ABSTRACT
Bamboo-shavings hemicellulose (BSH) is a water-extractable hemicellulose isolated from steamed-exploded bamboo shavings. The present study investigated the protective potential of BSH against cyclophosphamide (CPA)-induced immunosuppression in mice. Mice were orally administered BSH once daily at the dose of 50, 100 or 200 mg/kg body weight (BW) for 14 days, coupled with a hypodermic injection of CPA (70 mg/kg BW/day) on days 4, 8 and 12. The results showed that 14 days of oral administration of BSH at a dose of 100 or 200 mg/kg BW/day significantly accelerated the recoveries of serum hemolysin formation, phagocytic function of the reticuloendothelial system, splenocyte proliferation, splenic NK cell activity, secretion of splenic Th1/Th2 cytokines (IL-2, IL-12, TNF-α, INF-γ/IL-4) together with mRNA expression of Th1/Th2 transcription factors (T-bet/GATA-3) in CPA-induced immunosuppressive mice. Our results demonstrated that BSH could ameliorate CPA-induced immunosuppression in mice and may potentially serve as a supportive therapy in conventional chemotherapy.
1. Introduction
Cancer is part of the leading causes of death globally. Chemotherapeutics tend to inhibit the proliferation of cancer cells. Cyclophosphamide (CPA) is the most commonly used chemotherapeutic drug for treatment of a broad spectrum of cancers, but it has severe adverse effects on normal cells. The toxic effects of CPA are mainly due to the generation of two major metabolites, namely phosphoramide mustard with the antineoplastic moiety and acrolein metabolite with the most toxic agent (Sheweita, El-Hosseiny, & Nashashibi, Citation2016). CPA-induced immunosuppression leads to significant morbidity and mortality and remains a consistent obstacle to successful antitumor treatment (Yu et al., Citation2014). Discovery of novel agents with immunopotentiation and detoxification properties would bear a large significance for cancer treatment.
Polysaccharides represent a class of structurally diverse macromolecules and have many biological functions, especially immunomodulatory activity (Chen et al., Citation2014; Liu, Li, Xu, & Li, Citation2016; Navegantes, Albuquerque, Dalla-Santa, Soccol, & Monteiro, Citation2013; Sansone, Sansone, Shiga, & Nascimento, Citation2016). Recently, polysaccharides obtained from various natural sources have shown protective effects against CPA-induced immunosuppression in mice (Cho et al., Citation2015; Hao & Zhao, Citation2016; Hu, Jiang, Huang, & Sun, Citation2016; Yu et al., Citation2015), thus indicating ideal candidates for the development of chemotherapeutics with immunomodulatory action. Hemicelluloses, comprising the non-cellulose cell-wall polysaccharides of vegetative and storage tissues of annual and perennial plants, represent an immense renewable resource of biopolymers (Ebringerová, Hromádková, & Heinze, Citation2005). They occur in a large variety of structural types, divided into four general groups, that is, xylans, mannans, mixed linkage β-glucans and xyloglucans (Ebringerová et al., Citation2005). Hemicelluloses have a very wide range of uses. They can be converted into monose, fuel ethanol and biopolymers. Additionally, they are reported to possess immunomodulatory activity (Cao et al., Citation2011; Kumar & Tiku, Citation2016; Sahasrabudhe, Schols, Faas, & de Vos, Citation2016; Zhang, Li, Smith, & Musa, Citation2015; Zhang, Smith, Li, & Ashworth, Citation2016) or antitumor activity (Cao et al., Citation2011). In consideration of the abundance of hemicelluloses sources in the earth, it would be feasible and beneficial to explore hemicelluloses as immunomodulators.
Bamboo, belonging to Gramineae Bambusoideae, is a large perennial plant widely distributed in tropical and subtropical regions with a total annual production of 6–7 million tons (Li, Fan, Xu, & Sun, Citation2011). In Asian countries, different parts of bamboo have been used for diverse medicinal purposes (Panee, Citation2015). Bamboo shavings, the outer or intermediate layer of bamboo stems, have served as a traditional Chinese medicine for treating stomach ache, diarrhea and vomiting, chest diaphragm inflammation, restlessness and excessive thirst (Zhang, Yao, Bao, & Zhang, Citation2006). China is a rich country for producing bamboo in the world and is well developed in bamboo processing. During the processing of bamboo stems, sizable quantities of bamboo shavings are produced. Currently, aside from being directly used as folk medicine with quite small amounts, bamboo shavings have not been effectively utilized yet. Meanwhile, up to now, very little information is found on the composition and pharmacological details of bamboo shavings. In our previous study, we isolated a water-extractable hemicellulose (namely BSH, bamboo-shavings hemicellulose) from steam-exploded bamboo shavings. BSH could be separated in two fractions on a DEAE-sepharose Fast Flow column, an O-acetylated-arabinoxylan (molecular weight of 12,800 g/mol) and an O-acetylated-(4-O-methyl-glucurono)-arabinoxylan (molecular weight of 11,300 g/mol) (Huang et al., Citation2016). Meanwhile, BSH showed a robust immunostimulatory activity in vitro (Huang et al., Citation2016). The present study investigated the protective potential of BSH against CPA-induced immunosuppression in mice.
2. Materials and methods
2.1. Materials and reagents
BSH was prepared as described previously (Huang et al., Citation2016). Cyclophosphamide was from Shanxi Pude Pharmaceutical Co., Ltd. (Shanxi, China). Lentinan (LNT) was supplied by Hubei Chuangli Pharmaceutical Co., Ltd. (Hubei, China). Concanavalin A (ConA), lipopolysaccharide (LPS, from Escherichia coli 055:B5) and methylthiazolyldiphenyl-tetrazolium bromide (MTT) were from Sigma Chemical Co. Ltd. (St. Louis, MO, USA). RPMI-1640 medium, fetal bovine serum (heat-inactived), penicillin, streptomycin and phosphate buffer saline (PBS) were from Bioind Co., Ltd. (Israel). Erythrocyte lysis buffer was a product of eBioscience (San Diego, CA, USA). Mouse IL-2 (interleukin-2), IL-4 (interleukin-4), IL-10 (interleukin-10), IL-12 (interleukin-12), TNF-α (tumor necrosis factor-α) and IFN-γ (interferon-γ) quantitative sandwich enzyme immunoassay kits were from Hangzhou Multisciences Biotech Co., Ltd. (Hanzhou, China). Lactate dehydrogenase (LDH) assay kit was from Nanjing JianCheng Bioengineering Institute (Nanjing, China). RNAisoTM plus was from Takara Biotechnology Co., Ltd. (Dalian, China). RevertAid First Strand cDNA Synthesis kit was from Thermo Scientific (USA). FastStart Universal SYBR Green Master (ROX) was from Roche (Switzerland). Oligonucleotide primer sequences of T-bet (T-box expressed in T-cells), GATA-3 (GATA-binding protein-3) and β-actin for RT-qPCR (real-time quantitative polymerase chain reaction) were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). All chemicals used in the experiments were of analytical grade.
2.2. Animals and cells
Female-specific pathogen-free ICR mice weighing 18.0 ± 2.0 g were from Shanghai Laboratory Animal Center (Certificate No. SOXK. 2007-0005, Shanghai, China). The animals were maintained in a 12/12 h light/dark cycle room with a temperature of 24 ± 1°C and a humidity of 50 ± 10%. Standard laboratory animal feed and water were supplied ad libitum. All procedures related to the animals and their care were in line with the internationally accepted principles as found in the Guidelines for Keeping Experimental Animals issued by the government of China. The experiments were approved by the Ethics Committee of College of Biosystem Engineering & Food Science, Zhejiang University. Sterile fiber-free sheep red blood cells (SRBCs) were from Hangzhou Sinry Bio-engineering Co., Ltd. (Hangzhou, China). YAC-1 cells were from Cell Bank of Chinese Academy of Science (Shanghai, China).
2.3. Animal study
Mice were randomly divided into 7 groups. From day 1 to day 14, mice received intragastrical administration once daily of (i) NC (normal control) group, sterile distilled water; (ii) CPA group, sterile distilled water; (iii) CPA + LNT (50) group, 50 mg LNT/kg body weight (BW); (iv) CPA + BSH (50) group, 50 mg BSH/kg BW; (v) CPA + BSH (100) group, 100 mg BSH/kg BW; (vi) CPA + BSH (200) group, 200 mg BSH/kg BW; (vii) BSH (200) group, 200 mg BSH/kg BW. Mice in groups (ii)∼(vi) were subjected to immunosuppression by hypodermic injection of CPA (70 mg/kg BW/day) on days 4, 8 and 12 to establish the immunosuppressive animal model, while mice in groups (i) and (vii) had the same volume of sterile physiological saline. Each group contained 24 mice. Eight mice in each group were used for: (a) serum hemolysin formation test; (b) carbon clearance test; (c) determination of splenocyte proliferation, splenic natural killer (NK) cell activity, cytokine production and mRNA expression of transcription factors. Twenty-four hours after the last administration, mice were sacrificed by cervical dislocation and subjected to the following analyses.
2.3.1. Serum hemolysin formation test
The test was determined as described previously (Lv et al., Citation2013) with minor modifications. On day 10, mice were immunized by intraperitoneal injection of 0.2 mL suspensions of SRBCs (2%, v/v). After five days, blood samples were collected by retro-orbital sinus puncture, allowed to clot for 1 h, and then centrifuged at 1000 g for 10 min to obtain the serum. Serum samples were diluted 700-fold (for normal mice) or 80-fold (for CPA-treated mice) with the SA buffer (NaCl 8.38 g, C4H4N2O3 0.46 g, C8H11N2NaO3 0.3 g, NaHCO3 0.252 g, CaCl2·2H2O 0.2 g, and MgCl2 0.1 g, which were made up to 1000 mL with distilled water). An aliquot of 50 μL of SRBCs (10%, v/v) was mixed with 200 μL of diluted serum and 100 μL of fresh guinea pig serum (initially diluted with aforementioned SA buffer at 1:8 dilution). After incubation at 37°C for 25 min, the reaction mixture was immediately placed on an ice bath and centrifuged at 1000 g for 10 min. A 50 μL volume of supernatant was transferred into a 96-well plate, and 150 μL of Dush’s solution was added (NaHCO3 1.0 g, K3Fe(CN)6 0.2 g and KCN 0.05 g, which were made up to 1000 mL with distilled water). At the same time, 12.5 μL of 10% (v/v) SRBCs was mixed with 200 μL of Dush’s solution. After 10 min, the absorbance was read at a wavelength of 540 nm. The level of serum hemolysin was expressed as the half value of hemolysis (HC50), as calculated using the following formula.where OD1 and OD2 were the absorbance of the sample and SRBCs, respectively.
2.3.2. Carbon clearance test
A carbon clearance test was used to determine the phagocytic index based on the method described by a previous study (Lv et al., Citation2013) with minor modifications. On day 14, 2 hours after the last administration, mice were weighed and then injected with Indian ink (0.1 mL/10 g BW) via the tail vein. A 20 μL specimen of blood was drawn from the retro-orbital sinus at 2 and 10 min immediately after ink injection. Blood samples were mixed with 2 mL of 0.1% Na2CO3 solution, and the absorbance was measured at 650 nm. Afterwards, mice were sacrificed by cervical dislocation. The spleen and liver were excised and immediately weighed. The rate of carbon clearance (K) and the phagocytic index (α) were calculated as follows:
where t2 = 10 min, t1 = 2 min, OD1 and OD2 were the absorbance at 2 and 10 min, respectively.
2.3.3. Splenocyte proliferation assay
Splenocytes proliferation assay was performed as described previously (Huang et al., Citation2016). Briefly, spleens were removed under aseptic conditions from the sacrificed mice in serum-free RPMI-1640 medium, gently homogenized and passed through a 40 μm nylon cell strainer to obtain single-cell suspensions. After treatment with erythrocyte lysis buffer, the remaining cells were centrifuged (200 g at 4°C for 10 min) and washed by PBS three times. The cells were re-suspended to a final density of 2.5 × 106 cells/mL with RPMI-1640 complete medium (RPMI-1640 medium supplemented with 10% heat-inactived fetal bovine serum, 100 U/mL of streptomycin and penicillin). Splenocytes (100 μL/well) were seeded into 96-well plates, with ConA (final concentration: 2.5 μg/mL), LPS (final concentration: 7.5 μg/mL) and RPMI 1640 complete medium in a final volume of 200 μL. The plates were incubated at 37°C in a humidified atmosphere of 5% CO2 for 68 h. The optical density (OD) was determined at 570 nm and the stimulation index (SI) was calculated by the following formula
2.3.4. Measurement of splenic NK cell activity
Splenic NK cell activity was determined by cytotoxicity against YAC-1 cells using the LDH release assay (Chan, Moriwaki, & De Rosa, Citation2013). YAC-1 cells were used as the target cells, and splenocytes were used as the effector cells. The effector-to-target ratio was 50:1. The cells were co-incubated at 37°C in a humidified atmosphere of 5% CO2 for 4 h. After centrifugation (380 g for 10 min), the supernatants were collected. Cell-mediated cytotoxicity was calculated using the following formula
2.3.5. Determination of cytokine production
Splenocytes were seeded into 24-well plates (density: 2.5×106 cells/mL, 1 mL/well) with ConA (final concentration: 2.5 μg/mL). Plates were incubated at 37°C in a humid atmosphere of 5% CO2 for 48 h and then centrifuged at 380 g for 10 min. The levels of IL-2, IL-4, IL-10, IL-12, TNF-α and IFN-γ in the supernatants were determined by commercial assay kits according to the manufacturer’s instructions.
2.3.6. Quantification of T-bet and GATA-3 by RT-qPCR
Splenocytes were seeded into 24-well plates (density: 5×106 cells/mL, 2 mL/well) with ConA (final concentration: 2.5 μg/mL) and incubated at 37°C in a humid atmosphere of 5% CO2. After 18 h, cells were harvested by centrifugation (at 380 g for 10 min at 4°C), washed with ice-cold PBS and then subjected to RNA extraction. Splenocytes (1 × 107) were lysed in 1 mL of RNAisoTM Plus reagent (Takara, China) and the total RNA was isolated according to the manufacturer’s protocol. The concentration of total RNA was checked by determining the optical density at 260 nm. The reverse transcription was performed by mixing 5 μg of RNA with 1 μL of oligo(dT)18 primer, 4 μL of 5 × Reaction Buffer, 1 μL of RiboLock RNase Inhibitor (20 U/μL), 2 μL of 10 mM dNTP Mix, 1 μL of RevertAid M-MuLV Transcriptase (200 U/μL) (Thermo Scientific, USA) in a DEPC-treated tube; thereafter nuclease-free water was added to a final volume of 20 μL. The reaction conditions for reverse transcription were maintained according to the manufacture’s protocol (5 min at 25°C, 60 min at 42°C, 5 min at 70°C, hold at 4°C). Relative quantification of GATA-3, T-bet cDNA to β-actin message was conducted on ABI 7300 (PE Applied Biosystems, USA). Mouse primers of T-bet (forward: 5′-ATTGCCCGCGGGGTTG-3′, reverse: 5′-GACAGGAATGGGAACATTCGC-3′, 135 bp) and GATA-3 (forward: 5′-GGTCAAGGCAACCACGTC-3′, reverse: 5′-CATCCAGCCAGG GCAGAG-3′, 133 bp) genes were amplified. The housekeeping gene β-actin (forward: 5′AGCGGTTCCGATGCCCT-3′, reverse: 5′-AGAGGTCTTTACGGATGTCAACG-3′, 201 bp) was used. Amplification was carried out in a total volume of 20 μL containing 1 μL of cDNA template, 0.5 μL of form primer, 0.5 μL of reverse primer, 8 μL of nuclease-free water and 10 μL of FastStart Universal SYBR Green Master (ROX) (Roche, Switzerland). Reaction conditions were maintained as follows: pre-denaturation program (2 min at 50°C, 10 min at 95°C), amplification and quantification program (15 s at 95°C, 1 min at 60°C) with 40 cycles and melting curve program (15 s at 95°C, 1 min at 60°C, 15 s at 95°C). Relative quantification between samples was achieved by the 2−ΔΔCt method (Livak & Schmittgen, Citation2001) and reported as the n-fold difference relative to the target gene mRNA expression of the NC group.
2.4. Statistical analyses
Data were expressed as means ± standard deviations (SD). Independent sample t-test or one-way analysis of variance (ANOVA) followed by Dunnett’s test were used to compare the parameter between groups by using SPSS 17.0. P values of less than .05 were considered statistically significant.
3. Results
3.1. Effect of BSH on serum hemolysin formation in CPA-treated mice
The formation of serum hemolysin reflects humoral immunity and can be used as a measure to evaluate the functional status of the humoral immune response. It has been reported that CPA primarily suppresses humoral immunity (Wahab et al., Citation2014). As shown in , in the CPA-alone group, the production of serum hemolysin (expressed as HC50, representing the level of SRBCs-specific antibody) was noticeably lower than the NC group (p < .05). In comparison, serum level of hemolysin in the 100 mg/kg BSH, 200 mg/kg BSH or 50 mg/kg LNT-treated group was significantly higher than the CPA-alone group (p < .05 or p < .01). The result suggested that BSH or LNT could reverse CPA-induced humoral immune suppression in mice.
Table 1. Effect of BSH on serum hemolysin formation and NK cell activity in mice.
3.2. Effect of BSH on phagocytic function of reticuloendothelial system in CPA-treated mice
Reticuloendothelial system (RES) is a diffuse system comprising a group of cells having the capacity to take up and sequester inert particles and vital dyes, including macrophages, specialized endothelial cells, reticular cells and so on. Cells of RES have prominent roles in the clearance of particles from the bloodstream (Lv et al., Citation2013). In this study, the phagocytic function of RES was determined by the carbon clearance test. When Indian ink containing carbon particles was injected directly into the systemic circulation, macrophages located in the liver and the spleen vividly swallowed the particles bound with serum proteins (Li & Huang, Citation2009). The rate of clearance of carbon from the blood by macrophages was guided by an exponential equation (Lv et al., Citation2013). And the phagocytic activity was positively correlated with the removal rate of carbon particles (Lv et al., Citation2013). As shown in , the carbon clearance phagocytic index of CPA-treated mice was significantly lower than that of normal mice (p < .01), whereas administration of BSH (100 or 200 mg/kg) could significantly increase the phagocytic index of CPA-treated mice (p < .01 and p < .05), demonstrating that BSH was capable of enhancing the phagocytic function of RES in CPA-treated mice. A similar effect was observed in the LNT-positive control group (p < .05).
3.3. Effect of BSH on splenic NK cell activity in CPA-treated mice
NK cells are lymphocytes that have spontaneous and non-MHC (major-histocompatibility-complex)-restricted cytotoxic activity targeted against a variety of tumor, virus-infected or allogeneic cell targets (Kakutani et al., Citation2012). In this study, the cytotoxic activity of splenocytes against NK cell-sensitive cells (YAC-1) was evaluated. As shown in , CPA significantly inhibited splenic NK cell activity in mice (p < .05). In contrast, BSH treatment dose-dependently ameliorated CPA-induced decrease in splenic NK cell activity, with significant effects at a dose level of 100 mg/kg or 200 mg/kg as compared with the CPA-alone group (both p < .05). A similar result was seen in the LNT-positive control group (p < .05). The above data suggested that BSH could accelerate the recovery of splenic NK cell activity in CPA-treated mice.
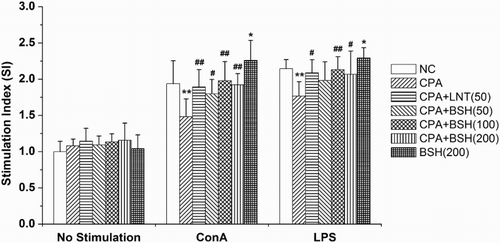
3.4. Effect of BSH on splenocyte proliferation in CPA-treated mice
Splenocyte proliferation is a pivotal event in the activation cascade of both cellular and humoral immune responses. T cells are primarily responsible for cellular immunity, and B cells are the cells capable of producing antibodies (Sun, Gao, Xiong, Huang, & Xu, Citation2014). They both play a crucial role in host defense. The proliferation of T cells and B cells is recognized as a response to the stimulation induced by antigen or mitogens. ConA stimulates T cell whereas LPS stimulates B cell proliferation (Sun et al., Citation2014). In this study, the co-mitogenic effects of BSH on the proliferation of splenocytes in response to ConA or LPS were investigated (). In the CPA-alone group, the splenocyte proliferative response to ConA or LPS was markedly weaker as compared with the NC group (both p < .01). On the contrary, BSH treatment (50, 100 and 200 mg/kg) could significantly recover the splenocyte proliferative response to ConA in CPA-treated mice (p < .05, p < .01 and p < .01). Meanwhile, in the 100 mg/kg or 200 mg/kg BSH-treated groups (but not the 50 mg/kg BSH-treated group), there were significantly stronger splenocyte proliferative responses to LPS as compared with mice treated with CPA alone (p < .01 or p < .05). The result indicated that BSH could restore CPA-induced T cell or B cell proliferation suppression in mice. Similar effect was seen in the LNT-positive control group ().
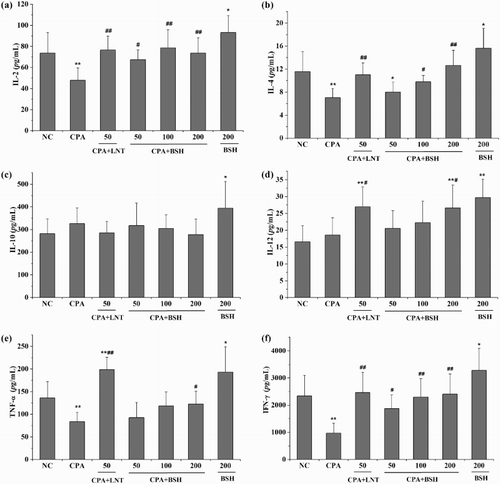
3.5. Effect of BSH on cytokine production by splenocytes in CPA-treated mice
Cytokines are a group of small proteins produced by a broad range of cells, including immune cells like T cells, B cells, macrophages and mast cells, as well as endothelial cells, fibroblasts and various stromal cells. As important cell-signaling molecules, cytokines play crucial roles in immune cell differentiation and development, immune regulation, inflammation and hematopoietic function (Lackie, Citation2010). Cytokines can be classified as interleukins, interferons, TNF, colony-stimulating factors, chemokines, growth factors and so on. Generally, the production of IL-2, IL-12, TNF-α and IFN-γ leads to Th1 type immune response, while the production of IL-4, IL-5 and IL-10 leads to Th2 type immune response (Raphael, Nalawade, Eagar, & Forsthuber, Citation2015; Vahedi et al., Citation2013). In this study, we investigated the effect of BSH on the secretion of both splenic Th1 and Th2 cytokines (IL-2, IL-12, TNF-α, IFN-γ, IL-4, IL-10) in mice. shows that mice treated with CPA alone secreted significantly lower levels of IL-2, IL-4, TNF-α and IFN-γ as compared with mice in the NC group (all p < .01). The levels of IL-10 and IL-12 were partially increased by CPA treatment with non-significant differences in comparison with the NC group (p > .05). All BSH-treated groups (at the dose of 50, 100 or 200 mg/kg) induced significant increases in IL-2 and IFN-γ when compared with the CPA-alone group (p < .05 or p < .01). Both 100 mg/kg and 200 mg/kg BSH-treated groups showed significantly higher levels of IL-4 as compared to the CPA-alone group (p < .05 and p < .01). The levels of IL-12 and TNF-α were also noticeably higher in the 200 mg/kg treated BSH group as compared with the CPA-alone group (both p < .05). The level of IL-10 was partly decreased by BSH treatment (at the dose of 50, 100 or 200 mg/kg) without significant differences as compared to the CPA-alone group (p > .05). For the positive control (LNT), it significantly enhanced the secretions of IL-2, IL-4, IL-12, TNF-α and IFN-γ (but not IL-10) when compared to the CPA-alone group (p < .05 or p < .01).
Figure 2. Effect of BSH on cytokine production from splenocytes in mice. (a) IL-2; (b) IL-4; (c) IL-10; (d) IL-12, (e) TNF-α; (f) IFN-γ. NC, normal control; CPA, cyclophosphamide; BSH, bamboo-shavings hemicellulose; LNT, lentinan (positive control). Values are expressed as mean ± SD of eight mice. *p < .05 and **p < .01 vs. NC group, #p < .05 and ##p < .01 vs. CPA group.
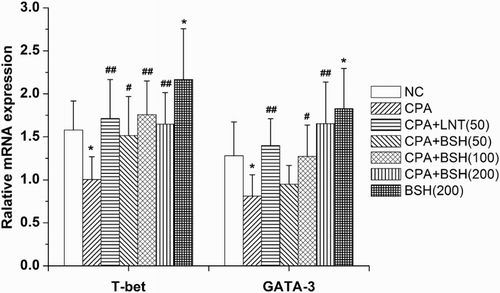
3.6. Effect of BSH on mRNA expression of T-bet and GATA-3 in splenocytes of CPA-treated mice
T-bet (T-box expressed in T-cells) is a Th1 cell-specific transcription factor that controls the expression of Th1 cytokines. GATA-3 (GATA-binding protein-3) belongs to the GATA family of transcription factors and induces the differentiation of naive Th cells toward the Th2 subtype (Yagi, Zhu, & Paul, Citation2011). The mRNA expression of T-bet and GATA-3 from mouse splenocytes was quantified. As shown in , mice treated with CPA alone induced a significantly lower level of T-bet and GATA-3 mRNA expression as compared to normal mice (both p < .05). Mice in all BSH-treated groups (50, 100 and 200 mg/kg) showed a significantly higher level of T-bet mRNA expression as compared with the CPA-alone group (p < .05, p < .01 and p < .01). Meanwhile, mice in the 100 mg/kg or 200 mg/kg BSH groups showed noticeably enhanced GATA-3 mRNA expression as compared to the CPA-alone group (p < .05 and p < .01). LNT treatment also significantly up-regulated the mRNA expression of T-bet and GATA-3 in CPA-treated mice (both p < .01).
Figure 3. Effect of BSH on T-bet and GATA-3 mRNA expression in splenocytes of mice. NC, normal control; CPA, cyclophosphamide; BSH, bamboo-shavings hemicellulose; LNT, lentinan (positive control). Values are expressed as mean ± SD of eight mice. *p < .05 and **p < .01 vs. NC group, #p < .05 and ##p < .01 vs. CPA group.
3.7. Effect of BSH on immune function in normal mice
It is generally accepted that the immunomodulatory effect of nourishing medicine is difficult to evaluate in healthy animals. In the present study, we investigated the effect of BSH on the immune function of normal mice as well. It was found that BSH alone at a dose of 200 mg/kg significantly increased serum level of hemolysin in normal mice (p < .05) (). In addition, there were significant differences in splenocyte proliferative response to ConA or LPS, secretion of cytokines (IL-2, IL-4, IL-10, IL-12, TNF-α and IFN-γ) and mRNA expression of T-bet and GATA-3 in splenocytes between the 200 mg/kg BSH-treated mice and the normal mice (–). These data indicated that BSH could also stimulate the immune function of normal mice.
4. Discussion
It is well known that CPA is an effective chemotherapeutic drug in cancer treatment, but it can cause immunosuppression to the organism. In this study, mice were treated with CPA to simulate a weakened immune system. As expected, CPA markedly reduced serum hemolysin formation, splenocyte proliferative response to ConA or LPS, as well as splenic NK cell activity. It also suppressed the secretion of splenocyte cytokines (IL-2, IL-4 IL-12, TNF-α and IFN-γ). The above data were in agreement with previous studies (Chen et al., Citation2012; Jang et al., Citation2013; Wang et al., Citation2011; Yu et al., Citation2014). Besides, we found that CPA significantly suppressed mRNA expression of T-bet/GATA-3 (Th1/Th2 transcription factors) in splenocytes. Traditionally, medicinal herbs are administered via the oral route, and oral use of immunomodulators can diminish the side effects found in parenteral administration. Thus, the present study investigated the alleviative effect of oral administration of BSH on CPA-induced immunosuppression in mice.
Lymphocytes are part of the subtypes of white blood cell in a vertebrate’s immune system. They include NK cells (for cytotoxic innate immunity), T cells (for cell-mediated, cytotoxic adaptive immunity) and B cells (for humoral, antibody-driven adaptive immunity). In this study, we found that BSH could dramatically increase the cytotoxic activity of NK cells in CPA-induced immunosuppressed mice, indicating that BSH could improve the body’s innate immune defenses and may have potential antitumor and anti-infection activities. Meanwhile, BSH could markedly enhance the proliferation of splenic T cells and B cells, as well as the formation of serum hemolysin in CPA-induced immunosuppressed mice, suggesting that BSH was capable of activating the body’s specific immune responses and had positive impacts on both cellular and humoral immune function in mice.
T cells are highly heterogeneous cells, which are known to be of different types, such as T-helper (Th) cells, T-cytotoxic (Tc) cells and T-regulatory (Treg) cells (Wang, Tong, Li, Cao, & Su, Citation2012), wherein Th cells are a group of cells that can help enhance the cellular and humoral immune responses. It is generally recognized that the precursor of Th cells firstly differentiated into naive Th cells by antigen stimulation, and then differentiated into Th1 or Th2 cells under different microenvironments (Vahedi et al., Citation2013). Th1 cells mainly release IL-2, IL-12 and IFN-γ, and enhance the cytotoxicity of killer cells or cell-mediated immunity response, while Th2 cells primarily secrete IL-4, IL-5 and IL-10, and promote the production of antibodies and mediate the humoral immune response (Raphael et al., Citation2015; Vahedi et al., Citation2013). Thus, the activated Th cells govern the activities of immune cells by releasing different types of cytokines. For instance, IL-2 is produced predominately by antigen-stimulated CD4+ T cells, and can also be produced by CD8+ cells or NK cells. It modulates Tcell differentiation programs in response to antigen and promotes the cytotoxicity activity of CD8+ cells and NK cells (Jiang, Zhou, & Ren, Citation2016). Therefore, the enhanced splenic T cell proliferation and NK cell activity induced by BSH might be partly related to its promotion on IL-2 secretion (, and (a)). IL-4 is a key regulator in humoral immunity and has many biological roles, including the stimulation of B and cytotoxic T cells proliferation, and the enhancement of MHC class II expression and antibody production (Turner, Nedjai, Hurst, & Pennington, Citation2014). Accordingly, the enhanced splenic B cell proliferation induced by BSH might be due to its promotion on splenic IL-4 secretion ( and (b)). IL-12, a proinflammatory cytokine produced by dendritic cells, macrophages and B cells in response to microbial pathogens, facilitates Th1 cells differentiation and can also induce the production of IFN-γ by NK cells (Vignali & Kuchroo, Citation2012). IFN-γ, also called type II interferon, is a key cytokine mainly secreted by T cells, and has a number of important roles including increasing the expression of toll-like receptors (TLRs) by innate immune cells, induction of chemokine secretion, macrophage activation and increased phagocytosis (Raphael et al., Citation2015). Consequently, the enhancement of phagocytic function of RES and splenic NK cell activity induced by BSH might be partially because of its promotion on splenic IL-12 and IFN-γ secretions (, (d) and (f)). Nevertheless, the regulation of various cytokines is usually not independent but interacted or synergetic. For the present study, IL-2 enhanced the proliferation of T cells, and the activated T cells promoted the secretion of IFN-γ. Both IL-2 and IL-12 could enhance the cytotoxic activity of NK cells, and activated NK cells also promoted the secretion of IFN-γ. IFN-γ could activate monocytes/macrophages and promoted them secreting TNF-α. However, IL-4 is a potent anti-inflammatory cytokine that could lower the expression of pro-inflammatory cytokines (such as TNF-α). As a consequence, the production of TNF-α was regulated to a moderate level ((e)).
The differentiation of naive Th cells toward Th1 or Th2 cells is controlled by the transcription factors T-bet and GATA-3 (Shih et al., Citation2014). T-bet for Th1 cells and GATA-3 for Th2 cells are prime candidates for key transcription factors of cytokine memory (Vahedi et al., Citation2013). To explore the possible mechanism on how BSH regulated the secretion of splenic Th1/Th2 cytokines, we further examined the effect of BSH on the mRNA expression of T-bet/GATA-3 from splenocytes in mice. It was found that CPA markedly down-regulated the mRNA expression of T-bet/GATA-3 in mice splenocytes, whereas BSH could restore them to normal level (). Therefore, we deduced that BSH might modulate Th1/Th2 cytokine secretion via the regulation of T-bet/GATA-3 mRNA expression in mice.
5. Conclusion
In conclusion, the present study has demonstrated that BSH could alleviate CPA-induced immunosuppression in mice, and it has the potential to be implemented in antineoplastic immunotherapy combined with chemotherapeutic agents. In view of the abundance of bamboo and its by-products in China as well as other tropical countries, our study may be useful to pave a way for the conversion of bamboo shavings as value-added materials.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Ju-qing Huang obtained her Doctor's degree in Food Science from Zhejiang University (Hangzhou, China) in 2015. She currently works as a research assistant in Fujian Academy of Agricultural Sciences (Fuzhou, China). Her research interests include bioactive carbohydrates and immunology.
Mei-rong Pang obtained her Master's degree in Food Science from Zhejiang University (Hangzhou, China) in 2015. She now works as a R & D personnel in COFCO Corporation (Beijing, China). Her research interests include chemicals in agricultural products and their safety.
Guang-yu Li is a Ph.D. candidate in Oncology in Zhejiang University (Hangzhou, China). His research interests include animal immunology and antitumor immunotherapy.
Nan Wang is a postgraduate student in Food Engineering in Zhejiang University (Hangzhou, China). She is interested in food processing and natural product research.
Lu Jin is a postgraduate student in Food Engineering in Zhejiang University (Hangzhou, China). His is interested in polysaccharides and human health.
Ying Zhang is a Ph.D. in Food Science. Presently, she holds a professor position in the Department of Food Science and Nutrition of Zhejiang University (Hangzhou, China). Her research interest includes the relationship between natural products and human health.
Additional information
Funding
References
- Cao, L., Liu, X., Qian, T., Sun, G., Guo, Y., Chang, F., … Sun, X. (2011). Antitumor and immunomodulatory activity of arabinoxylans: A major constituent of wheat bran. International Journal of Biological Macromolecules, 48(1), 160–164. doi:10.1016/j.ijbiomac.2010.10.014
- Chan, F. K., Moriwaki, K., & De Rosa, M. J. (2013). Detection of necrosis by release of lactate dehydrogenase activity. Immune Homeostasis: Methods and Protocols, 65–70. doi:10.1007/978-1-62703-290-2_7
- Chen, P. B., Wang, H., Liu, Y., Lin, S., Chou, H., & Sheen, L. (2014). Immunomodulatory activities of polysaccharides from Chlorella pyrenoidosa in a mouse model of Parkinson’s disease. Journal of Functional Foods, 11, 103–113. doi:10.1016/j.jff.2014.08.019
- Chen, X., Nie, W., Fan, S., Zhang, J., Wang, Y., Lu, J., … Jin, L. (2012). A polysaccharide from Sargassum fusiforme protects against immunosuppression in cyclophosphamide-treated mice. Carbohydrate Polymers, 90(2), 1114–1119. doi:10.1016/j.carbpol.2012.06.052
- Cho, C., Han, C., Rhee, Y. K., Lee, Y., Shin, K., Shin, J., … Hong, H. (2015). Cheonggukjang polysaccharides enhance immune activities and prevent cyclophosphamide-induced immunosuppression. International Journal of Biological Macromolecules, 72, 519–525. doi:10.1016/j.ijbiomac.2014.09.010
- Ebringerová, A., Hromádková, Z., & Heinze, T. (2005). Advances in polymer science. Advances in Polymer Science, 186, 1–67. doi:10.1007/b136816
- Hao, L., & Zhao, X. (2016). Immunomodulatory potentials of the water-soluble yam (Dioscorea opposita Thunb) polysaccharides for the normal and cyclophosphamide-suppressed mice. Food and Agricultural Immunology, 27(5), 667–677. doi:10.1080/09540105.2016.1148666
- Hu, T., Jiang, C., Huang, Q., & Sun, F. (2016). A comb-like branched β-d-glucan produced by a Cordyceps sinensis fungus and its protective effect against cyclophosphamide-induced immunosuppression in mice. Carbohydrate Polymers, 142, 259–267. doi:10.1016/j.carbpol.2016.01.036
- Huang, J., Qi, R., Pang, M., Liu, C., Li, G., & Zhang, Y. (2016). Isolation, chemical characterization and immunomodulatory activity of naturally acetylated hemicelluloses from bamboo shavings. Journal of Zhejiang University-SCIENCE B, 1. doi:10.1631/jzus.B1500274
- Jang, S., Joh, E., Ahn, Y., Huh, C., Han, M. J., & Kim, D. (2013). Lactobacillus casei HY7213 ameliorates cyclophosphamide-induced immunosuppression in mice by activating NK, cytotoxic t cells and macrophages. Immunopharmacology and Immunotoxicology, 35(3), 396–402. doi:10.3109/08923973.2013.789055
- Jiang, T., Zhou, C., & Ren, S. (2016). Role of IL-2 in cancer immunotherapy. OncoImmunology, 5(6), e1163462. doi:10.1080/2162402X.2016.1163462
- Kakutani, R., Adachi, Y., Kajiura, H., Takata, H., Kuriki, T., & Ohno, N. (2012). The effect of orally administered glycogen on anti-tumor activity and natural killer cell activity in mice. International Immunopharmacology, 12(1), 80–87. doi:10.1016/j.intimp.2011.10.017
- Kumar, S., & Tiku, A. B. (2016). Immunomodulatory potential of Acemannan (polysaccharide from Aloe vera) against radiation induced mortality in Swiss albino mice. Food and Agricultural Immunology, 27(1), 72–86. doi:10.1080/09540105.2015.1079594
- Lackie, J. (2010). A dictionary of biomedicine. Oxford: Oxford University Press.
- Li, M. F., Fan, Y. M., Xu, F., & Sun, R. C. (2011). Structure and thermal stability of polysaccharide fractions extracted from the ultrasonic irradiated and cold alkali pretreated bamboo. Journal of Applied Polymer Science, 121(1), 176–185. doi:10.1002/app.33491
- Li, S. D., & Huang, L. (2009). Nanoparticles evading the reticuloendothelial system: Role of the supported bilayer. Biochimica et Biophysica Acta (BBA) – Biomembranes, 1788(10), 2259–2266. doi:10.1016/j.bbamem.2009.06.022
- Liu, L., Li, H., Xu, R., & Li, P. (2016). Expolysaccharides from Bifidobacterium animalis RH activates RAW 264.7 macrophages through toll-like receptor 4. Food and Agricultural Immunology, 1–13. doi:10.1080/09540105.2016.1230599
- Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods, 25(4), 402–408. doi:10.1006/meth.2001.1262
- Lv, Y., Huang, J., Cai, M., Li, C., Zhang, D., Hu, Y., … Li, Z. (2013). A health food high-peptide meal alleviates immunosuppression induced by hydrocortisone and cyclophosphamide in mice. Food & Function, 4(9), 1352–1359. doi:10.1039/C3FO30230J
- Navegantes, K. C., Albuquerque, R. F. V., Dalla-Santa, H. S., Soccol, C. R., & Monteiro, M. C. (2013). Agaricus brasiliensis mycelium and its polysaccharide modulate the parameters of innate and adaptive immunity. Food and Agricultural Immunology, 24(4), 393–408. doi:10.1080/09540105.2012.691089
- Panee, J. (2015). Potential medicinal application and toxicity evaluation of extracts from bamboo plants. Journal of Medicinal Plant Research, 9(23), 681–692. doi: 10.5897/JMPR2014.5657
- Raphael, I., Nalawade, S., Eagar, T. N., & Forsthuber, T. G. (2015). T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine, 74(1), 5–17. doi:10.1016/j.cyto.2014.09.011
- Sahasrabudhe, N. M., Schols, H. A., Faas, M. M., & de Vos, P. (2016). Arabinoxylan activates Dectin-1 and modulates particulate β-glucan-induced Dectin-1 activation. Molecular Nutrition & Food Research, 60(2), 458–467. doi:10.1002/mnfr.201500582
- Sansone, M., Sansone, A. C. M. B., Shiga, T. M., & Nascimento, J. R. O. D. (2016). The water-soluble non-starch polysaccharides from bananas display immunomodulatory properties on cultured macrophages. Food Research International, 87, 125–133. doi:10.1016/j.foodres.2016.07.003
- Sheweita, S. A., El-Hosseiny, L. S., & Nashashibi, M. A. (2016). Protective effects of essential oils as natural antioxidants against hepatotoxicity induced by cyclophosphamide in mice. PLoS One, 11(11), e165667. doi:10.1371/journal.pone.0165667
- Shih, H. Y., Sciumè, G., Poholek, A. C., Vahedi, G., Hirahara, K., Villarino, A. V., … Muljo, S. A. (2014). Transcriptional and epigenetic networks of helper T and innate lymphoid cells. Immunological Reviews, 261(1), 23–49. doi:10.1111/imr.12208
- Sun, X., Gao, R., Xiong, Y., Huang, Q., & Xu, M. (2014). Antitumor and immunomodulatory effects of a water-soluble polysaccharide from Lilii Bulbus in mice. Carbohydrate Polymers, 102, 543–549. doi:10.1016/j.carbpol.2013.12.002
- Turner, M. D., Nedjai, B., Hurst, T., & Pennington, D. J. (2014). Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1843(11), 2563–2582. doi:10.1016/j.bbamcr.2014.05.014
- Vahedi, G., C Poholek, A., Hand, T. W., Laurence, A., Kanno, Y., O’shea, J. J., … Hirahara, K. (2013). Helper T-cell identity and evolution of differential transcriptomes and epigenomes. Immunological Reviews, 252(1), 24–40. doi:10.1111/imr.12037
- Vignali, D. A. A., & Kuchroo, V. K. (2012). IL-12 family cytokines: Immunological playmakers. Nature Immunology, 13(8), 722–728. doi:10.1038/ni.2366
- Wahab, S., Hussain, A., Ahmad, M. P., Rizvi, A., Ahmad, M. F., & Farooqui, A. H. A. (2014). The ameliorative effects of Averroha carambola on humoral response to sheep erythrocytes in non-treated and cyclophosphamide-immunocompromised mice. Journal of Acute Disease, 3(2), 115–123. doi:10.1016/S2221-6189(14)60027-5
- Wang, H., Wang, M., Chen, J., Tang, Y., Dou, J., Yu, J., … Zhou, C. (2011). A polysaccharide from Strongylocentrotus nudus eggs protects against myelosuppression and immunosuppression in cyclophosphamide-treated mice. International Immunopharmacology, 11(11), 1946–1953. doi:10.1016/j.intimp.2011.06.006
- Wang, J., Tong, X., Li, P., Cao, H., & Su, W. (2012). Immuno-enhancement effects of Shenqi Fuzheng injection on cyclophosphamide-induced immunosuppression in Balb/c mice. Journal of Ethnopharmacology, 139(3), 788–795. doi:10.1016/j.jep.2011.12.019
- Yagi, R., Zhu, J., & Paul, W. E. (2011). An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. International Immunology, 23(7), 415–420. doi:10.1093/intimm/dxr029
- Yingjian, L., Junming, H., Min, C., Chenyue, L., Dachao, Z., Yuanhua, H., & Zhi, L. (2013). A health food high-peptide meal alleviates immunosuppression induced by hydrocortisone and cyclophosphamide in mice. Food & Function, 4(9), 1352–1359. doi:10.1039/C3FO30230J
- Yu, Q., Nie, S., Wang, J., Huang, D., Li, W., & Xie, M. (2015). Molecular mechanism underlying chemoprotective effects of Ganoderma atrum polysaccharide in cyclophosphamide-induced immunosuppressed mice. Journal of Functional Foods, 15, 52–60. doi:10.1016/j.jff.2015.03.015
- Yu, Q., Nie, S., Wang, J., Liu, X., Yin, P., Huang, D., … Xie, M. (2014). Chemoprotective effects of Ganoderma atrum polysaccharide in cyclophosphamide-induced mice. International Journal of Biological Macromolecules, 64, 395–401. doi:10.1016/j.ijbiomac.2013.12.029
- Zhang, S., Li, W., Smith, C. J., & Musa, H. (2015). Cereal-derived arabinoxylans as biological response modifiers: Extraction, molecular features, and immune-stimulating properties. Critical Reviews in Food Science and Nutrition, 55(8), 1035–1052. doi:10.1080/10408398.2012.705188
- Zhang, Y., Yao, X., Bao, B., & Zhang, Y. (2006). Anti-fatigue activity of a triterpenoid-rich extract from Chinese bamboo shavings (Caulis bamfusae in taeniam). Phytotherapy Research, 20(10), 872–876. doi:10.1002/ptr.1965
- Zhang, Z., Smith, C., Li, W., & Ashworth, J. (2016). Characterization of nitric oxide modulatory activities of alkaline-extracted and enzymatic-modified arabinoxylans from corn bran in cultured human monocytes. Journal of Agricultural and Food Chemistry, 64(43), 8128–8137. doi:10.1021/acs.jafc.6b02896