bai, J. C., Bonaz, B., Bouma, G., Calabrò, A., Carroccio, A., … Fasano, A. (2013). Non-celiac gluten sensitivity: The new frontier of gluten related disorders. Nutrients, 5, 3839–3853. doi: 10.3390/nu5103839
- Ciacci, C., Cavallaro, R., Iovino, P., Sabbatini, F., Palumbo, A., Amoruso, D., … Mazzacca, G. (2004). Allergy prevalence in adult celiac disease. Journal of Allergy and Clinical Immunology, 113(6), 1199–1203. doi: 10.1016/j.jaci.2004.03.012
- Cielecka, E. K., Dereń, K., & Grzegorczyk, A. (2010). Food hypersensitivity. Annals of Allergy, Asthma & Immunology, 15(3), 118–124.
- Cornell, H. J. (1996). Coeliac disease: A review of the causative agents and their possibly mechanisms of action. Amino Acids, 10(1), 1–19. doi: 10.1007/BF00806090
- Cornell, H. J., & Mothes, T. (1993). The activity of wheat gliadin peptides in in vitro assays for coeliac disease. Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease, 1181(2), 169–173. doi: 10.1016/0925-4439(93)90107-C
- De Ritis, G., Auricchio, S., Jones, H. W., Lew, E. J., Bernardin, J. E., & Kasarda, D. D. (1988). In vitro (organ culture) studies of the toxicity of specific A-gliadin peptides in celiac disease. Gastroenterology, 94, 41–49. doi: 10.1016/0016-5085(88)90607-5
- Dewar, D., Pereira, S. P., & Ciclitira, P. J. (2004). The pathogenesis of coeliac disease. The International Journal of Biochemistry & Cell Biology, 36(1), 17–24. doi: 10.1016/S1357-2725(03)00239-5
- Ensari, A., Marsh, M. N., Moriarty, K. J., Moore, C. M., Fido, R. J., & Tatham, A. S. (1998). Studies in vivo of ω-gliadins in gluten sensitivity (coeliac sprue disease). Clinical Science, 95(4), 419–424. doi: 10.1042/cs0950419
- Fränken, J., Stephan, U., Meyer, H. E., & König, W. (1994). Identification of alpha-amylase inhibitor as a major allergen of wheat flour. International Archives of Allergy and Immunology, 104(2), 171–174. doi: 10.1159/000236726
- Haraszi, R., Chassaigne, H., Maquet, A., & Ulberth, F. (2011). Analytical methods for detection of Gluten in food – method developments in support of food labeling legislation. Journal of AOAC International, 94(4), 1006–1025.
- Hausch, F., Shan, L., Santiago, N. A., Gray, G. M., & Khosla, C. (2002). Intestinal digestive resistance of immunodominant gliadin peptides. American Journal of Physiology – Gastrointestinal and Liver Physiology, 283(4), G996–G1003. doi: 10.1152/ajpgi.00136.2002
- Huebener, S., Tanaka, C. K., Uhde, M., Zone, J. J., Vensel, W. H., Kasarda, D. D., … Alaedini, A. (2015). Specific nongluten proteins of wheat are novel target antigens in celiac disease humoral response. Journal of Proteome Research, 14(1), 503–511. doi: 10.1021/pr500809b
- James, J. M., Sixbey, J. P., Helm, R. M., Bannon, G. A., & Burks, A. W. (1997). Wheat α-amylase inhibitor: A second route of allergic sensitization. Journal of Allergy and Clinical Immunology, 99(2), 239–244. doi: 10.1016/S0091-6749(97)70103-9
- Kagnoff, M. F. (2005). Overview and pathogenesis of celiac disease. Gastroenterology, 128(4), S10–S18. doi: 10.1053/j.gastro.2005.02.008
- Khan, M. R., Anjum, F. M., Din, A., Hussain, S., Shabbir, M. A., & Nadeem, M. (2010). Immunochemical characteristics of wheat proteins. Food and Agricultural Immunology, 21(4), 279–294. doi: 10.1080/09540105.2010.511151
- Kimoto, M., Yoshikawa, M., Takahashi, K. Bando, N., Okita, M., & Tsuju, H. (1998). Identification of allergen in cereals and their hypoallergenization. I. Screening of allergens in wheat and identification of an allergen, Tri a Bd 17K. Annual Report of Interdisciplinary Research Institute of Environmental Sciences, 17, 53–60.
- Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227(5259), 680–685. doi: 10.1038/227680a0
- Räsänen, L., Lehto, M., Turjanmaa, K., Savolainen, J., & Reunala, T. (1994). Allergy to ingested cereals in atopic children. Allergy, 49, 871–876. doi: 10.1111/j.1398-9995.1994.tb00790.x
- Rewers, M. (2005). Epidemiology of celiac disease: What are the prevalence, incidence, and progression of celiac disease? Gastroenterology, 128(Suppl 4), S47–S51. doi: 10.1053/j.gastro.2005.02.030
- Sánchez-Monge, R., Gomez, L., Barber, D., Lopez-Otin, C., Armentia, A., & Salcedo, G. (1992). Wheat and barley allergens associated with baker’s asthma. Glycosylated subunits of the α-amylase inhibitor family have enhanced IgE-binding capacity. Biochemical Journal, 281(2), 401–405. doi: 10.1042/bj2810401
- Schöll, I., & Jensen-Jarolim, E. (2004). Allergenic potency of spices: Hot, medium hot, or very hot. International Archives of Allergy and Immunology, 135, 247–261. doi: 10.1159/000081950
- Shan, L., Molberg, Ø., Parrot, I., Hausch, F., Filiz, F., Gray, G. M., … Khosla, C. H. (2002). Structural basis for gluten intolerance in celiac sprue. Science, 297(5590), 2275–2279. doi: 10.1126/science.1074129
- Sollid, L. M. (2000). Molecular basis of celiac disease. Annual Review of Immunology, 18, 53–81. doi: 10.1146/annurev.immunol.18.1.53
- Sollid, L. M. (2002). Coeliac disease: Dissecting a complex inflammatory disorder. Nature Reviews Immunology, 2(9), 647–655. doi: 10.1038/nri885
- Sollid, L. M., & Lundin, K. E. A. (2009). Diagnosis and treatment of celiac disease. Mucosal Immunology, 2, 3–7. doi: 10.1038/mi.2008.74
- Tanabe, S., Arai, S., Yanagihara, Y., Mita, H., Takahashi, K., & Watanabe, M. (1996). A major heat allergen has a Gln-Gln-Gln-Pro-Pro motif identified as an IgE-binding epitope. Biochemical and Biophysical Research Communications, 219(2), 290–293. doi: 10.1006/bbrc.1996.0225
- Tsuji, H., Kimoto, M., & Natori, Y. (2001). Allergens in major crops. Nutrition Research, 21(6), 925–934. doi: 10.1016/S0271-5317(01)00291-3
- Varjonen, E., Petman, L., & Makinen-Kiljunen, S. (2000). Immediate contact allergy from hydrolysed wheat in a cosmetic cream. Allergy, 55, 294–296. doi: 10.1034/j.1398-9995.2000.00516.x
- Varjonen, E., Vainio, E., & Kalimo, K. (1997). Life-threatening, recurrent anaphylaxis caused by allergy to gliadin and exercise. Clinical and Experimental Allergy, 27, 162–166. doi: 10.1111/j.1365-2222.1997.tb00688.x
- Weber, D., Cléroux, C., & Godefroy, S. B. (2009). Emerging analytical methods to determine gluten markers in processed foods – method development in support of standard setting. Analytical and Bioanalytical Chemistry, 395(1), 111–117. doi: 10.1007/s00216-009-2943-1
