ABSTRACT
Botryoid-shaped Au/Ag nanoparticles (BSNPs) were prepared with a tailored galvanic reaction. Ag nanoprisms–BSA complex were employed as a template to react with HAuCl4 in the presence of ascorbic acid. The BSNPs–HRP–IgG about 40 nm were used as the carriers of HRP–IgG for amplifying the detection signal of an indirect competitive ELISA against ochratoxin A. This BSNPs-enhanced ELISA method achieved an IC20 of 0.016 ng/mL and an IC50 of 0.05 ng/mL, which were 30 times more sensitive than conventional ELISA. The application of BSNPs to the enhanced ELISA showed acceptable reproducibility, stability and could be applied to determine other harmful small molecules in food.
Introduction
ELISA method has been widely used owing to its high sensitivity, simplicity and low cost (Cao, Meng, Lu, & Xi, Citation2011; Peng, Duan, Song, & Xue, Citation2013; Xu et al., Citation2015; Yu, Liu, Song, Kuang, & Xu, Citation2016; Zhan, Wu, Yang, & Huang, Citation2014; Zhou et al., Citation2012; Zhu, Song, Liu, Kuang, & Xu, Citation2016). The analytical performances of ELISA are highly dependent on the activity of antibody and enzyme label. In biomedical, feed/food and environmental settings, many chemicals, such as toxins, antibiotics and some synthetic dyes, are inspected through ELISA methods (Cao et al., Citation2013; Du et al., Citation2016; Jiang et al., Citation2013; Peng, Duan, Khamba, & Xie, Citation2014; Wang, Li, Zhang, Hu, & Zhang, Citation2016; Xu et al., Citation2012). Nevertheless, the analytical performances of the conventional ELISA methods are needed to be improved in order to facilitate their application in practice (Lei & Ju, Citation2012).
Nanomaterials used as the carriers to load recognition molecule and/or signal molecule have been much experimented with to develop ultrasensitive biosensors, such as fluorescence, chemiluminescence and electrochemical biosensors especially for proteins and pathogens detection (Cao, Ye, & Liu, Citation2011; Chou, Liu, & Wu, Citation2013; He & Cui, Citation2012; Ling, Hao, Lei, & Ju, Citation2015; Zhao et al., Citation2012; Zhou et al., Citation2012). The above nanomaterial-based strategy is also attractive for developing highly sensitive ELISA (Chou et al., Citation2013; Chunglok, Wuragil, Oaew, Somasundrum, & Surareungchai, Citation2011; Gao, Xu, Hou, Chen, & Tang, Citation2013; Liang et al., Citation2015; Lin et al., Citation2013; Liu et al., Citation2014; Lu, Wang, Ye, Chen, & Yang, Citation2012; Nie et al., Citation2014; Peng, Liu, Song, & Liu, Citation2014; Peng et al., Citation2013; Peng et al., Citation2014; Zhan et al., Citation2014; Zhang et al., Citation2011; Zhou et al., Citation2012). For example, single-walled and multi-walled carbon nanotubes were employed as a labeling platform for antibody and horseradish peroxidase (HRP) co-immobilizing (Chunglok et al., Citation2011). Antibody-functionalized graphene oxide sheets and AuNPs for serum protein Hsp70 and 7-aminonitrazepam were reported (Peng et al., Citation2013; Zhan et al., Citation2014). Hierarchical flower-like AuNPs have been used to label IgG for signal amplification of lateral flow immnochromatographic assay (Zhang, Huang et al., Citation2015). Although markedly the progress of ELISA has been achieved through nanomaterial-based signal amplification, the additional steps and cost compared with conventional ELISA still hampered their application in nanomaterial-based ELISA methods in food hazards analysis. In addition, some of nanomaterials with big sizes in the range of 200 nm–2 μm may impair the reproducibility of ELISA because these nanomaterials with big sizes may be harder to be absorbed onto solid surface than those nanomaterials with small sizes and are apt to aggregate together (Li, Duan, Xu, Huang, & Xiong, Citation2016; López-Muñoz, Pescador-Rojas, Ortega-Lopez, Salazar, & Balderas-López, Citation2012; Zhang, Leem, Srisombat, & Lee, Citation2008). Recently, gold nanoparticles (AuNPs) were used as the carriers of the signaling antibody anti-respiratory syncytial virus-HRP and achieved a significant amplification of the signal of ELISA (Zhan et al., Citation2014). We also developed a gold nanoflowers-enhanced ELISA for bisphenol (Peng et al., Citation2016). It was reasoned that other nanomaterials with a similar nanoscale to AuNPs and nanoflowers but a larger surface area-to-volume ratio than them possibly are better loading carriers of IgG–HRP for developing ELISA.
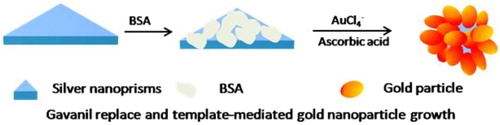
Herein, we synthesized a kind of novel botryoid-shaped Au/Ag nanoparticles (BSNPs) by controlling AuNPs growth and galvanic replacement reaction on the surface of Ag nanoprisms for the first time. The BSNPs were utilized as the carriers to load IgG–HRP molecules. Ochratoxin A (OTA), a kind of mycotoxin in food and strictly monitored in many states (Radoi, Dumitru, Barthelmebs, & Marty, Citation2009), was selected as a model molecule to develop a BSNPs-enhanced ELISA. Compared with the conventional ELISA for OTA, this BSNPs-based ELISA showed much higher sensitivity.
Experimental procedures
Reagents and materials
All chemicals were used as obtained without further treatment. Hydrogen peroxide (H2O2, 30wt-%) was purchased from Sinopharm. Silver nitrate (AgNO3, 99+%), chloroauric acid tetrahyrate (HAuCl4,99+%) and ascorbic acid(AA,99+%) were obtained from J&K. Sodium borohydride (NaBH4, 99%), sodium citrate tribasic dehydrate (TSC, 99%), 3, 3, 5, 5-tetramethylbenzidine (TMB), bovine serum albumin (BSA, 98%) and polyethylene glycol (PEG, Mw 6000) were bought from Sigma-Aldrich. Monoclonal antibody (mouse) anti-OTA was prepared and purified by our laboratory, and monoclonal goat-anti-mouse second antibody was purchased from Shanghai Sangon Biotech Company. 96-well microplates were purchased from Wuxi Jiecheng Biotech Company. All solutions were prepared with double-deionized water.
About 0.05 M carbonate/bicarbonate buffer (pH 9.6) was used as the coating buffer. Ten millimolar phosphate-buffered saline (PBS, pH 7.4) was used as the dilution to OTA standard substance. About 0.001% (w/v) PEG 6000 aqueous water was used as the blocking buffer. PBS with 0.05% Tween 20 was used as the washing buffer. PBS with 0.05% (v/v) Tween 20 and 0.1% (w/v) gelatin was used as the antibody dilution buffer.
Apparatus
The NPs were dropped on a 300 mesh carbon grid and dried. Transmission electron microscopy (TEM) images, high-resolution TEM (HRTEM) images and EDX spectrum of NPs were acquired using a JEOL JEM-2100 operating at 200 kV. A Biotek Eon microplate reader was employed to record the absorbance values of reaction solutions.
Synthesis and characterization of BSNPs
The Ag nanoprisms were prepared by the method reported previously (Zhang, Li, Goebl, Lu, & Yin, Citation2011). Then the BSA solution (200 μL, 1.0 mg/mL) was added into the above-prepared Ag nanoprisms (10 mL) and incubated overnight. After ascorbic acid solution (825 μL, 1.5 mM) was added to the above Ag nanoprisms under stirring, and the HAuCl4 solution (10 mL, 0.08 mM) was added via a syringe pump at a speed of 1.0 mL/min under stirring. The solution color changed from blue to purple, indicating the formation of BSNPs.
Preparation of IgG–HRP conjugate
Briefly, 10 μL of NaIO4 (0.06 M) was mixed with HRP (10 mg/mL, 200μL) at 4 °C for 30 min. The excess NaIO4 in the mixture was eliminated by adding glycol (0.16 M, 75 μL) and incubating at room temperature for 30 min. Then 200 μL of anti-mouse IgG goat antibody (10 mg/mL) was adjusted to pH 9.0 with 0.05 M carbonate buffer and mixed with the above oxidized HRP solution. After the mixture was incubated at 4°C for 20 h, the IgG–HRP conjugate was obtained by adding 400 μL of NaBH4 solution (5 mg/mL) to reduce the Schiff base of the IgG–HRP conjugate. Finally, the IgG–HRP conjugate was precipitated by adding an equal volume of saturated ammonium sulfate solution and the sediment was dialyzed with 0.01 M PBS (pH 7.4) for 3 d. All the reactions and dialysis were protected from light.
Preparation of the BSNPs–IgG–HRP conjugate
The as-prepared BSNPs (1.0 mL) were centrifuged at 8500 rpm for 15 min and the supernatant was discarded. The obtained pellet was re-suspended with 1.0 mL of IgG–HRP (0.1 mg/mL) and 8 μL of 0.1 M K2CO3. The mixture was shaken gently at 4°C for 3 h. After centrifuged at 8500 rpm for 15 minutes, the prepared BSNPs–IgG–HRP complex was re-suspended in 500 μL of antibody dilution buffer containing 0.05 mg/mL BSA.
Preparation of coating antigen
The coating antigen, OVA-OTA, was prepared via the N,N′-carbonyldiimidazole (CDI) method (Peng et al., Citation2008). About 1.0 mg OTA was dissolved in anhydrous dimethyl sulfoxide, and then CDI (2.0 mg) was added and stirred at room temperature for 10 min. At the same time, the activated OTA solution was added slowly by dropwise into1.0 mL of 5.0 mg/mL OVA in carbonate buffer (0.01 M, pH 9.6) and stirred at room temperature for 4 h under dark. Then the reaction solution was dialyzed with PBS (0.01 M, pH 7.4) at 4 °C for 2 d. The obtained coating antigen, OTA–OVA, was stored at −20 °C until use.
Indirect competitive ELISA for OTA
For OTA detection, a 96-well micro plate was first modified with OTA–OVA coating antigen in the coating buffer (100 μL) at 37 °C for 2 h. Then the plate was washed with the washing buffer for three times. Thereafter the plate was blocked with the blocking buffer at 37°C for 2 h. Then the plate was washed three times with the washing buffer. The sensitized plate was sealed in a plastic bag and stored at 4°C until use. In order to establish the calibration curve, 50 μL of OTA standard solutions (0, 0.01, 0.02, 0.05, 0.1,0. 2, 0.5, 1 ng/mL) diluted with PBS (0.01M, pH 7.4) was added to each microplate well, followed by adding 50 μL of anti-OTA antibody diluted with the antibody dilution buffer. Then the wells were kept at 37 °C for 30 min. After the wells were washed four times with the washing buffer, 100 μL of IgG–HRP or BSNPs–IgG–HRP complex was added to each well and incubated at 37°C for 30 min. Then the wells were washed with the washing buffer again and patted dry. A volume of 100 μL of substrate solutions consisting of 0.3 mM TMB and 64 mM H2O2 was added to each well and reacted at 37 °C for 30 min. The reaction was stopped by adding 50μL of H2SO4 (2.0 M) to each well and the absorbance values at 450 nm were recorded by a microplate reader.
Sample preparation for ELISA
The negative wheat flour sample used for the spiking and recovery study was collected from a local supermarket in Wuxi, China. The sample was pretreated as described by Wang et al. (Citation2015) Briefly, 1 g of samples was weighed and spiked with 1.0, 2.0 and 5.0 µg/kg OTA. The spiked samples were mixed with 4.0 mL of 50% methanol in water (v/v) and extracted under an ultrasonic cleaner for 20 min. The mixture was centrifuged (10,000 × g) at 4°C for 10 min, and the supernatant was 10-fold diluted with PBS for the above ELISA.
Results and discussion
Synthesis and characterization of BSNPs
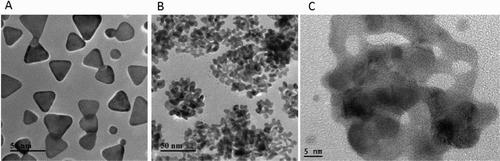
Taking advantage of seed-mediated metallic nanoparticle growth, bimetallic nanostructures, such as raspberry-like bimetal Ag@Cu bimetal nanoparticles, hierarchical flower-like AuNPs and Au@Pt nanospheres can be controllably synthesized (Zhang, Huang et al., Citation2015). Meanwhile, the galvanic displacement reaction has been widely used to synthesize many heteromorphic nanostructures, such as Pt/Ag nanopopcorns, Au/Ag hollow nanostructures and Ag@Au core-shell nanocubes (Yang, Zhang, Fu, & Qin, Citation2014). Taking Ag nanoprisms as the template nanoparticles, edge-gold-coated Ag nanoprisms also was obtained (Shahjamali, Salvador, Bosman, Ginger, & Xue, Citation2014). Here the botryoid-shaped Ag/Au NPs were synthesized by combining the galvanic displacement reaction with seed-mediated metallic nanoparticle growth, as illustrated in . Initially, 30 nm Ag nanoprisms were prepared without covering any macromolecules ((a)) and then covered by BSA molecules. HAuCl4 was injected slowly to the BSA covered Ag nanoprisms in the presence of ascorbic acid. In this step, two reactions happened at the same time. One reaction was BSA-templated growth of AuNPs, and another one was the galvanic displacement reaction between ions and Ag nanoprisms. As seen in (b) and (c), the BSNPs have an average diameter of about 40 nm. Meanwhile, many small holes were found in which attributed to the corrosion of Ag nanoprisms by
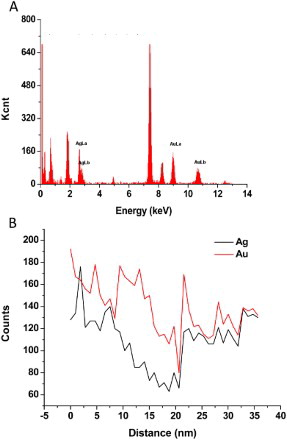
ions. And tens of NPs also can be observed clearly in each of a BSNP, which indicated the growth of small AuNPs. EDX analysis showed that the BSNPs mainly consisted of both Ag and Au elements ((a)). The cross-section line profiles of the BSNPs composition demonstrated that high ratio of Au accompanied with Ag ((b)), which further confirmed the galvanic displacement between
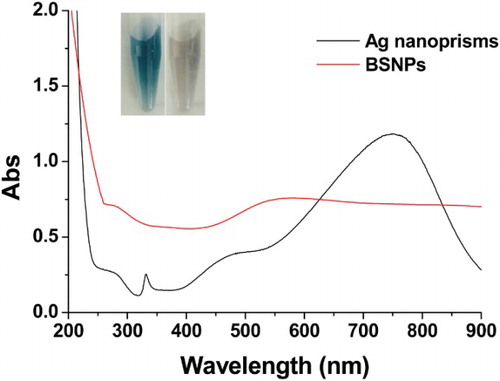
ions and Ag nanoprisms. Another interesting phenomenon was that the BSNPs have very broad optical adsorption from 250 nm to 900 nm while the Ag nanoprisms only have an adsorption peak at 750 nm (). The BSNPs with the above unique optical property may be useful in developing optical sensors, such as SERS-based sensor (Zhang, Liu et al.,, Citation2015).
Preparation of HRP–IgG–BSNPs complex
Similar to the colloidal AuNPs, high concentrations of salt could induce irreversible aggregation of such BSNPs and the BSNPs covered by the protein can become more stable and withstand high concentration of salt. Since the pH affects the adsorption of protein onto the surface of metallic NPs, the pH of BSNPs solution was adjusted by adding different amounts of 0.1 M K2CO3 (6, 7, 8, 9 and 10 μL) before incubation with HRP–IgG (Figure S1). After incubation the salt-induced BSNPs aggregation test was performed to verify the optimal pH for the preparation of HRP–IgG–BSNPs. It was found that the HRP–IgG–BSNPs complex obtained with the addition of 8 μL of K2CO3 was most stable and appeared gray while the color of others was less stable appeared light gray or colorless.
BSNPs-enhanced ELISA
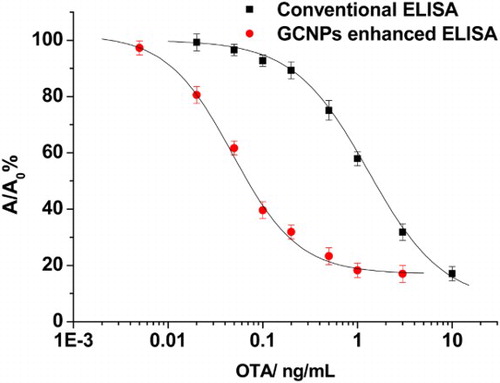
In order to ensure that the OTA–OVA conjugate was suitable for developing ELISA for OTA, the working conditions including the coating antigen (OTA–OVA), anti-OTA antibody and HRP–IgG of the traditional ELISA were selected by double-checkerboard method titration experiments (Table S1). Under the selected conditions of OTA–OVA, anti-OTA antibody and HRP–IgG, the EC20 (concentration for 20% of maximal effect) and EC50 (concentration for 50% of maximal effect) of the traditional ELISA were 0.48 ng/mL and 1.6 ng/mL, respectively ().
In order to apply the HRP–IgG–BSNPs complex to the traditional ELISA for OTA, the concentration of anti-OTA antibody and the dilution of HRP–IgG–BSNPs complex were also selected by double-checkerboard titration experiments. It should be pointed out that the selected concentration of anti-OTA antibody was obviously lower than that in the traditional ELISA (Table S1). This result was reasoned because only the lower concentration of anti-OTA antibody can demonstrate the competitiveness of lower concentration of OTA in competitive ELISA format. Meanwhile, the lower concentration of anti-OTA antibody can be signal-amplified by the BSNPs–IgG–HRP since a BSNP was covered by many HRP–IgG–BSNPs molecules.
Since the HRP–IgG–BSNPs complex was much bigger than HRP–IgG, the adsorption of the complex onto the surface of microplate may be different from HRP–IgG. Thus, some factors such as the concentration of PEG in the blocking buffer, the dilution of the BSNPs–IgG–HRP complex and the incubation time of the IgG–HRP–BSNPs complex were investigated. When 0.001% of PEG was selected to block the microplate, the lowest A/A0 (A and A0 were the optimal intensity in the presence and absence of OTA, respectively) was obtained (Figure S2). The optimal dilution of the IgG–HRP–BSNPs complex was found to be 1:4 (Figure S3). In addition, the optimal incubation time of the IgG–HRP–BSNPs complex was 30 min, which was the same as that of IgG–HRP in the conventional ELISA (Figure S4). Under the above optimal working conditions, the EC20 and EC50 of the BSNPs-based ELISA were 0.016 ng/mL and 0.05 ng/mL, respectively (). Thus, the BSNPs-based ELISA for OTA was over 30 times more sensitive than the conventional ELISA.
It is important to point out that the above HRP–IgG–BSNPs complex can be easily synthesized and the cost of the BSNPs-enhanced ELISA is just slightly higher than that of traditional ELISA. Moreover, the operation procedure of the BSNPs-enhanced ELISA is the same as the traditional ELISA.
To demonstrate the practical application of the BSNPs-based ELISA, the negative wheat flour samples confirmed using the HPLC-FD method were spiked with 1.0, 2.0 and 5.0 µg/kg OTA, pretreated and tested by the ELISA method. The recoveries ranging from 89.5–103.6% were obtained and the relative standard deviations (RSD) were lower than 10% (). The preliminary evaluation showed that this BSNPs-based ELISA was suitable for rapid detection of this wheat flour sample with simple pretreatment.
Table 1. Recovery measurements of OTA in wheat flour by the BSNPs-based ELISA (n = 3).
Conclusions
In summary, we used BSNPs as the carriers of the HRP–IgG and developed an enhanced ELISA for OTA detection. The sensitivity of this assay for OTA was found to be more than 30 times higher than the conventional ELISA method. The improved analytical performance should be ascribed to the high loading amount of HRP–IgG onto the BSNPs surfaces. Since the IgG–HRP–BSNPs complex can be used in indirect competitive ELISA for various small molecules, this BSNPs-enhanced ELISA format was expected to be applied in broad fields. In addition, the IgG–HRP–BSNPs complex also was deserved to be investigated in developing ultrasensitive sandwich ELISA methods for macromolecules and pathogens.
Supplementary_file.zip
Download Zip (95.5 KB)Notes on contributors
Yan Zhu is currently working toward the master degree at the State Key Lab of Food Science and Technology, School of Food Science and Technology, Jiangnan University. His major research areas focus on the nanomaterials-based chemical sensing.
Chun-Li Liu is currently working toward the master degree at the State Key Lab of Food Science and Technology, School of Food Science and Technology, Jiangnan University. His major research areas focus on the nanomaterials-based chemical sensing.
Zheng-Jun Xie is an associate professor in the State Key Lab of Food Science and Technology, School of Food Science and Technology, Jiangnan University. His major research interests include sensing method for food nutritional ingredients and hazards.
Li-Qiang Liu is an associate research fellow in the State Key Lab of Food Science and Technology, School of Food Science and Technology, Jiangnan University. His major research interests include sensing method for food nutritional ingredients and hazards.
Chi-Fang Peng is a professor in the State Key Lab of Food Science and Technology, the School of Food Science and Technology, Jiangnan University. He received his B.S. degree in Fine Chemicals from Wuhan University of Technology, and Ph.D. degree in Food Science from Jiangnan University. Now, his major research interests include functional nanomaterial fabrication and chemical/biological sensing technology.
Feng Xue is a research fellow in Animal, Plant and Food Inspection Center, Jiangsu Entry-Exit Inspection and Quarantine. He received his B.S. degree and Ph.D. degree in College of Veterinary Medicine, Yangzhou University. Now, his major research interests include developing biosensors of food hazards.
Additional information
Funding
References
- Cao, B., He, G., Yang, H., Chang, H., Li, S., & Deng, A. (2013). Development of a highly sensitive and specific enzyme-linked immunosorbent assay (ELISA) for the detection of phenylethanolamine a in tissue and feed samples and confirmed by liquid chromatography tandem mass spectrometry (LC–MS/MS). Talanta, 115, 624–630. doi:10.1016/j.talanta.2013.06.026
- Cao, X., Ye, Y., & Liu, S. (2011). Gold nanoparticle-based signal amplification for biosensing. Analytical Biochemistry, 417(1), 1–16. doi:dx.doi.org/10.1016/j.ab.2011.05.027
- Cao, Z., Meng, M., Lu, S., & Xi, R. (2011). Development of an indirect chemiluminescent competitive ELISA to detect danofloxacin residues in milk. Analytical Letters, 44(6), 1077–1084. doi:10.1080/00032719.2010.507295
- Chou, C.-Y., Liu, S.-R., & Wu, S.-P. (2013). A highly selective turn-on fluorescent sensor for Cu(II) based on an NSe2 chelating moiety and its application in living cell imaging. The Analyst, 138(11), 3264–3270. doi:10.1039/C3AN00286A
- Chunglok, W., Wuragil, D. K., Oaew, S., Somasundrum, M., & Surareungchai, W. (2011). Immunoassay based on carbon nanotubes-enhanced ELISA for Salmonella enterica serovar typhimurium. Biosensors and Bioelectronics, 26(8), 3584–3589. doi:10.1016/j.bios.2011.02.005
- Du, X.-J., Zhou, X.-N., Li, P., Sheng, W., Ducancel, F., & Wang, S. (2016). Development of an immunoassay for chloramphenicol based on the preparation of a specific single-chain variable fragment antibody. Journal of Agricultural and Food Chemistry, 64(14), 2971–2979. doi:10.1021/acs.jafc.6b00639
- Gao, Z., Xu, M., Hou, L., Chen, G., & Tang, D. (2013). Magnetic bead-based reverse colorimetric immunoassay strategy for sensing biomolecules. Analytical Chemistry, 85(14), 6945–6952. doi:10.1021/ac401433p
- He, Y., & Cui, H. (2012). Fabrication of luminol and lucigenin bifunctionalized gold nnanoparticles/graphene oxide nanocomposites with dual-wavelength chemiluminescence. The Journal of Physical Chemistry C, 116(23), 12953–12957. doi:10.1021/jp303304z
- Jiang, W., Zhang, H., Li, X., Liu, X., Zhang, S., Shi, W., … Wang, Z. (2013). Monoclonal antibody production and the development of an indirect competitive enzyme-linked immunosorbent assay for screening spiramycin in milk. Journal of Agricultural and Food Chemistry, 61(46), 10925–10931. doi:10.1021/jf404027b
- Lei, J., & Ju, H. (2012). Signal amplification using functional nanomaterials for biosensing. Chemical Society Reviews, 41(6), 2122–2134. doi:10.1039/C1CS15274B
- Li, J., Duan, H., Xu, P., Huang, X., & Xiong, Y. (2016). Effect of different-sized spherical gold nanoparticles grown layer by layer on the sensitivity of an immunochromatographic assay. RSC Advances, 6(31), 26178–26185. doi:10.1039/c6ra03695c
- Liang, J., Yao, C., Li, X., Wu, Z., Huang, C., Fu, Q., … Tang, Y. (2015). Silver nanoprism etching-based plasmonic ELISA for the high sensitive detection of prostate-specific antigen. Biosensors and Bioelectronics, 69, 128–134. doi: 10.1016/j.bios.2015.02.026
- Lin, H., Liu, Y., Huo, J., Zhang, A., Pan, Y., Bai, H., … Qian, X. (2013). Modified enzyme-linked immunosorbent assay strategy using graphene oxide sheets and gold nanoparticles functionalized with different antibody types. Analytical Chemistry, 85(13), 6228–6232. doi:10.1021/ac401075u
- Ling, P., Hao, Q., Lei, J., & Ju, H. (2015). Porphyrin functionalized porous carbon derived from metal–organic framework as a biomimetic catalyst for electrochemical biosensing. Journal of Materials Chemistry B, 3(7), 1335–1341. doi:10.1039/c4tb01620c
- Liu, D., Yang, J., Wang, H.-F., Wang, Z., Huang, X., Wang, Z., … Chen, X. (2014). Glucose oxidase-catalyzed growth of gold nanoparticles enables quantitative detection of attomolar cancer biomarkers. Analytical Chemistry, 86(12), 5800–5806. doi:10.1021/ac500478g
- López-Muñoz, G. A., Pescador-Rojas, J. A., Ortega-Lopez, J., Salazar, J. S., & Balderas-López, J. A. (2012). Thermal diffusivity measurement of spherical gold nanofluids of different sizes/concentrations. Nanoscale Research Letters, 7(1), 423. doi:10.1186/1556-276x-7-423
- Lu, C.-H., Wang, Y.-W., Ye, S.-L., Chen, G.-N., & Yang, H.-H. (2012). Ultrasensitive detection of Cu2+ with the naked eye and application in immunoassays. NPG Asia Materials, 4, e10. doi: 10.1038/am.2012.18
- Nie, X.-M., Huang, R., Dong, C.-X., Tang, L.-J., Gui, R., & Jiang, J.-H. (2014). Plasmonic ELISA for the ultrasensitive detection of Treponema pallidum. Biosensors and Bioelectronics, 58, 314–319. doi:10.1016/j.bios.2014.03.007
- Peng, C., Duan, X., Khamba, G. W., & Xie, Z. (2014). Highly sensitive “signal on” plasmonic ELISA for small molecules by the naked eye. Analytical Methods, 6(24), 9616–9621. doi:10.1039/c4ay01993h
- Peng, C., Duan, X., Song, S., & Xue, F. (2013). Parts per trillion detection of 7-aminonitrazepam by nano-enhanced ELISA. International Journal of Molecular Sciences, 14(10), 19474–19483. doi: 10.3390/ijms141019474
- Peng, C. F., Chen, Y. W., Chen, W., Xu, C. L., Kim, J. M., & Jin, Z. Y. (2008). Development of a sensitive heterologous ELISA method for analysis of acetylgestagen residues in animal fat. Food Chemistry, 109(3), 647–653. doi:10.1016/j.foodchem.2007.12.072
- Peng, C.-F., Liu, C.-L., Song, S.-S., & Liu, L.-Q. (2014). Highly sensitive nano-ELISA for detecting 19-nortestosterone in beef. Food and Agricultural Immunology, 25(3), 423–431. doi:10.1080/09540105.2013.821599
- Peng, C., Pan, N., Xie, Z., Liu, L., Xiang, J., & Liu, C. (2016). Determination of bisphenol A by a gold nanoflower enhanced enzyme-linked immunosorbent assay. Analytical Letters, 49, 1492–1501. doi:10.1080/00032719.2015.1113420
- Radoi, A., Dumitru, L., Barthelmebs, L., & Marty, J. L. (2009). Ochratoxin A in some French Wines: Application of a direct competitive ELISA based on an OTA–HRP conjugate. Analytical Letters, 42(8), 1187–1202. doi:10.1080/00032710902890447
- Shahjamali, M. M., Salvador, M., Bosman, M., Ginger, D. S., & Xue, C. (2014). Edge-gold-coated silver nanoprisms: Enhanced stability and applications in organic photovoltaics and chemical sensing. The Journal of Physical Chemistry C, 118(23), 12459–12468. doi:10.1021/jp501884s
- Wang, Y., Li, P., Zhang, Q., Hu, X., & Zhang, W. (2016). A toxin-free enzyme-linked immunosorbent assay for the analysis of aflatoxins based on a VHH surrogate standard. Analytical and Bioanalytical Chemistry, 408(22), 6019–6026. doi:10.1007/s00216-016-9370-x
- Xu, N., Xu, L., Ma, W., Liu, L., Kuang, H., & Xu, C. (2015). An ultrasensitive immunochromatographic assay for non-pretreatment monitoring of chloramphenicol in raw milk. Food and Agricultural Immunology, 26(5), 635–644. doi:10.1080/09540105.2014.998640
- Xu, Z.-L., Dong, J.-X., Wang, H., Li, Z.-F., Beier, R. C., Jiang, Y.-M., … Sun, Y.-M. (2012). Production and characterization of a single-chain variable fragment linked alkaline phosphatase fusion protein for detection of O,O-diethyl organophosphorus pesticides in a one-step enzyme-linked immunosorbent assay. Journal of Agricultural and Food Chemistry, 60(20), 5076–5083. doi:10.1021/jf300570q
- Yang, Y., Zhang, Q., Fu, Z.-W., & Qin, D. (2014). Transformation of Ag nanocubes into Ag–Au hollow nanostructures with enriched Ag contents to improve SERS activity and chemical stability. ACS Applied Materials & Interfaces, 6(5), 3750–3757. doi:10.1021/am500506j
- Yu, L., Liu, L., Song, S., Kuang, H., & Xu, C. (2016). Development of an immunochromatographic test strip and ic-ELISA for tetrabromobisphenol: A detection in lake water and rice pudding samples. Food and Agricultural Immunology, 27(4), 460–470. doi:10.1080/09540105.2015.1126234
- Zeng, J., Cao, Y., Lu, C. H., Wang, X. D., Wang, Q., Wen, C. Y., … Chen, X. (2015). A colorimetric assay for measuring iodide using Au@Ag core-shell nanoparticles coupled with Cu(2.). Analytica Chimica Acta, 891, 269–276. doi:10.1016/j.aca.2015.06.043
- Zhan, L., Wu, W. B., Yang, X. X., & Huang, C. Z. (2014). Gold nanoparticle-based enhanced ELISA for respiratory syncytial virus. New Journal of Chemistry, 38(7), 2935–2940. doi:10.1039/C4NJ00253A
- Zhang, L., Huang, Y., Wang, J., Rong, Y., Lai, W., Zhang, J., & Chen, T. (2015). Hierarchical flowerlike gold nanoparticles labeled immunochromatography test strip for highly sensitive detection of escherichia coli O157:H7. Langmuir, 31(19), 5537–5544. doi:10.1021/acs.langmuir.5b00592
- Zhang, N., Liu, K., Liu, Z., Song, H., Zeng, X., Ji, D., … Gan, Q. (2015). Ultrabroadband metasurface for efficient light trapping and localization: A universal surface-enhanced Raman spectroscopy substrate for “all” excitation wavelengths. Advanced Materials Interfaces, 2, 1500142. doi:10.1002/admi.201500142
- Zhang, Q., Li, N., Goebl, J., Lu, Z., & Yin, Y. (2011). A systematic study of the synthesis of silver nanoplates: Is citrate a “magic” reagent? Journal of the American Chemical Society, 133(46), 18931–18939. doi:10.1021/ja2080345
- Zhang, Q., Zhao, B., Yan, J., Song, S., Min, R., & Fan, C. (2011). Nanotube-based colorimetric probe for ultrasensitive detection of ataxia telangiectasia mutated protein. Analytical Chemistry, 83(23), 9191–9196. doi:10.1021/ac2023684
- Zhang, S., Leem, G., Srisombat, L.-O., & Lee, T. R. (2008). Rationally designed ligands that inhibit the aggregation of large gold nanoparticles in solution. Journal of the American Chemical Society, 130(1), 113–120. doi:10.1021/ja0724588
- Zhao, J., Lin, F., Yi, Y., Huang, Y., Li, H., Zhang, Y., & Yao, S. (2012). Dual amplification strategy of highly sensitive thrombin amperometric aptasensor based on chitosan-Au nanocomposites. The Analyst, 137(15), 3488–3495. doi:10.1039/C2AN35340G
- Zhou, F., Wang, M., Yuan, L., Cheng, Z., Wu, Z., & Chen, H. (2012). Sensitive sandwich ELISA based on a gold nanoparticle layer for cancer detection. The Analyst, 137(8), 1779–1784. doi:10.1039/C2AN16257A
- Zhu, Y., Song, S., Liu, L., Kuang, H., & Xu, C. (2016). An indirect competitive enzyme-linked immunosorbent assay for acrylamide detection based on a monoclonal antibody. Food and Agricultural Immunology, 27(6), 796–805. doi:10.1080/09540105.2016.1160369