ABSTRACT
Polysaccharides isolated from mushrooms have recently attracted attention due to its potential immune-stimulatory activity. The aim of this study was to validate the in vitro immune-stimulatory activities of various mushroom extracts. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay revealed that Pleurotus eryngii, with the highest β-glucan (18.94%) content, displayed highest viability on macrophage cells of 62.59% at 200 μg/ml concentration. Pleurotus cystidiosus, with 18.16% β-glucan, content showed highest activation of NF-kB (0.7 µg/ml) at a concentration of 100 µg/ml. Termitomyces heimii, with the lowest percentage of β-glucan (0.51%), exhibited highest phagocytosis index of 9.38 at 12.5 µg/ml. The brown strain of Agaricus bisporus with 1.54% of β-glucan stimulates the highest nitric oxide (NO) production of 12.39 µM nitrite oxide at 100 µg/ml. This study revealed that hot water extracts of mushrooms have different β-glucan contents and produced varying immune-stimulatory activities. Among these, Pleurotus spp. demonstrated the highest percentage of β-glucan content and viability of macrophage cells. Pleurotus spp. are deemed immune-stimulatory by increasing phagocytic activity, NO production, and triggered the activation of NF-kB.
Introduction
Mushrooms are foods with well-known medicinal properties due to their antioxidants and immunomodulatory attributes (Abdullah, Ismail, Aminudin, Shuib, & Lau, Citation2012; Raaman et al., Citation2011). Nowadays, mushrooms are often consumed more for their nutritional and medicinal properties than for their texture and flavour (Abidin, Abdullah, & Abidin, Citation2016b). There is a huge attraction on mushrooms for their immunoceutical properties not only for crude and semi-crude extracts but also for their complex carbohydrate-biological response modifiers (Morris et al., Citation2007).
The human immune system combats a range of invasive microorganisms and cancers through macrophages, the body’s first line of defence. Macrophages are a type of differentiated tissue cell that originate as blood monocytes. The cells have several functions, such as the removal of cell debris, killing pathogenic microorganisms, and processing antigen presentation to lymphocytes (Cutolo, Citation1999; Gordon, Citation2002). Activation of macrophages is a key event for effective innate and adaptive immunity. NF-κB, one of the most important transcription factors in macrophages, regulates the transcription of a large number of genes in immune and inflammatory response. The stimulation of a wide variety of cell-surface receptors by mushroom polysaccharides has been demonstrated to lead directly to NF-κB activation and fairly rapid changes in gene expression (Chen, Lu, Qu, Wang, & Zhang, Citation2010). Nitrite oxide (NO) production is a major mediator of macrophages cells which is activated by NF-κB inflammatory signalling mechanism (Chan et al., Citation2015). The NO production may destroy bacterial infections and tumour cells due to its immune response capability (Iuvone, Esposito, Capasso, & Izzo, Citation2003). Macrophage phagocytosis activity is yet another important immune defence in the body against microbial infections. It is also acted as a clearance mechanism to engulf any apoptotic or necrosis cells (Sauter et al., Citation2000).
Edible mushrooms are a potential source of dietary fibre such as chitin and other hemicelluloses, mannans, and polysaccharides namely β-glucan. Beta-glucans are the most abundant forms of polysaccharides and are a major component of mushrooms which display immune-stimulatory potency. Muller et al. observed that β-glucan may enhance immune system cells (e.g. macrophages) by binding to particular cell receptors and directly activating the cells (Mueller et al., Citation2000). Wasser added that instead of killing cancer cells directly, β-glucan actually activates cells of the immune system which then kill the cancer cell (Wasser, Citation2002). Some β-glucans extracted from mushrooms have been shown to have immune-stimulatory properties. Lentinan from Lentinula edodes, grifolan from Grifola frondosa, and schizophyllan from Schizophyllum commune have all been identified as immunostimulating components upregulating the functions of macrophages and natural killer cells (Daba & Ezeronye, Citation2003; Hong, Weiyu, Qin, Shuzhen, & Lebin, Citation2013; Jong, Birmingham, & Pai, Citation1991). These compounds, homo- and hetero-glucans with β(1->3), β(1->4), and β(1->6) glucosidic linkages, are present in the cell walls of mushrooms and can be extracted by boiling in water (Daba & Ezeronye, Citation2003; Jong et al., Citation1991). Mushrooms are typically not eaten raw. They are usually boiled at high temperatures in water. Hence, information obtained from analysis of hot water extraction that mimics common cooking conditions would be useful in evaluating the immune-stimulatory potential of mushrooms upon consumption.
The aim of this research is to determine the level of β-glucans in hot water extracts from different edible mushrooms and to evaluate the potential of these extracts to activate macrophage-mediated innate immune responses as such as NO production, phagocytic ability, and activation of NF-κB.
Materials and methods
Mushroom collection
The 16 species of culinary medicinal mushrooms used in this study () were obtained from mushroom farms and supermarkets in Selangor and Kuala Lumpur, Malaysia. These include Agaricus bisporus (brown strain); A. bisporus (white strain); Agrocybe sp.; Auricularia auricula-judae; Flammulina velutipes; Hericeum erinaceus; L. edodes; Pleurotus giganteus; Pleurotus citrinopileatus; Pleurotus cystidiosus; Pleurotus eryngii; Pleurotus flabellatus; Pleurotus florida; Pleurotus pulmonarius; S. commune; and Termitomyces heimii. The samples were identified and authenticated by experts at the Mushroom Research Centre, University of Malaya.
Table 1. Composition of β-glucan in hot water extracts of mushrooms.
Preparation of mushroom hot water extracts
All mushrooms fruiting bodies were washed, dried out, cut into smaller pieces, and boiled in distilled water at a ratio of 1:10 (w/v) at 100°C for 30 min. Boiled mushrooms were cooled to room temperature and filtered using Whatman No. 1 filter paper and lyophilized. Dried hot water extract at stock concentration of 1 mg/ml was then sterilized using a Millipore filter (10 um) into a sterile vial and kept as a stock solution at 4°C. For the assay, concentrations were prepared by diluting the stock at 12.5, 25, 50, and 100 µg/ml under sterile conditions.
Preparation of macrophage (RAW264.7) cells
The RAW264.7 cells used in this study were purchased from American Type Cell Collection (Catalog number TIB-71™) and cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 10% foetal bovine serum. Cells were subcultured every 2–3 days and incubated at 37 ± 2°C in a 5% CO2 humidified incubator. The cells for MTT, phagocytosis, and NF-κB assays were grown for 3 days until reaching 60–70% confluence.
Estimation of β-glucan level
The measurement of β-glucan level was performed using a Mushroom and Yeast Beta-Glucan (YBG) Assay Kit (Megazyme International). The main principle of this study is based on the measurement of β-d-glucan, a polysaccharide yielding d-glucose only via acid hydrolysis with a mixture of highly purified exo-1,3-β-glucanase and β-glucosidase. Absorbance was read in triplicate at 510 nm via a UV–Vis spectrophotometer (Shimadzu, Japan). The results were expressed as a percentage of β-glucan in the extract (w/w). β-glucan and Lentinan were included in this assay as a comparison to mushroom extracts.
Cells viability assay
The assay was performed following Abidin et al. method with some modification (Abidin, Abdullah, & Abidin, Citation2016a). A 96-well plate was seeded with 1.5 × 104 of RAW264.7 cells per well and incubated for overnight to allow the cells to settle down. The cells were then treated with various concentrations of extracts and incubated for 24 h. β-Glucan and Lentinan were included as comparison and the untreated cells served as a negative control. The plates were incubated at 37°C in 5% CO2 humidified incubator. The viability study for RAW264.7 cells was carried out after treatment was done via the 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. The measurement of cell viability by this assay is based on the capability of living cells to reduce MTT tetrazolium salt into formazan crystals. The test was performed in triplicate. After 48 h incubation, 20 µl MTT solution was added to each well, then incubated for 4 h at room temperature. The medium from the wells was then discarded, followed by the addition of 100 µl of Dimethyl sulfoxide. The plate was incubated for another 20 min to dissolve the purple formazon product with live cells. The absorbance was read at 570 nm using an ELISA micro-plate reader. The percentage of cell viability was calculated based on the following equation: V% = [(A–B)/(A)*100], where A indicates the absorbance value of untreated and B indicates the absorbance value of treated cells with extracts.
Phagocytosis assay
The cells prepared as described above were treated with extracts and incubated at 37°C in a 5% CO2 humidified incubator. The test was done in triplicate. After 48 h incubation, 100 µl of 0.075% neutral red solution was added into each well, then incubated for another 30 min at room temperature. The supernatant was discarded and the plate rinsed with 1× potassium buffer sulphate to remove excess dye. Then, the cell lysate (50% ethanol and 1% acetic acid) was added to lyse the cells overnight. The absorbance was read at 540 nm using an ELISA micro-plate reader. Index of the phagocytic activity was calculated using phagocytic activity index = Abssample/Abscontrol.
NF-κB level
The treated cells at various concentrations of extracts were incubated at 37 ± 2°C in a 5% CO2 humidified incubator for 24 h incubation. Assays were performed based on the NF-κB/p65 Activ ELISA Kit (IMGENEX Corporation). The basis of this study is the measurement of free NF-κB in the nucleus of cells, which can be seen by the pink colour development. The absorbance was read at 405 nm via an ELISA micro-plate reader in triplicate. A standard curve was plotted with the absorbance value at 405 nm against NF-κB concentration (µg/ml). The calculation for concentration of NF-kB level (µg/ml) in the cell = A/0.0935, where A indicated the absorbance value of the cell at 405 nm.
NO assay
In this study, the NO standard curve was prepared by diluting 0.1 M sodium nitrate 1000 fold. To set up a standard curve, 50 µl of the medium was added into wells B through H and 100 µl of diluted nitrite solution was pipetted into well A. The test was performed in triplicate. Then, 50 µl of NO solution from well A was dispersed to well B and serial dilution was continued until well G. After mixing, 50 µl of solution from well G was discarded and well H was left empty. The standard curve for nitric oxide was generated at 100, 50, 25, 12.5, 6.25, 3.13, and 1.56 µM. The treated cells with various concentrations of extracts were incubated at 37 ± 2°C in 5% CO2 humidified incubator for 24 h incubation. Fifty microlitre of the extracts was transferred into 96-well plates which already contained 50 µl of the medium. Finally, 50 µl of the Griess reagent was added to each well and incubated for 10 min under dark conditions at room temperature. Positive results appeared immediately as a purple or magenta in colour. Absorbance was measured at 540 nm.
Statistical analysis
Results were given as mean ± standard deviation (SD) in triplicates with their 95% confidence intervals. All analysis was conducted using one-way analysis of variance in Statgraphics Plus for Windows 3.0.
Results and discussion
Estimation of β-glucan level in hot water extract of mushrooms
β-d-glucan are linear polymers of glucose with other d-monosaccharides and have been reported to have higher anticancer activity compared to α-d-glucans (Carbonero et al., Citation2006; Mizuno, Citation1999). Mizuno et al. also proved that glucans with higher molecular weight are more effective than those of low molecular weight against tumours (Mizuno, Citation1999). These differences are due to the ability of the polysaccharide molecule to solubilize in water, size of the molecules, branching rate, and form (Wasser, Citation2002). β-d-glucan has also shown the ability to activate and reinforce the host immune system by inhibiting the growth of cancer cells (Mizuno, Citation1999). In this study, hot water extracts of edible-medicinal mushrooms were investigated to determine their β-glucan levels. From the results, commercial YBG showed the highest percentage of β-glucan (78.09%) compared to commercial Lentinan (29.52%), which is also known to have an anticancer effect on animal and human carcinomas (Mizuno, Citation1999). depicts that among the mushroom species, β-glucan levels of Pleurotus species are higher than other mushroom species. P. eryngii and P. cystidiosus exhibited the highest level of β-glucan with 18.19% followed by P. florida (18.16%). Carbonero et al. (Citation2006) stated that the anti-tumour activity of P. eryngii may be in part due to β-glucan. Mushroom extracts like T. heimii, A. bisporus (white), and A. bisporus (brown) had very low β-glucan content at 0.51%, 1.41%, and 1.54%, respectively.
Effect of the extracts on macrophages cells (RAW264.7) viability
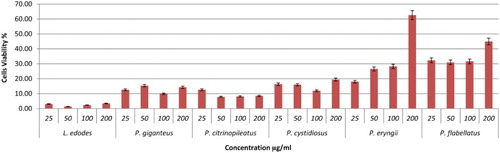
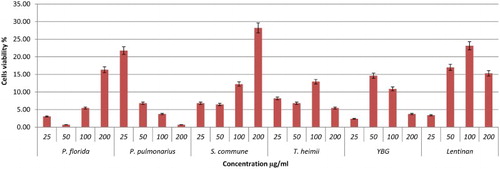
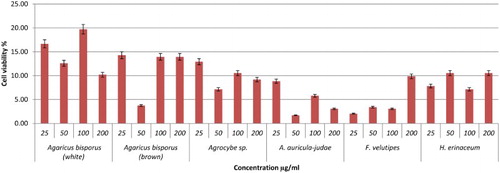
The viability of the cells treated with mushroom hot water extracts was investigated using MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. The activity involves reducing water-soluble tetrazolium salt to formazon, which can be seen by the colour change from yellow to purple. Lentinan and YBG served as positive control. Referring to , P. eryngii exhibited highest cell viability of 62.59% at 200 µg/ml after 48 h incubation. Other mushrooms showed low cell viability, with viability percentages below 40% except for P. flabellatus, with 44.90% at 200 µg/ml (–). The positive controls Lentinan and YBG exhibited lower cell viability probably due to the high β-glucan that could kill the cells. In another report, in accordance with our study, polysaccharides extracted from Cordyceps militaris demonstrated a decrease in macrophages cell viability percentage with concentration ranging from 10 to 1000 µg/ml (Lee et al., Citation2015). However, hot water extracts of P. eryngii and P. flabellatus are potential immune-stimulatory agents since they exhibited high viability towards RAW264.7 macrophages cells.
Figure 1. Viability effects of different concentrations of mushroom hot water extracts (i.e., Agaricus bisporus (white and brown), Agrocybe sp., A. auricula-judae, F.velutipes, H.erinaceum) on RAW264.7 macrophage cells for 48 h incubation. Values are expressed in triplicate as mean ± SD.
Phagocytosis activity
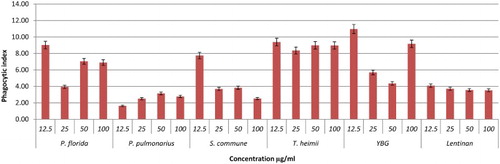
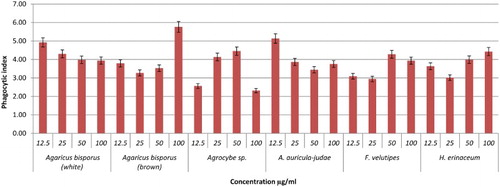
As shown in –, after 48 h of incubation YBG (12.5 and 100 µg/ml); T. heimii (all concentrations); P. florida (12.5, 50, and 100 µg/ml); and S. commune (12.5 µg/ml) extracts significantly stimulated phagocytosis activity of RAW264.7 cells after 48 h incubation. Overall, the phagocytosis activity from treated macrophage cells was shown to be higher than untreated cells, however not dose dependent. According to the results, T. heimii at 12.5 µg/ml concentration showed the highest phagocytosis index of 9.38 (–), however, these values are lower than YBG (10.97, 12.5 µg/ml). Among Pleurotus species, P. florida exhibited the highest phagocytosis activity index at 9.02 at 12.5 µg/ml after 48 h incubation (). This might be due to the high level of β-glucan in P. florida hot water extract. As proven by Rout, Mondal, Chakraborty, Pramanik, and Islam (Citation2005), it was found that β-glucan compound isolated from P. florida was able to activate phagocytic activity of macrophage cells. In addition, glucans isolated from P. florida were able to activate the phagocytosis of fish leukocytes (Kamilya, Ghosh, Bandyopadhyay, Mal, & Maiti, Citation2006). However, the high phagocytosis activity of T. heimii even when the level of β-glucan was low may be due to its structure and size. Mushrooms had a wide variety of β-glucan structure and size. Mizuno et al. also proved that glucans with higher molecular weight are more effective than those of low molecular weight against tumours (Mizuno, Citation1999). These differences are due to the ability of the polysaccharide molecule to solubilize in water, size of the molecules, branching rate and form (Wasser, Citation2002).
Figure 4. Effects of different concentrations of mushroom hot water extracts (i.e., Agaricus bisporus (white and brown), Agrocybe sp., A. auricula-judae, F.velutipes, H.erinaceum) on phagocytosis of RAW264.7 macrophages cells for 48 h incubation. Values are expressed in triplicate as mean ± SD.
NF-κB activation
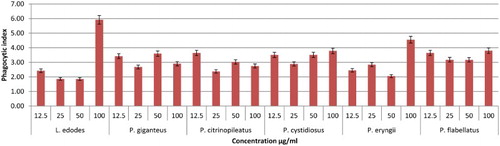
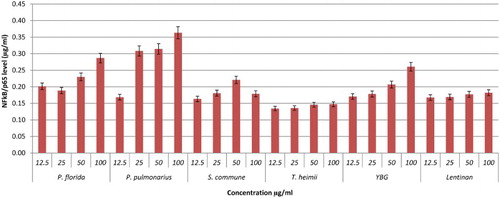
NF-κB is a complex protein that controls the transcription of DNA. NF-κB is an important protein in modulating the expression of immunoregulatory genes that are relevant in critical illness, inflammatory diseases, apoptosis, and cancer (Neurath, Becker, & Barbulescu, Citation1998). Thus, the activation of NF-kB pathway can be a very important target for therapeutic intervention and the design of new treatments for chronic inflammation diseases (Lubberts, Joosten, Helsen, & van den Berg, Citation1998). In this study, the activation of NF-κB by hot water extracts of mushrooms and YBG towards RAW264.7 cells lines were investigated via an IMK-503;NF-κB/p65 Activ ELISA™ Kit purchased from IMGENEX Corporation. The results were compared to the NF-κB/p65 standard reference curve. Based on these results, most of the hot water extracts showed dose-dependent activation of Nf-kB with P. cystidiosus extract exhibiting the highest activation of 0.71 µg/ml of NF-κB/p65 at 100 µg/ml concentration (–). It is interesting to note that the activation of NF-kB by P. cystidiosus was better than YBG and also Lentinan at all concentrations tested.
Figure 7. Activation of NF-kB from RAW264.7 macrophage cells by different concentrations of mushroom hot water extracts . The results were analysed based on the standard reference graph. Values are expressed in triplicate as mean ± SD.
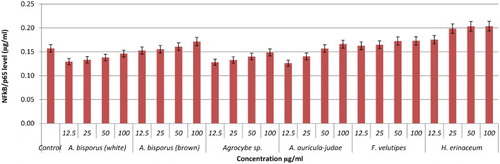
Figure 8. Activation of NF-kB from RAW264.7 macrophage cells by different concentrations of mushroom hot water extracts (i.e., L. edodes, P. giganteus, P. citrinopileatus, P. cystidiosus, P. eryngii, P. flabellatus). The results were analysed based on the standard reference graph. Values are expressed in triplicate as mean ± SD.
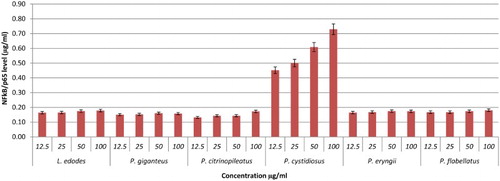
Figure 9. Activation of NF-kB from RAW264.7 macrophage cells by different concentrations of YBG, Lentinan and mushroom hot water extracts (i.e., P. florida, P. pulmonarius, S. commune, T. heimii). The results were analysed based on the standard reference graph. Values are expressed in triplicate as mean ± SD.
Effects of hot water extracts on NO production
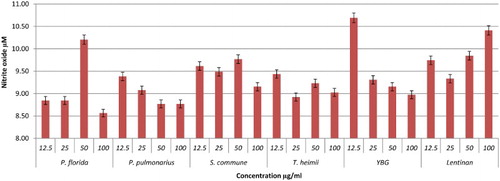
Nitric oxide is secreted in huge amount and is believed to act as a major mediator of macrophages cells, which can destroy any infections and ameliorate inflammation (Ghazanfari, Yaraee, Farahnejad, Hakimzadeh, & Danialy, Citation2009). In this study, the effect of hot water extracts of mushrooms on the NO production of RAW264.7 cells was determined using a Griess reagent assay. The results showed that most of the mushroom extracts up-regulated NO levels significantly. Brown strain of A. bisporus is the only mushroom shown to be dose dependent in triggering NO production, maximizing at 100 µg/ml with 12.39 µM (). This is followed by A. auricular judae (50 µg/ml with 11.46 µM) and white strain A. bisporus (12.5 µg/ml with 11.44 µM; 25 µg/ml with 11.03 µM) (). Among the extracts in the Pleurotus spp., P. florida possessed the highest level of NO production with 10.21 µM at 50 µg/ml concentration (–). Volman et al. (Hong et al., Citation2013) found that A. bisporus was better able to stimulate NO production by macrophage cells than Coprinus comatus and Ganoderma lucidum. They thus concluded that A. bisporus is a good candidate for stimulating immune response. According to a research done by Ghazanfari et al. (Abidin et al., Citation2016a), P. florida was found to be able to increase the NO production of macrophage cells significantly. A report from Lee and his group displayed that a polysaccharide of 1,6-branched-glucogalactomannan with 36 kDa molecular weight was able to stimulate high NO production from macrophages (Lee et al., Citation2010). In a recent work on Chinese medicinal mushroom Coriolus versicolor it was proved that it has β-glucan capability in giving protection against bacterial infection by stimulating high release of NO production (Shi et al., Citation2016).
Figure 10. Activation of NO from RAW264.7 macrophage cells by different concentrations of mushroom hot water extracts (i.e., Agaricus bisporus (white and brown), Agrocybe sp., A. auricula-judae, F.velutipes, H.erinaceum). The results were analysed based on the standard reference graph. Values are expressed in triplicate as mean ± SD.
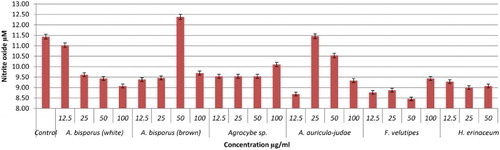
Figure 11. Activation of NO from RAW264.7 macrophage cells by different concentrations of mushroom hot water extracts (i.e., L. edodes, P. giganteus, P. citrinopileatus, P. cystidiosus, P. eryngii, P. flabellatus). The results were analysed based on the standard reference graph. Values are expressed in triplicate as mean ± SD.
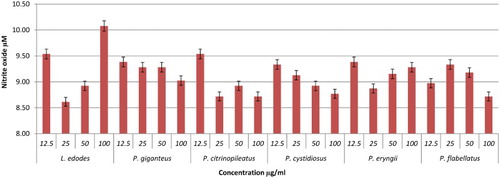
Figure 12. Activation of NO from RAW264.7 macrophage cells by different concentrations of YBG, Lentinan and mushroom hot water extracts (i.e., P. florida, P. pulmonarius, S. commune, T. heimii). The results were analysed based on the standard reference graph. Values are expressed in triplicate as mean ± SD.
Conclusion
This study has demonstrated that hot water extracts of different mushrooms were found to exhibit different immune-stimulatory activities. This work also revealed that P. eryngii (100 µg/ml), P. cystidiosus (100 µg/ml), T. heimii (12.5 µg/ml), and brown strain of A. bisporus (100 µg/ml) were the most potent extracts in terms of cell viability, activation of NF-kB, phagocytosis, and NO production of macrophage cells (RAW264.7), respectively. In addition, compared to other species of mushroom, Pleurotus demonstrated a high viability of macrophage cells, increase phagocytic activity and the production of nitric oxide (NO), and trigger the activation of NF-κB. This work reveals that purification and characterization of β-glucans of T. heimii, A. bisporus and the Pleurotus spp. is worth pursuing as potential immune-stimulatory agents.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Noorlidah Abdullah is a Professor at the Institute of Biological Sciences, University of Malaya (Malaysia). Her research interests are Fungal Taxonomy and Biotechnology, Medicinal Mushrooms and Food Mycology.
Rosnina Abdulgani obtained her Doctors degree in Mushroom Biology from University of Malaya (Malaysia). She currently works as a lecturer at Department of Agroecotechnology of Agriculture Faculty, University of Malikussaleh, Lhokseumawe, Aceh, Indonesia.
Siti Marjiana Ismail obtained her Masters in Biotechnology degree from University of Malaya (Malaysia). She currently works as a Research Officer at Mushroom Research Centre, University of Malaya. She is currently a PhD candidate in the Department of Physiology, Faculty of Medicine at University of Malaya, in the field of biomedical sciences.
Mohamad Hamdi Zainal Abidin has received his Bachelor and Master degrees in Biotechnology from Institute of Science Biology, University of Malaya. He is currently a PhD candidate under the Department of Chemical Engineering, Faculty of Engineering at University of Malaya. His main interest is in the application of ionic liquids in drug delivery design.
Additional information
Funding
References
- Abdullah, N., Ismail, S. M., Aminudin, N., Shuib, A. S., & Lau, B. F. (2012). Evaluation of selected culinary-medicinal mushrooms for antioxidant and ACE inhibitory activities. Evidence-Based Complementary and Alternative Medicine, 13, 1–12. doi:10.1155/2012/464238
- Abidin, M. H., Abdullah, N., & Abidin, N. Z. (2016a). Protective effect of antioxidant extracts from grey oyster mushroom, Pleurotus pulmonarius (Agaricomycetes), against human low-density lipoprotein oxidation and aortic endothelial cell damage. International Journal of Medicinal Mushrooms, 18(2), 109–121. doi:10.1615/IntJMedMushrooms.v18.i2.20 doi: 10.1615/IntJMedMushrooms.v18.i2.20
- Abidin, M. H. Z., Abdullah, N., & Abidin, N. Z. (2016b). Therapeutic properties of Pleurotus species (Oyster Mushrooms) for atherosclerosis: A review. International Journal of Food Properties, 1–11. doi:10.1080/10942912.2016.1210162
- Carbonero, E. R., Gracher, A. H. P., Smiderle, F. R., Rosado, F. R., Sassaki, G. L., Gorin, P. A., & Iacomini, M. (2006). A β-glucan from the fruit bodies of edible mushrooms Pleurotus eryngii and Pleurotus ostreatoroseus. Carbohydrate Polymers, 66(2), 252–257. doi: 10.1016/j.carbpol.2006.03.009
- Chan, P.-M., Tan, Y.-S., Chua, K.-H., Sabaratnam, V.Kuppusamy, U. R., & Palaniyandi, S. (2015). Attenuation of inflammatory mediators (TNF-α and nitric oxide) and up-regulation of IL-10 by wild and domesticated basidiocarps of Amauroderma rugosum (Blume & T. Nees) Torrend in LPS-Stimulated RAW264. 7 Cells. PLoS One, 10(10), e0139593. doi: 10.1371/journal.pone.0139593
- Chen, H. X., Lu, X. M., Qu, Z. S., Wang, Z. S., & Zhang, L. P. (2010). Glycosidase inhibitory activity and antioxidant properties of a polysaccharide from the mushroom inonotus obliquus. Journal of Food Biochemistry, 34, 178–191. doi:10.1111/j.1745-4514.2009.00322.x
- Cutolo, M. (1999). Macrophages as effectors of the immunoendocrinologic interactions in autoimmune rheumatic diseases. Neuroendocrine Immune Basis of the Rheumatic Diseases, 876, 32–42. doi:10.1111/j.1749-6632.1999.tb07620.x
- Daba, A., & Ezeronye, O. (2003). Anti-cancer effect of polysaccharides isolated from higher basidiomycetes mushrooms. African Journal of Biotechnology, 2(12), 672–678. doi: 10.5897/AJB2003.000-1123
- Ghazanfari, T., Yaraee, R., Farahnejad, Z., Hakimzadeh, H., & Danialy, F. (2009). In vitro effect of Pleurotus florida on macrophage cell viability and nitric oxide production. Food and Agricultural Immunology, 20(2), 105–110. doi:10.1080/09540100902838198
- Gordon, S. (2002). Pattern recognition receptors: Doubling up for the innate immune response. Cell, 111(7), 927–930. doi:10.1016/S0092-8674(02)01201-1
- Hong, L., Weiyu, W., Qin, W., Shuzhen, G., & Lebin, W. (2013). Antioxidant and immunomodulatory effects of a α-glucan from fruit body of maitake (Grifola frondosa). Food and Agricultural Immunology, 24(4), 409–418. doi:10.1080/09540105.2012.704901
- Iuvone, T., Esposito, G., Capasso, F., & Izzo, A. A. (2003). Induction of nitric oxide synthase expression by Withania somnifera in macrophages. Life Sciences, 72(14), 1617–1625. doi: 10.1016/S0024-3205(02)02472-4
- Jong, S., Birmingham, J., & Pai, S. (1991). Immunomodulatory substances of fungal origin. Eos-Rivista di immunologia ed immunofarmacologia, 11(3), 115–122.
- Kamilya, D., Ghosh, D., Bandyopadhyay, S., Mal, B. C., & Maiti, T. K. (2006). In vitro effects of bovine lactoferrin, mushroom glucan and Abrus agglutinin on Indian major carp, catla (Catla catla) head kidney leukocytes. Aquaculture, 253(1–4), 130–139. doi:10.1016/j.aquaculture.2005.07.038
- Lee, J. S., Kwon, D. S., Lee, K. R., Park, J. M., Ha, S.-J., & Hong, E. K. (2015). Mechanism of macrophage activation induced by polysaccharide from Cordyceps militaris culture broth. Carbohydrate Polymers, 120, 29–37. doi:10.1016/j.carbpol.2014.11.059
- Lee, J. S., Kwon, J. S., Won, D. P., Lee, K. E., Shin, W. C., & Hong, E. K. (2010). Study on macrophage activation and structural characteristics of purified polysaccharide from the liquid culture broth of Cordyceps militaris. Carbohydrate Polymers, 82(3), 982–988. doi: 10.1016/j.carbpol.2010.06.025
- Lubberts, E., Joosten, L. A., Helsen, M. M., & van den Berg, W. B. (1998). Regulatory role of interleukin 10 in joint inflammation and cartilage destruction in murine streptococcal cell wall (SCW) arthritis. More therapeutic benefit with IL-4/IL-10 combination therapy than with IL-10 treatment alone. Cytokine, 10(5), 361–369. doi: 10.1006/cyto.1997.0298
- Mizuno, T. (1999). The extraction and development of antitumor-active polysaccharides from medicinal mushrooms in Japan (review). International Journal of Medicinal Mushrooms, 1(1), 9–29. doi: 10.1615/IntJMedMushrooms.v1.i1.20
- Morris, H. J., Lebeque, Y., Fontaine, R., Bermúdez, R. C., Llauradó, G., & Marcos, J. (2007). A note on the in vitro macrophage-stimulating activity of water-soluble extracts from mycelium of Pleurotus spp. Food and Agricultural Immunology, 18(1), 31–37. doi:10.1080/09540100701248631
- Mueller, A., Raptis, J., Rice, P. J., Kalbfleisch, J. H., Stout, R. D., Ensley, H. E., … Williams, D. L. (2000). The influence of glucan polymer structure and solution conformation on binding to (1→ 3)-β-D-glucan receptors in a human monocyte-like cell line. Glycobiology 10(4), 339–346. doi: 10.1093/glycob/10.4.339
- Neurath, M., Becker, C., & Barbulescu, K. (1998). Role of NF-kappa B in immune and inflammatory responses in the gut. Gut, 43(6), 856–860. doi: 10.1136/gut.43.6.856
- Raaman, N., Jegadeesh, R., Hariprasath, L., Kumaresan, K., Srikumar, R., & Ayyappan, S. R. (2011). Effect of Pleurotus djamor var. roseus, an edible mushroom on neutrophil functions. Food and Agricultural Immunology, 22(3), 229–234. doi:10.1080/09540105.2011.556712
- Rout, D., Mondal, S., Chakraborty, I., Pramanik, M., & Islam, S. S. (2005). Chemical analysis of a new (1 -> 3)-, (1 -> 6)-branched glucan from an edible mushroom, Pleurotus florida. Carbohydrate Research, 340(16), 2533–2539. doi:10.1016/j.carres.2005.08.006
- Sauter, B., Albert, M. L., Francisco, L., Larsson, M., Somersan, S., & Bhardwaj, N. (2000). Consequences of cell death: Exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. Journal of Experimental Medicine, 191(3), 423–434. doi: 10.1084/jem.191.3.423
- Shi, S.-H., Yang, W.-T., Huang, K.-Y., Jiang, Y.-L., Yang, G.-L., Wang, C.-F., & Li, Y. (2016). β-glucans from Coriolus versicolor protect mice against S. typhimurium challenge by activation of macrophages. International Journal of Biological Macromolecules, 86, 352–361. doi:10.1016/j.ijbiomac.2016.01.058
- Wasser, S. (2002). Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Applied Microbiology and Biotechnology, 60(3), 258–274. doi: 10.1007/s00253-002-1076-7