ABSTRACT
Myrica rubra Sieb. et Zucc. is a valuable fruit tree that is used in Chinese, Japanese and Taiwanese traditional medicine. We investigated the anti-inflammatory activity of M. rubra leaves extracted with four different solvents. Total phenolics were determined using the Folin–Ciocalteu method. Extracts were investigated for their inhibitory activity toward the pro-inflammatory enzymes cyclooxygenase-1 and -2 (COX-1, COX-2) and 5-lipoxygenase (5-LOX). The ethanol extract of M. rubra leaves demonstrated a strong inhibition of prostaglandin E2 (PGE2) biosynthesis catalyzed by both COX-1 (93.42%) and COX-2 (75.71%) and leukotriene B4 (LTB4) formation catalyzed by 5-LOX (82.72%). Further we identified selective COX-1 inhibition by the n-butanol and aqueous fractions of the ethanol extract (with an IC50 for COX-1 inhibition of 1.07 and 0.71 µg mL−1 , respectively) and dual 5-LOX/COX inhibition by the ethyl acetate fraction (with an IC50 of 3.29 for COX-1, 2.54 for COX-2 and 8.30 µg mL−1 for 5-LOX).
1. Introduction
Natural products from medicinal plants are considered a promising source for developing new and effective anti-inflammatory drugs (Chakraborti, Garg, Kumar, Motiwala, & Jadhavar, Citation2010). Chinese bayberry (Myrica rubra Sieb. et Zucc.) is an evergreen fruit tree with considerable economic value in China. Chinese bayberry fruit acquired local popularity for its unique and delicious sweet and sour taste (Kang, Li, Xu, Jiang, & Tao, Citation2012). M. rubra bark and leaves are commonly used as an astringent, antidiarrheic, analgesic and antidote in traditional medicine in China, Japan and Taiwan (Tong, Zhou, Wang, Yang, & Cao, Citation2009). M. rubra leaves are also traditionally used to treat inflammatory disorders in China (Sun, Huang, Xu, Li, & Chen, Citation2013; Wang et al., Citation2010). The anti-inflammatory activity of M. rubra leaves has been supported by some recent studies (Kim et al., Citation2013; Kim, Oh, Park, Heo, & Lee, Citation2014). M. rubra leaves and bark were reported to be rich in flavonoids (Shimosaki, Tsurunaga, Itamura, & Nakamura, Citation2011) and in tannins, triterpenes and diarylheptanoids (Nonaka, Muta, & Nishioka, Citation1983; Tong et al., Citation2009). The main pharmacologically active components identified in M. rubra leaves were myricetin (Masuda, Someya, & Fujimoto, Citation2010) and myricitrin (Shimosaki et al., Citation2011). Myricitrin was reported to be a significant down-regulator of the pro-inflammatory cytokine TNFα production in lipopolysaccharide (LPS) stimulated RAW 264.7 (Mouse leukaemic monocyte macrophage cell line) cells (Shimosaki et al, Citation2011). Myricetin showed analgesic activity when tested in vivo, with a suggested mechanism of inhibiting cyclooxygenase-1 (COX-1) (Tong et al., Citation2009). Recently, two galloyl myricetin glycosides, one galloyl quercetin glycoside and two sulfate-containing phenolic compounds isolated from M. rubra leaves were reported to inhibit the production of prostaglandin E2 (PGE2) by suppressing cyclooxygenase-2 (COX-2) gene expression in LPS-stimulated RAW 264.7 macrophages (Kim, et al., Citation2013, Citation2014). However, the inhibitory activity of M. rubra leaves toward COX-1, COX-2 and 5-lipoxygenase (5-LOX) catalytic activity has never been studied.
Both cyclooxygenases and 5-LOX are key enzymes that are involved in inflammation. Cyclooxygenases catalyze the conversion of arachidonic acid to prostacyclins, prostaglandins and thromboxanes. There are two forms of cyclooxygenase enzymes: COX-1 and COX-2. Compared to COX-1, which is a constitutive isoform that is expressed constantly in almost all tissues and has a gastrointestinal-protective effect, COX-2 is an inducible isoform that produces pro-inflammatory cytokines and effectors of pain signaling as a response to inflammation. Therefore, the beneficial anti-inflammatory effects seem to be due to the inhibition of COX-2; thus, several compounds have been developed to block COX-2 selectively (Chakraborti et al., Citation2010; Sostres, Gargallo, & Lanas, Citation2013). However, it appears that the long-term use of known selective COX-2 inhibitors, such as celecoxib, rofecoxib or parecoxib, have severe adverse effects, including sudden myocardial infarction and thrombosis. Recently, there is increasing evidence that prostaglandins generated by COX-2 are involved in both inflammation and homoeostatic processes and that COX-1 is a modulator of inflammatory reactions (Armstrong et al., Citation2011; Ren, Lin, Mou, & Dong, Citation2013).
Prostaglandins, which are induced by COX enzymes during the inflammatory process, are not the only products of the arachidonic acid pathway. Arachidonic acid is also a substrate for leukotriene B4 (LTB4) and the cysteinyl leukotrienes generated by the 5-lypoxygenase (5-LOX) pathway. Activation of the 5-LOX pathway results in allergy, inflammation and gastric damage (Pergola & Werz, Citation2010). Recently, the inhibited production of both prostaglandins and leucotrienes was reported as a new promising approach that results in the development of dual 5-LOX/COX inhibitors (Leval, Julemont, Delarge, Pirotte, & Dogne, Citation2002). According to this approach, dual 5-LOX/COX inhibitors may enhance the anti-inflammatory effects while avoiding the cardiovascular problems associated with selective COX-2 inhibitors and inducing less gastrointestinal damage than what is associated with non-selective, nonsteroidal anti-inflammatory drugs (Burnett & Levy, Citation2012; Davies, Smith, Windmeijer, & Martin, Citation2013; Fiorucci, Meli, Bucci, & Cirino, Citation2001). Therefore, in our study, we used in vitro enzyme assays to determine the inhibition of PGE2 production, catalyzed individually by COX-1 or COX-2, and the inhibition of LTB4, biosynthesis, catalyzed by 5-LOX, in stimulated human neutrophil granulocytes.
2. Materials and methods
2.1. Chemicals
Flavonoids myricetin (≥ 96.0%, PubChem CID: 5281672), myricitrin (≥ 99.0%, PubChem CID: 5281673), quercetin (≥ 95.0%, PubChem CID: 5280343) and proanthocyanidin epigallocatechin 3-O-gallate (≥ 95.0%, PubChem CID: 65064) were purchased from Sigma–Aldrich chemicals (Prague, Czech Republic). Dextran T-500 and methanol for high-performance liquid chromatography (HPLC) were purchased from Roth (Karlsruhe, Germany). Ammonium chloride, disodium hydrogen phosphate, sodium chloride and potassium dihydrogen phosphate were purchased from Lachner s.r.o. (Neratovice, Czech Republic). Zileuton (ZIL) was donated by Farmak a.s., (Olomouc, Czech Republic). Potassium chloride and sodium hydroxide were purchased from Lachema a.s. (Brno, Czech Republic). Tris was purchased from Bio-Rad (Prague, Czech Republic). Calcium chloride and acetic acid were purchased from Penta (Prague, Czech Republic). The LTB4 ELISA kit and PGE2 ELISA kit (Enzo Life Sciences, USA) were purchased from GeneTiCA (Prague, Czech Republic). All other solvents (HPLC or analytical grade) and all other chemicals for assays (Ibuprofen, 99% and Indomethacin, ≥ 99%) were purchased from Sigma-Aldrich (Prague, Czech Republic).
2.2. Plant material and extraction
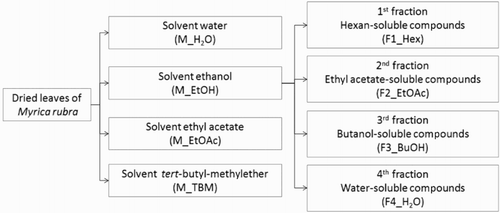
Leaves of M. rubra (var. Dong kui) were collected and identified by Prof. Ji Dong Lou from China Jiliang University on plantations in Zheijang province, China, in 2011. The specimens were authenticated by Prof. Li Sufang from the Department of Botany, China Jiliang University, where a voucher specimen is deposited (number: TD20130129). Collected leaves were air dried and pulverized. This material was separately extracted in water (M_H2O), ethanol (M_EtOH), ethyl acetate (M_EtOAc) and tert-butyl-methylether (M_TBM). Ten grams of pulverized leaves was soaked 3 times in 200 mL of each of the four solvents for 12 hours at room temperature. Extracts were evaporated to dryness with a vacuum rotary evaporator. Each of the four crude extracts was pre-dissolved in DMSO and stored at −20°C before use.
The ethanol extract (M_EtOH) was further fractionated () using the method outlined by Masuda et al. (Citation2010), with modifications. Briefly, the dry ethanol extract was dissolved in 91% methanol in H2O and washed with hexane to remove the lipophilic substances. We obtained a hexane-soluble fraction, F1_Hex. The residual solution was evaporated to dryness and was then re-dissolved in H2O and extracted subsequently with ethyl acetate (ethyl acetate-soluble fraction, F2_EtOAc), and water-saturated n-butanol (butanol-soluble fraction, F3_BuOH). The residual aqueous solution was collected as a water-soluble fraction (F4_H2O). All obtained fractions were evaporated to dryness, pre-dissolved in DMSO at a concentration of 10 mg mL−1 and stored at −20°C before use.
2.3. Total phenol content
The concentration of total phenolic compounds was determined using a modified Folin–Ciocalteu colorimetric method (Singleton, Orthofer, & Lamuela-Raventos, Citation1999). Briefly, 100 µL of diluted extract, blank (DMSO) or standard (gallic acid) was mixed with 25 µL of Folin–Ciocalteu reagent in a 96-well microplate. Samples were shaken at 200 rpm at room temperature for 10 min, and 75 µL of sodium carbonate (12% w/v) was added. The decrease in absorbance was measured using a microplate reader Tecan Infinite M200 (Tecan Group) at 760 nm after 1 hour of incubation in the dark at 37°C. The calculation of the phenolic content was based on a calibration curve obtained with gallic acid and expressed as milligrams of gallic acid equivalents per gram of dry weight extract (mg GA g−1).
2.4. Evaluation of anti-inflammatory activity in vitro
2.4.1. COX-1/2 inhibition
COX inhibitory activity was determined using a cell-free system enzymatic assay according to a previously reported method (Reininger & Bauer, Citation2006). Briefly, 1.0 unit of COX-1 or 0.2 units of COX-2 per reaction was added to 180 µL of incubation mixture. The incubation mixture consisted of 100 mM Tris buffer (pH 8.0), 18 mM L-epinephrine, 5 µM hematin porcine and 50 µM Na2EDTA. Samples diluted in DMSO (or the positive control or DMSO alone, as a blank) were added to the incubation mixture in the 96-well microplate. The reaction was started by adding 10 µM arachidonic acid, and after 20 min of incubation at 37°C, it was stopped with 10% formic acid. The concentration of formed PGE2 was determined using a PGE2 ELISA kit according to the manufacturer’s instructions in samples that were diluted 1:15 in assay buffer. The absorbance was measured with a microplate reader Tecan Infinite M200 at 405 nm. The results were expressed as percentage inhibition of PGE2 formation compared to untreated samples (DMSO alone). The IC50 values were calculated from 4 concentrations. Indomethacin and ibuprofen were used as reference inhibitors.
2.4.2. LOX inhibition
The inhibition of LTB4 produced by the 5-LOX enzyme in human neutrophil granulocytes was determined using a previously described method (Cusan et al., Citation2005; Kutil et al., Citation2015), with some modifications. Neutrophil granulocytes were isolated from the blood of healthy donors and incubated with dextrane solution (6% dextran T-500, 1% NaCl) at 4°C. After one hour, the supernatant was collected and centrifuged at 1600 rpm at 4°C. Pellets were washed with phosphate buffered saline and centrifuged. Pellets were then lysed and centrifuged to remove erythrocytes. Subsequently, the viability of the obtained neutrophil granulocytes was checked, and the cell concentration was determined. The assay mixture was placed in a 96-well microplate and consisted of 225 µL of cell suspension (4500 cells µL−1), 10 µL of 2 mM CaCl2, 10 µM of eicosatetraynoic acid, 5 µL of extract dissolved appropriately in DMSO (or positive control, or DMSO alone as a blank), 10 µL of 21 µM calcium ionophor A23187, and 5 µL of 120 µM arachidonic acid. After 10 min of incubation at 37°C, the reaction was stopped with 10% formic acid. The concentration of LTB4 was determined using an LTB4 ELISA kit according to the manufacturer’s instructions in samples that were diluted 1:40 in assay buffer. The absorbance was measured with a microplate reader Tecan Infinite M200 at 405 nm. The results were expressed as percentage inhibition of LTB4 formation compared to untreated samples (DMSO alone). IC50 values were calculated from 4 concentrations. ZIL was used as a reference inhibitor.
2.5. Analytical studies
An HPLC-PDA system (Midas, Spark, Netherlands) equipped with a Kinetex (2.6 µm) PFP, 100A (150 × 4.6 mm) column (Phenomenex, USA) was used to determine the myricitrin content (Tauchen et al., Citation2015). Mobil phases A (0.5% acetic acid in water) and B (0.5% acetic acid in acetonitrile) were used for gradient elution (% B in A): 96% at 0 min; 85% at 10 min; 79% at 14 min; 78% at 25 min; 59% at 34 min and then 100% B from 38 min to 48 min followed by 10 min of 96% B for equilibration. Other settings were as follows: injection volume, 10 µL; flow rate, 1 mL·min−1; temperature, 33°C. Absorption was monitored between 194 and 500 nm; quantification under 260 and 300 nm. The data were processed using the Clarity software (DataApex, CZ) and EZ-Chrom Elite software (ThermoFinigan, USA).
The absence of fatty acids in M. rubra active fractions (Reininger & Bauer, Citation2006) was confirmed by GC×GC-TOFMS analysis, performed on a LECO Pegasus 4D GC×GC-TOFMS system (Leco Corporation, St. Joseph, MI, USA) containing an Agilent 7890 gas chromatograph (Agilent technologies, Santa Clara, CA, USA) with a LECO dual-jet thermal modulator, Gerstel MultiPurpose Sampler and a temperature programmed CIS4 inlet (Gerstel GmbH & Co. KG, Mülheim an der Ruhr, Germany). The data were processed using the LECO ChromaTOF® software version 4.44.0.0. Fatty acid identification was based on a comparison of their spectra with those in the existing mass libraries (NIST, LECO/Fiehn Metabolomics Library).
2.6. Statistical analysis
The COX-1 and COX-2 measurements were obtained with at least two replicates in three to five independent experiments. The 5-LOX measurements were obtained with at least three replicates in five independent experiments. IC50 values were calculated by regression analysis using Microsoft Excel involving at least four concentrations. The percentage inhibition of enzyme activity and IC50 values are presented as the mean values ± SD. Statistical significance among the samples was analyzed by one-way ANOVA followed by the Tukey test (p ≤ .01) using STAISTICA 12 (StatSoft).
3. Results and discussion
There is a continuing need to search for safe and effective anti-inflammatory compounds from natural sources due to the side effects that are associated with commonly used nonsteroidal anti-inflammatory drugs (NSAIDs). NSAIDs such as indomethacin, aspirin or ibuprofen are known to cause adverse effects in the liver, kidneys and digestive tract (Chakraborti et al., Citation2010). In our study, we tested the potential of M .rubra leaves to inhibit COX-1, COX-2 and 5-LOX enzymes. We aimed to find a potential dual 5-LOX/COX inhibitor in M. rubra leaves.
Screening our crude extracts at a concentration of 50 µg mL−1 revealed that the ethanol extract (M_EtOH) had the best potential to inhibit 5-LOX, COX-1 and COX-2 (). Therefore, we prepared four fractions from the ethanol extract and tested their inhibitory activity on the COX-2 enzyme at concentrations of 10 and 1 µg mL−1 . We observed significant activity (data not shown) with all of the tested fractions, except for the hexane-soluble fraction (F1_Hex). Therefore, IC50 values were determined for the three active fractions (). The significant anti-inflammatory activity of the ethyl acetate and n-butanol fractions has also been observed in other studies on plants such as Siegesbeckia glabrescens (Lee, Kang, Hwang, & Kim, Citation2011) and Solanum melongena (Im et al., Citation2016). In our study on M. rubra, the ethyl acetate-soluble fraction (F2_EtOAc) showed a strong inhibition of all of the tested enzymes, with an IC50 = 8.30 µg mL−1 for 5-LOX, 3.29 µg mL−1 for COX-1 and 2.54 µg mL−1 for COX-2. The butanol (F3_BuOH) and aqueous-soluble (F4_H2O) fractions showed strong inhibitory activity of the COX-1 enzyme with an IC50 = 1.07 and 0.71 µg mL−1, respectively. Selective COX-1 inhibitors have been found to be effective in the prevention of cardiovascular diseases, as demonstrated by the application of low doses of aspirin, which is a preferential COX-1 inhibitor (Patrignani, Filabozzi, & Patrono, Citation1982). Recently, increasing evidence has confirmed that the previous classification of COX-1 as a homeostatic enzyme and COX-2 as an inflammatory enzyme was hasty and inaccurate. More recent studies have demonstrated that the prostaglandins generated by COX-2 are involved in both inflammation and homoeostatic processes with cardioprotective effects. In contrast, the thromboxane formed by COX-1 has been associated with an increased risk of coronary heart disease (Armstrong et al., Citation2011). Moreover, there is increasing evidence that COX-1 is a potent mediator of neuroinflammation (Choi, Aid, & Bosetti, Citation2009). It has been suggested that treatment with selective COX-1 inhibitors may also be effective in the prevention of neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease and traumatic brain injury (Perrone, Scilimati, Simone, & Vitale, Citation2010).
Table 1. Inhibition of inflammatory enzymes by M. rubra extracts at 50 µg mL−1 tested concentration, and by individual compounds at 50 µM tested concentration.
Table 2. Inhibition of inflammatory enzymes by fractions isolated from M. rubra leaf ethanol extract.
In our study, the ethanol extract (M_EtOH) and all biologically active fractions (F2_EtOAc, F3_BuOH and F4_H2O) were rich in phenolics (). Selected phenolic compounds that are present in M. rubra leaves and were reported previously for their biological activity (Du, Xiao, & Li, Citation2011; Shimosaki et al., Citation2011; Tong et al., Citation2009; Yang, Chang, Chen, & Wang, Citation2003) were tested for their inhibitory activity toward COX enzymes at a concentration of 50 µM to determine their contribution to the biological activity of M. rubra leaves. Only quercetin demonstrated a perceptible inhibition of COX-1 (78.48%), and epigallocatechin 3-O-gallate exhibited a moderate inhibition of both COX-1 and COX-2 (). However, when quercetin was tested by Kutil et al. (Citation2014) at lower concentrations, it appeared to be inactive. On the other hand, Kutil et al. (Citation2014) found quercetin and myricetin were 5-LOX inhibitor with an IC50 = 3.26 and 4.02 µM, respectively. Additionally, myricitrin was found to be a significant inhibitor of 5-LOX, with an IC50 = 7.8 µM (Winekenstadde et al., Citation2015). Tong et al. (Citation2009) suggested that the analgesic mechanism of myricetin might involve the inhibition of PGE2 catalyzed by COX-1. However, our results indicate that the basal inhibitory ability of M. rubra leaf extracts toward COX enzymes is probably determined by other compounds. In our study, myricetin showed only a weak inhibition of COX-1 and no inhibition of COX-2. HPLC analyses () showed a high amount of myricitrin in the butanol-soluble fraction (67.31 mg g−1). Nevertheless, the same concentration was observed in the ethylacetate-soluble fraction (66.98 mg g−1), although both fractions revealed different biological activities when tested, as described above. Furthermore, the phenolic acids contained in the fractions () were not able to inhibit the activity of the COX or LOX enzymes in our previous study (Kutil et al., Citation2014). In fact, we were not able to identify the compounds responsible for the anti-inflammatory activity. Possible candidate compounds are proanthocyanidins, which were previously identified in M. rubra leaves (Fu et al., Citation2014). Proanthocyanidin dimers and trimers, such as the gallocatechin dimer, gallocatechin–epigallocatechin dimer and gallocatechin trimer, significantly inhibited both COX isoforms (Garbacki, Angenot, Bassleer, Damas, & Tits, Citation2002; Mansoor, Matalka, Qa'dan, Awad, & Schmidt, Citation2016), and tannins were able to inhibit 5-LOX (Hartisch, Kolodziej, & von Bruchhausen, Citation1997).
Table 3. Evaluation of total phenol content (expressed as gallic acid equivalent per gram of dry weight of the extract) determined by Folin–Ciocalteu assay; and content of compounds identified using HPLC analysis, in leaf extracts and fractions from the ethanol extract of M. rubra.
GC×GC-TOFMS analysis confirmed the absence of fatty acids in the ethyl acetate and butanol fractions. The presence of fatty acids in plant extracts may misrepresent the results obtained in the COXs and 5-LOX assays that we used (Reininger & Bauer, Citation2006).
4. Conclusion
In summary, the ethyl acetate fraction of the M. rubra leaf ethanol extract showed good potential for inhibiting both COX isoforms and 5-LOX. The development of dual 5-LOX/COX inhibitors is currently a unique approach for developing compounds with enhanced anti-inflammatory effects and attenuated cardiovascular and gastrointestinal side effects. Moreover, we found a potential source of selective COX-1 inhibitors in the butanol and aqueous fractions. The discovery of new selective COX-1 inhibitors may be useful in preventing cardiovascular and neurodegenerative diseases.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Lenka Langhansova is an Associate Researcher in the Laboratory of Plant Biotechnologies (LPB) at the Institute of Experimental Botany, Czech Academy of Sciences (IEB CAS). Her recent scientific research pursues study of biosynthetic pathways of valuable secondary metabolites and their biological activity; she searches particularly for anti-oxidative, anti-inflammatory and anticancer activity. Further, she focus on micropropagation of endangered medicinal plants, production of biologically active compounds in plant tissue cultures and phytoremediation studies including plant stress responses.
Premysl Landa is an Associate Researcher in LPB IEB CAS. Within his research he focuses among all on anti-inflammatory activity of compound isolated from plants and synthetized compounds.
Zsofia Kutil was a Ph.D. student in LPB IEB CAS during the research presented in this manuscript. Her research pursued study on inhibition of COXs and 5-LOX enzymes and in-silico modeling.
Jan Tauchen was a Ph.D. student in LPB IEB CAS during the research presented in this manuscript. His research pursued study on anti-oxidative activity of flavonoids.
Petr Marsik is an Associate Researcher in LPB IEB CAS. Within his research he focuses among all on analytical techniques, mainly on HPLC and LC MS systems.
Jan Rezek is an Associate Researcher in LPB IEB CAS. Within his research he focuses among all on analytical techniques, mainly on GCXGC-TOFMS.
Ji Dong Lou is a Profesor at China Jiliang University, College of Life Sciences. He is a Chinese joint project coordinator of the research presented in this manuscript. His team focuses mainly on bioactive compounds from plants used in Chinese traditional medicine.
Zhu Li Yun is an Associate Researcher at China Jiliang University, College of Life Sciences. Within her research she focuses mainly on bioactive compounds from Chinse plants, their extraction, isolation and purification.
Tomas Vanek is a senior scientist and a head of LPB IEB CAS and he is a coordinator of the research presented in this manuscript. His research activities include isolation and identification of biologically active compounds from plants and plant tissue cultures, production and biotransformation of biologically active compounds in plant tissue cultures, phytoremediation studies, uptake and distribution of xenobiotics in plants, and plant stress response.
Additional information
Funding
References
- Armstrong, P. C., Kirkby, N. S., Zain, Z. N., Emerson, M., Mitchell, J. A., & Warner, T. D. (2011). Thrombosis is reduced by inhibition of COX-1, but unaffected by inhibition of COX-2, in an acute model of platelet activation in the mouse. Plos One, 6(5), e20062. doi:10.1371/journal.pone.0020062
- Burnett, B. P., & Levy, R. M. (2012). 5-Lipoxygenase metabolic contributions to NSAID-induced organ toxicity. Advances in Therapy, 29(2), 79–98. doi:10.1007/s12325-011-0100-7
- Chakraborti, A. K., Garg, S. K., Kumar, R., Motiwala, H. F., & Jadhavar, P. S. (2010). Progress in COX-2 inhibitors: A journey so far. Current Medicinal Chemistry, 17(15), 1563–1593. doi:10.2174/092986710790979980
- Choi, S. H., Aid, S., & Bosetti, F. (2009). The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: Implications for translational research. Trends in Pharmacological Sciences, 30(4), 174–181. doi:10.1016/j.tips.2009.01.002
- Cusan, C., Spalluto, G., Prato, M., Adams, M., Bodensieck, A., Bauer, R., … Da Ros, T. (2005). Synthesis and biological evaluation of new phenidone analogues as potential dual cyclooxygenase (COX-1 and COX-2) and human lipoxygenase (5-LOX) inhibitors. Il Farmaco (Lausanne), 60(1), 7–13. doi:10.1016/j.farmac.2004.09.002
- Davies, N. M., Smith, G. D., Windmeijer, F., & Martin, R. M. (2013). COX-2 selective nonsteroidal anti-inflammatory drugs and risk of gastrointestinal tract complications and myocardial infarction: An instrumental variable analysis. Epidemiology, 24(3), 352–362. doi:10.1097/EDE.0b013e318289e024
- Du, F.-Y., Xiao, X.-H., & Li, G.-K. (2011). Ionic liquid aqueous solvent-based microwave-assisted hydrolysis for the extraction and HPLC determination of myricetin and quercetin from Myrica rubra leaves. Biomedical Chromatography, 25(4), 472–478. doi:10.1002/bmc.1470
- Fiorucci, S., Meli, R., Bucci, M., & Cirino, G. (2001). Dual inhibitors of cyclooxygenase and 5-lipoxygenase. A new avenue in anti-inflammatory therapy? Biochemical Pharmacology, 62(11), 1433–1438. doi:10.1016/S0006-2952(01)00747-X
- Fu, Y., Qiao, L. P., Cao, Y. M., Zhou, X. Z., Liu, Y., & Ye, X. Q. (2014). Structural elucidation and antioxidant activities of proanthocyanidins from Chinese bayberry (Myrica rubra Sieb. et Zucc.) leaves. PLoS ONE, 9(5), e96162. doi:10.1371/journal.pone.0096162
- Garbacki, N., Angenot, L., Bassleer, C., Damas, J., & Tits, M. (2002). Effects of prodelphinidins isolated from Ribes nigrumon chondrocyte metabolism and COX activity. Naunyn-Schmiedeberg’s Archives of Pharmacology, 365(6), 434–441. doi:10.1007/s00210-002-0553-y
- Hartisch, C., Kolodziej, H., & von Bruchhausen, F. (1997). Dual inhibitory activities of tannins from Hamamelis virginiana and related polyphenols on 5-lipoxygenase and lyso-PAF: Acetyl-CoA acetyltransferase. Planta Medica, 63(2), 106–110. doi:10.1055/s-2006-957623
- Im, K., Lee, J. Y., Byeon, H., Hwang, K. W., Kang, W., Whang, W. K., & Min, H. (2016). In vitro antioxidative and anti-inflammatory activities of the ethanol extract of eggplant (Solanum melongena) stalks in macrophage RAW 264.7 cells. Food and Agricultural Immunology, 27, 758–771. doi:10.1080/09540105.2016.1150427
- Kang, W., Li, Y., Xu, Y., Jiang, W., & Tao, Y. (2012). Characterization of aroma compounds in Chinese bayberry (Myrica rubra Sieb. et Zucc.) by gas chromatography mass spectrometry (GC-MS) and olfactometry (GC-O). Journal of Food Science, 77(10), C1030–C1035. doi:10.1111/j.1750-3841.2012.02747.x
- Kim, H. H., Kim, D. H., Kim, M. H., Oh, M. H., Kim, S. R., Park, K. J., & Lee, M. W. (2013). Flavonoid constituents in the leaves of Myrica rubra sieb. et zucc. with anti-inflammatory activity. Archives of Pharmacal Research, 36(12), 1533–1540. doi:10.1007/s12272-013-0147-x
- Kim, H. H., Oh, M. H., Park, K. J., Heo, J. H., & Lee, M. W. (2014). Anti-inflammatory activity of sulfate-containing phenolic compounds isolated from the leaves of Myrica rubra. Fitoterapia, 92, 188–193. doi:10.1016/j.fitote.2013.10.007
- Kutil, Z., Kvasnicova, M., Temml, V., Schuster, D., Vanek, T., Fernandez, E., … Landa, P. (2015). The influence of the quinone antioxidants tert-butylhydroquinone and 2,5-di-tert-butylhydroquinone on the arachidonic acid metabolism in vitro. Food and Agricultural Immunology, 26, 504–511. doi:10.1080/09540105.2014.988126
- Kutil, Z., Temml, V., Maghradze, D., Pribylova, M., Dvorakova, M., Schuster, D., … Landa, P. (2014). Impact of wines and wine constituents on cyclooxygenase-1, cyclooxygenase-2, and 5-lipoxygenase catalytic activity. Mediators of Inflammation, 2014, 1–8. doi:10.1155/2014/178931
- Lee, J. H., Kang, B. S., Hwang, K. H., & Kim, G. H. (2011). Evaluation for anti-inflammatory effects of Siegesbeckia glabrescens extract in vitro. Food and Agricultural Immunology, 22, 145–160. doi:10.1080/09540105.2010.549210
- Leval, X. D., Julemont, F., Delarge, J., Pirotte, B., & Dogne, J.-M. (2002). New trends in dual 5-LOX/COX inhibition. Current Medicinal Chemistry, 9(9), 941–962. Retrieved from WOS:000175310600004 doi: 10.2174/0929867024606713
- Mansoor, K. A., Matalka, K. Z., Qa'dan, F. S., Awad, R., & Schmidt, M. (2016). Two new proanthocyanidin trimers isolated from Cistus incanus L. demonstrate potent anti-inflammatory activity and selectivity to cyclooxygenase isoenzymes inhibition. Natural Product Research, 30(17), 1919–1926. doi:10.1080/14786419.2015.1089242
- Masuda, T., Someya, T., & Fujimoto, A. (2010). Phenolic inhibitors of chemical and enzymatic oxidation in the leaves of Myrica rubra. Bioscience, Biotechnology, and Biochemistry, 74(1), 212–215. doi:10.1271/bbb.90697
- Nonaka, G. I., Muta, M., & Nishioka, I. (1983). Tannins and related-compounds. 6. Myricatin, a galloyl flavanonol sulfate and prodelphinidin gallates from Myrica rubra. Phytochemistry, 22(1), 237–241. doi:10.1016/S0031-9422(00)80097-7
- Patrignani, P., Filabozzi, P., & Patrono, C. (1982). Selective cumulative inhibition of platelet thromboxane production by Low-dose aspirin in healthy subjects. Journal of Clinical Investigation, 69(6), 1366–1372. doi:10.1172/JCI110576
- Pergola, C., & Werz, O. (2010). 5-Lipoxygenase inhibitors: A review of recent developments and patents. Expert Opinion on Therapeutic Patents, 20(3), 355–375. doi:10.1517/13543771003602012
- Perrone, M. G., Scilimati, A., Simone, L., & Vitale, P. (2010). Selective COX-1 inhibition: A therapeutic target to be reconsidered. Current Medicinal Chemistry, 17(32), 3769–3805. Retrieved from WOS:000284715400001 doi: 10.2174/092986710793205408
- Reininger, E. A., & Bauer, R. (2006). Prostaglandin-H-synthase (PGHS)-1 and -2 microtiter assays for the testing of herbal drugs and in vitro inhibition of PGHS-isoenzyms by polyunsaturated fatty acids from Platycodi radix. Phytomedicine, 13(3), 164–169. doi:10.1016/j.phymed.2005.03.006
- Ren, H., Lin, D., Mou, Z., & Dong, P. (2013). The adverse effect of selective cyclooxygenase-2 inhibitor on random skin flap survival in rats. Plos One, 8(12), e82802. doi:10.1371/journal.pone.0082802
- Shimosaki, S., Tsurunaga, Y., Itamura, H., & Nakamura, M. (2011). Anti-allergic effect of the flavonoid myricitrin from Myrica rubra leaf extracts in vitro and in vivo. Natural Product Research, 25(4), 374–380. doi:10.1080/14786411003774320
- Singleton, V. L., Orthofer, R., & Lamuela-Raventos, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Oxidants and Antioxidants, Pt A, 299, 152–178. Retrieved from WOS:000079431900014 doi: 10.1016/S0076-6879(99)99017-1
- Sostres, C., Gargallo, C. J., & Lanas, A. (2013). Nonsteroidal anti-inflammatory drugs and upper and lower gastrointestinal mucosal damage. Arthritis Research & Therapy, 15(Suppl. 3), S3. doi:10.1186/ar4175
- Sun, C., Huang, H., Xu, C., Li, X., & Chen, K. (2013). Biological activities of extracts from Chinese bayberry (Myrica rubra Sieb. et Zucc.): A review. Plant Foods for Human Nutrition, 68(2), 97–106. doi:10.1007/s11130-013-0349-x
- Tauchen, J., Marsik, P., Kvasnicova, M., Maghradze, D., Kokoska, L., Vanek, T., & Landa, P. (2015). In vitro antioxidant activity and phenolic composition of Georgian, Central and West European wines. Journal of Food Composition and Analysis, 41, 113–121. doi:10.1016/j.jfca.2014.12.029
- Tong, Y., Zhou, X.-M., Wang, S.-J., Yang, Y., & Cao, Y.-L. (2009). Analgesic activity of myricetin isolated from Myrica rubra Sieb. et Zucc. leaves. Archives of Pharmacal Research, 32(4), 527–533. doi:10.1007/s12272-009-1408-6
- Wang, S.-J., Tong, Y., Lu, S., Yang, R., Liao, X., Xu, Y.-F., & Li, X. (2010). Anti-inflammatory activity of myricetin isolated from Myrica rubra Sieb. et Zucc. leaves. Planta Medica, 76(14), 1492–1496. doi:10.1055/s-0030-1249780
- Winekenstadde, D., Angelis, A., Waltenberger, B., Schwaiger, S., Tchoumtchoua, J., Konig, S., … Stuppner, H. (2015). Phytochemical profile of the aerial parts of sedum sediforme and anti-inflammatory activity of myricitrin. Natural Product Communications, 10(1), 83–88. Retrieved from WOS:000348825700020.
- Yang, L. L., Chang, C. C., Chen, L. G., & Wang, C. C. (2003). Antitumor principle constituents of Myrica rubra var. acuminata. Journal of Agricultural and Food Chemistry, 51(10), 2974–2979. doi:10.1021/jf026188i