ABSTRACT
Despite the numerous benefits of milk constituents for human health a considerable number of the general population follow a milk-restricted diet due to clinically confirmed or self-assessed adverse reactions to cow’s milk consumption. Recurrent aphthous ulcers (RAU) are currently one of the most common oral disorders, with a worldwide distribution and insufficiently defined etiology, which, among other factors, implies the immunological reaction to food proteins. The aim of this study was to determine the immune-reactivity to donkey’s milk proteins in patients with RAU and compare it to the reactivity towards the proteins from cow’s and goat’s milks, in a set of simultaneous experiments. Levels of serum IgA, IgG and IgE antibodies to the same quantity of the examined antigens were determined by enzyme-linked immunosorbent assay. The results indicate that patients with RAU with increased immunity to cow’s milk proteins could consider the use of donkey’s milk as the best protein source.
Introduction
Milk has been used worldwide for centuries, as the first food introduced after birth and consumed through the whole life. Due to its composition rich in proteins, fats and micronutrients it has been considered one of the most natural and highly nutritive parts of an optimal diet. Beyond being a rich source of nutrients essential for normal growth and development, emerging scientific data provide evidence of both preventive and therapeutic effects of milk and its constituents. The bioactive compounds of milk, milk-based products and milk-derived constituents include proteins and peptides, prebiotic carbohydrates, probiotic bacteria and lipids, with beneficial effects supported by vitamins and minerals present. Recent scientific data have shown antihypertensive, antimicrobial, antithrombic, immunomodulatory, opioid, mineral binding effects of bioactive milk-derived peptides as the proposed mechanism of their beneficial effects on growth and development, gastrointestinal health, immunity, the cardiovascular system, cognitive function that rationalize their role in health promotion and the prevention of chronic diseases (Nagpal et al., Citation2012).
Despite the numerous benefits of milk constituents for human health a considerable number of the general population follow a milk-restricted diet due to clinically confirmed or self-assessed adverse reactions to milk consumption, primarily to cow’s milk as the most frequently consumed. The broad term of adverse reactions to milk covers immunologically based reactions – milk allergies (both IgE and non-IgE mediated), and non-immunological reactions, that is, milk intolerance (Taylor, Truelove, & Wright, Citation1964). Disturbances of the gastrointestinal tract associated with food allergies often include oral manifestations such are aphthous oral ulcers, glossitis or stomatitis (Shakeri et al., Citation2009). Additionally, inflammatory bowel diseases, both Cronh’s disease and ulcerative colitis, could be manifested as aphthous-like ulceration (Jurge, Kuffer, Scully, & Porter, Citation2006) and at the same time are shown to be associated with increased levels of antibodies to milk proteins in circulation (Lerner, Rossi, Park, Albini, & Lebenthal, Citation1989). These findings rationalize the assessment of milk allergies as part of differential diagnosis of the diseases of oral and gastrointestinal mucosa, the role of food proteins in pathogenesis of the diseases and the rationale of restricted diets as part of therapy. At the same time, if they can be considered safe the consumption of milk proteins from other animal species, especially from those phylogenetically distant from cows and with lower shown cross-reactivity (Restani et al., Citation1999), can provide both nutritional and health benefits and compensate the deprivation of cow’s milk in the diet of vulnerable population.
Recurrent aphthous ulcers (RAU) are currently one of the most common oral disorders, with a worldwide distribution and insufficiently defined etiology (Scully, Citation2006), which among other factors implies the immunological reaction to food proteins. The association between immunity to cow’s milk proteins (CMP) and oral diseases was described previously (Besu et al., Citation2009; Taylor et al., Citation1964; Thomas, Ferguson, Mclennan, & Mason, Citation1973). We have reported that subjects with RAU, without any gastrointestinal or other systemic disease or abnormalities other than recurrent aphthous ulcerations, had increased humoral (IgA and/or IgG and/or IgE) immunity to CMP (Besu et al., Citation2009; Besu, Jankovic, Konic-Ristic, Raskovic, et al., Citation2013). Successful therapy depends on completely eliminating CMP from the patient’s diet. However, this implies the restriction of all beneficial effects of milk proteins. Ideally, the replacement food should be hypo- or anallergenic, as well as non-cross-reactive with cow’s milk.
Donkey’s milk, the milk from a specie phylogenetically distant to cow’s, has recently attracted attention for its nutritional and functional properties. Based on its physicochemical properties such as pH value and viscosity, and the content of proteins, sugars and lipids, donkey’s milk appeared to be the most similar to human milk (El-Hatmi et al., Citation2015). Regarding the composition of proteins, donkey’s milk is characterized by the low content of casein, especially of αs2-casein, and relatively high content of whey protein, with a specific structure of β-lactoglobulin (Gubic et al., Citation2015; Guo et al., Citation2007; Tesse, Paglialunga, Braccio, & Armenio, Citation2009). In animal studies donkey and human milk consumption induced the reduction of pro-inflammatory stimuli, increase in antioxidant defense and the beneficial effects on gut microbiota and their metabolism, while none of the effects were shown in animals treated with cow’s milk (Lionetti et al., Citation2012; Trinchese et al., Citation2015) The observed effects are partly attributed to the specific composition of lipids and their functional properties (Chiofalo, Dugo, Bonaccorsi, & Mondello, Citation2011). However, it is considered hypoallergenic and, thus, compared to soy-protein-based formulas, extensively hydrolysed formulas and amino-acid formulas, it has been proposed as the optimal alternative to cow’s milk in the population of children with cow’s milk protein allergy (Monti et al., Citation2007; Vincenzetti et al., Citation2014).
The aim of this study was to determine the immune-reactivity to donkey’s milk proteins (DMP) in patients with RAU and compare it to the reactivity towards CMP and the proteins from goat’s milk, in a set of simultaneous in vitro experiments.
Material and methods
Milk samples
The samples of donkey`s milk were donated from the Special Nature Reserve ‘Zasavica’, Serbia, the specialized farm of Balkan donkeys as an autochthonous breed (Kugler, Grunenfelder, & Broxham, Citation2008). Milk samples were fresh composite samples obtained from animals in different periods of lactation. The contents of individual protein fractions of the composite sample of donkey milk from the Reserve Zasavica in different periods of lactation are reported to be: 1.38–1.89 g/L of αs1-casein, up to 0.11 g/L of αs2-casein, 0.1–0.55 g/L of β-casein, 1.57–2.73 g/L of α-lactalbumin, 0.26–0.20 g/L β-lactoglobulin. It also contains 2.97–2.49 g/L lysozyme and 0.01–0.25 g/L of lactoferrin (Gubic et al., Citation2015). Goat’s milk used was the composite sample of three fresh samples of milk obtained from the local open market and combined in equal volume ratio. Both donkey’s and goat’s milk samples were defatted by centrifugation on the day of collection, aliquoted and immediately stored at −20°C until analysis. Commercial skimmed cow’s pasteurized milk powder (ICN Biomedicals, Inc. Cosa Mesa, CA.) was used for testing immunoreactivity towards cow’s milk.
Studied population
Sera from 50 subjects (19 males and 31 females; average age ± SD, 39.4 ± 19.39 years) with RAU were used in the study. The study protocol was approved by the Ethical Committee of the Faculty of Stomatology, University of Belgrade and all subjects enrolled in the study gave written informed consent. They all were Caucasians and Serbian inhabitants. The clinical examination and the blood sampling were performed at the Clinics of Periodontology and Oral Medicine, Faculty of Stomatology, University of Belgrade, between 2007 and 2009.
The main inclusion criterion was a history of recurrent aphthous ulcerations, and the exclusion of etiological factors that could lead to ulceration of the oral mucosa confirmed by anamnestic data and clinical examination. In brief, all enrolled subjects had no gastrointestinal or other systemic disease or abnormalities, including changes at the skin and other mucosa and they were not using drugs that could cause oral changes. Blood was sampled in the phase of active ulcers, which was confirmed by clinical examination. In 86% (43/50) of subjects, aphthous ulcers were oval in shape with a diameter (2r) smaller than 10 mm while in 14% (7/50) of subjects ulcerations had a diameter bigger than 10 mm.
Sera of these subjects with RAU (who are involved in this research) were tested earlier to CMP, specific CMP, fresh goat’s milk (FGM), fresh cow’s milk, boiled goat’s milk and boiled cow’s milk (Besu et al., Citation2009, Besu, Jankovic, Konic-Ristic, Damjanovic, et al., Citation2013, Besu, Jankovic, Konic-Ristic, Raskovic, et al., Citation2013). In this study we used the same methodology as in prior studies, because we wanted the research to be conducted under the same experimental conditions.
Enzyme-linked immunosorbent assay
Three types of antigens were used: skimmed cow’s milk pasteurized powder, FGM and fresh donkey’s milk (FDM). Wells of the highabsorbent immunoplate (Nunctm) were coated with the same quantity of antigen proteins (10 µg). Determination of IgA, IgG and IgE serum’s immunoreactivity to CMP, FGM and FDM has been done by home-made enzyme-linked immunosorbent assay tests, using sheep antihuman IgA, IgG (Binding Site, Birmingham, England) and IgE (Sigma Chemicals Co, Saint Louis, MO), with HRP-labelled antibodies as secondary antibodies. Blocker was 1% bovine serum albumin (BSA – Sigma Chemical Co, Saint Louis, MO).
A substrate solution TMB (3,3,5,5-tetramethylbenzidine) (INEP, Zemun, Serbia) was added to the wells and after the incubation for 15 min, the enzyme reaction was terminated by addition of H2SO4. The absorbance of developed color was measured using microplate reader (Multiskan EX; ThermoLabsystems, Finland) at 450 nm.
The absorbance of blank (the sample with primary and secondary antibodies but without tested antigens) was subtracted from the absorbance of the tested sample. Obtained values of OD were standardized based on the values obtained for the sera of selected patients with high immunoreactivity to the same type of milk, which were used in each assay as the positive control. Obtained data are expressed as arbitrary units representing the ratio in OD of analysed and control samples.
Statistical analysis
Differences between parameters were evaluated by paired t test and Mann Whitney test.
Statistical package R (2.8.1 (2008-12-22); Copyright (C) 2008; The R Foundation for Statistical Computing; ISBN 3-900051-07-0) was used for data processing. Microsoft Office Excel was used to prepare all graphics.
Results
Humoral immunoreactivity to different types of milk in RAU+ patients
Sera of 36 subjects with RAU, who earlier showed increased immunoreactivity (RAU+) to CMP were tested for CMP again, FGM and FDM. Statistical analysis of the obtained data reveals that the level of anti-CMP IgA immunoreactivity estimated using paired t test was significantly higher than the anti-FGM immunoreactivity in RAU+ subjects (p = .0028). The level of anti-FDM IgA immunoreactivity was significantly lower than the anti-CMP immunoreactivity in RAU+ subjects (p = .0276). Statistical analyses show that there were no statistically significant differences between data for FGM and FDM ((A)).
Figure 1. Serum IgA (A), IgG (B), IgE (C) immunoreactivity with different types of milk (CMP, cow’s milk proteins; FGM, fresh goat’s milk; FDM, fresh donkey’s milk) determined for subjects with RAU with proven increased immunoreactivity to CMP (RAU+).
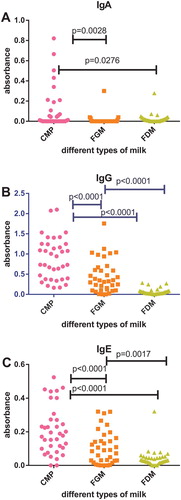
Statistical analysis of IgG immunoreactivity to different types of milk in RAU+ patients shows statistically significant differences between data for CMP vs. FGM, CMP vs. FDM, FGM vs. FDM. The level of anti-CMP IgG immunoreactivity, estimated using paired t test was significantly higher than the anti-FGM and anti-FDM IgG immunoreactivity in RAU+ subjects (p < .0001). The level of anti-FDM IgG immunoreactivity was significantly lower than the level of anti-FGM IgG immunoreactivity in RAU+ subjects (p < .0001) ((B)).
Testing of IgE immunoreactivity to different types of milk in RAU+ patients shows statistically significant differences between data for CMP vs. FGM, data for CMP vs. FDM and data for FGM vs. FDM. The level of anti-CMP IgE immunoreactivity was significantly higher than the levels of anti-FGM IgE immunoreactivity (p < .0001) and anti-FDM IgE immunoreactivity (p < .0001). The level of anti-FDM IgE immunoreactivity was significantly lower than the level of anti-FGM immunoreactivity in RAU+ subjects (p = .0017) ((C)).
Humoral immunoreactivity to different types of milk in RAU+ and RAU− subjects
Sera of 36 subjects with RAU, who earlier showed increased immunoreactivity (RAU+) to CMP and sera of 14 subjects with RAU but without increased immunity to CMP (RAU−), were tested to CMP again, and then to FGM and FDM.
Comparing the data for IgA immunoreactivity to CMP, we detect statistically significant differences between results for RAU+ and RAU− subjects ((A)). The level of anti-CMP IgA immunoreactivity, estimated using the Mann Whitney test, was significantly lower in RAU − subjects in comparison to RAU+ subjects (p = .0039). Statistical analyses show that there were no statistically significant differences between levels of anti-FGM and anti-FDM IgA immunity for these two tested groups of subjects ((A)).
Figure 2. Serum IgA (A), IgG (B), IgE (C) immunoreactivity with different types of milk (CMP, cow’s milk proteins; FGM, fresh goat’s milk; FDM, fresh donkey’s milk) determined for subjects with RAU with proven increased immunoreactivity to CMP (RAU+) and subjects with RAU without increased immunoreactivity to CMP (RAU−).
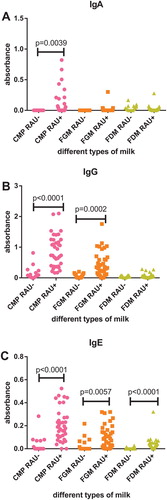
Statistical analysis of IgG immunoreactivity to different types of milk in RAU+ and RAU− subjects shows statistically significant differences between levels of IgG immunity to CMP and FGM for these two tested groups of subjects ((B)). The levels of anti-CMP and anti-FGM IgG immunoreactivity, estimated using the Mann Whitney test, were significantly lower in RAU− subjects in comparison to RAU+ subjects (p < .0001; p = .0002). There were no differences between levels of anti-FDM IgG immunity for RAU+ and RAU− subjects.
Comparing the data for IgE immunoreactivity to CMP, FGM and FDM, we detect statistically significant differences between levels of immunoreactivity for the two tested groups of subjects ((C)). The levels of anti-CMP, anti-FGM and anti-FDM IgE immunoreactivity, estimated using the Mann Whitney test, were significantly lower in RAU− subjects in comparison to RAU+ subjects (p < .0001; p = .0057; p < .0001), but with the lowest level of anti-FDM IgE antibodies.
Discussion
Immunity to CMP occurs in the patients with RAU (Besu et al., Citation2009). Due to frequent ulcerations the of the patients with RAU has been changed since they experience pain and suffer. CMP-restricted diet could be considered rational for these patients as a strategy to mitigate the immunological component of the disease. Results from our earlier study show that the exclusion of cow’s milk protein from the diet of these subjects with RAU, with increased humoral immunoreactivity to CMP, led to the disappearance of oral changes (Besu et al., Citation2009). The possibility for RAU patients to safely use milk from other mammalian species has been examined and it was concluded that goat’s milk could be a replacement for most of the population although some of the RAU patients tested have shown increased level of antibodies to GMP, especially IgG and IgE isotypes (Besu et al., Citation2013). It was shown by the immunization of Guinea pigs with fresh cow and goat milk that cow milk possesses a greater sensitization capacity than goat milk than even in other mammalian species (Ceballos, Sampelayo, Extremera, & Osorio, Citation2010).
In search for optimal source of milk proteins for patients with RAU with increased immunoreactivity to CMP, we investigated immunoreactivity of RAU patients’ sera to DMP.
The main nutritional and bioactive component of milk is proteins, which at the same time represent the predominant factors causing milk protein allergies. Milk proteins are divided into casein complexes and whey protein fractions. Depending of the species, the ratio between casein and whey proteins is different. The content of casein in cow milk is 2.46–2.80 g/100 g (Guo et al., Citation2007; Zicarelli, Citation2004); goat milk – 2.81 g/100 g (Leitner et al., Citation2004), while donkey and human milk are characterized by a low content of casein fraction 0.64–1.03 g/100 g and 0.32–0.42 g/100 g, respectively (Gubic et al., Citation2015; Guo et al., Citation2007). Guo et al. (Citation2007) reported that the content of whey proteins in human milk is in the range of 0.68–0.83 g/100 g; in cow milk 0.55–0.70; and 0.49–0.80 in donkey milk. Donkey milk has the most comparable protein composition with human milk and the ratio between casein and whey proteins (Gubic et al., Citation2015).
The data obtained in this study could be, at least partly, explained by the lower amount of αS1-casein in donkey’s milk which was previously hypothesized to underlie tolerance of goat’s milk by some children allergic to CMP, as reported previously (Ellis, Short, & Heiner, Citation1991; Webber, Graham-Brown, Hutchinson, & Burns, Citation1989).
Lara-Villoslada, Olivares, and Xaus (Citation2005) explained that the lower allergenicity of goat milk compared to cow milk is due to the fact that a lower share of αs1-casein reduces the sensitivity to another allergen protein, namely, β-lactoglobulin. According to Cunsolo et al. (Citation2009), considerable differences might be found between the primary structure of donkey and bovine as1-casein, which could be related to the already demonstrated low allergenic properties of donkey milk and could contribute to better tolerance of donkey milk.
The study of Monti et al. (Citation2007, Citation2012) found donkey milk to be a valid feeding solution in a selected population of children with cow’s milk protein allergy, for whom soy-protein-based formulas, extensively hydrolysed formulas and amino-acid formulas could not be used to replace cow’s milk and who, because of their concomitant multiple food allergies, required a substitute food that was palatable and tolerated, as well as being nutritionally valid.
Conclusion
Our study found FDM to be a valid feeding solution in a selected population of patients with RAU with immunoreactivity to CMP.
Additionally we have shown that there are no statistically significant differences between IgA immunoreactivity against FGM and FDM in RAU subjects who have increased immunoreactivity to CMP. This implies that patients with this type of IgA immunoreactivity could safely consume any goat’s or donkey’s milk. Finally, data presented that aligned with our previous data indicate that nutritional concealing is of great importance in the therapy of RAU, and that personalized nutrition should include restrictions only if it is supported with the assessment of immunological phenotype. After exploration of the impact of milk from different animal species on patients with RAU with proven immunoreactivity to CMP, it was concluded that for every patient with this problem it would be good to do (on the basis of its results) an individual nutrition programme and select the best choice.
Acknowledgements
The authors wish to thank Mrs Tatjana Petrovic for her excellent technical assistance. They also wish to thanks Mr Slobodan Simic (‘Special Reserve Zasavica’) from the Republic of Serbia for donkey’s milk samples and cooperation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Dr Irina Besu is Research Associate at the Laboratory for Biological Response Modifiers, Department of Experimental Oncology at the Institute for Oncology and Radiology of Serbia. She obtained her PhD degree at the Faculty of Stomatology, University of Belgrade. Dr Besu is engaged in the national scientific projects. She is interested in food compounds and their effect on health, especially in effect of milk (from different animal species) and gliadin. Dr Besu studied the role of food proteins in pathogenesis of the diseases and the rationale of restricted diets as part of therapy. She has authored or coauthored a number of scientific papers in this field. Dr Besu is also a specialist in orthodontics.
Dr Tatjana Srdic-Rajic is Principal Research Fellow at the Institute for Oncology and Radiology of Serbia. She obtained her PhD degree in biochemical science at the Faculty of Chemistry (University of Belgrade). Srdic-Rajic is actively engaged in basic medical and nutrition research in the national/international scientific projects. She has authored or coauthored a number of scientific papers. Her research interests are biological effects of food compounds (nutritive and nonnutritive dietary compounds), with focus on antioxidative and anti-inflammatory action and putative mechanisms and their implication in health promotion and prevention of cancer and cardiovascular diseases; development of immunotherapy against AIDS, autoimmune disorders, and atherosclerosis; and investigation of new drugs in the treatment of cancer.
Dr Ivana Matić, postdoctoral Research Associate, at the Laboratory for Biological Response Modifiers, Department of Experimental Oncology of the Institute of Oncology and Radiology of Serbia. She graduated and obtained doctoral degree – PhD in Biological Science/Molecular Biology at the Faculty of Biology, University of Belgrade. Her research interests focus on molecular biology of cancer, anticancer drug discovery and tumor immunology. Ivana has published 34 research articles in international journals.
Prof. Dr Ljiljana Jankovic is Chief of Oral Medicine, Clinic for Periodontology and Oral Medicine, Faculty of Stomatology, University of Belgrade. Dr Jankovic is also engaged in the national scientific projects. She has authored or coauthored a number of scientific papers.
Dr Valeri Besu is dentist, specialist for oral and dental diseases. He has decades of experience in working with patients. He is interested in effect of food components on oral health.
Dr Aleksandra Konic Ristic has received both MSc and DSc in Pharmaceutical Sciences/Food Science, Nutrition and Dietetics, the scientific area that she has been working in for the past 12 years. Before joining University of Leeds in 2016, she was a Senior Research Fellow at the Centre of Excellence in Nutrition and Metabolism of the Institute for Medical Research, University of Belgrade. She was a PI or a co-investigator in a number of clinical trials investigating the effects of plant bioactives on human health and also serves as an external member of two Ethics Committees in Serbia. She published more than 30 papers in international peer-reviewed journals and participated in many national and international projects and networks in the area of food and health (LCP FP 7 Project Bacchus, COST Action Positive, FP7 Project BaSeFood etc.)
Dr Zorica Juranic is Principal Research Fellow at the Institute for Oncology and Radiology of Serbia. She graduated physical-chemistry, (Faculty of Natural Sciences), and obtained doctoral degree –PhD in chemical science at the Faculty of Chemistry (University of Belgrade). Working at the Medical School (University of Belgrade) till 1982, she was involved in the research within experimental oncology that continued within various scientific projects at the Institute of Oncology and Radiology of Serbia. Although retired 2014, Juranic actively continued her engagement (now as volunteer) as a PI of the project “Biological Response Modifiers’ dealing with the research of the action various BRM in experimental oncology, immunology. She has authored or coauthored more than 100 papers in international peer-reviewed journals and was co-mentor for ten master or PhD, thesis. Till now, she was PI in four scientific projects. Her main research interest besides tumor immunology is nutritional immunology.
Additional information
Funding
References
- Besu, I., Jankovic, L., Konic-Ristic, A., Damjanovic, A., Besu, V., & Juranic, Z. (2013). Good tolerance to goat’s milk in patients with recurrent aphthous ulcers with increased immunoreactivity to cow’s milk proteins. Journal of Oral Pathology and Medicine, 42, 523–527. doi: 10.1111/jop.12052
- Besu, I., Jankovic, L., Konic-Ristic, A., Raskovic, S., Besu, V., Djuric, M., … Juranic, Z. (2013). The role of specific cow’s milk proteins in the etiology of recurrent aphthous ulcers. Journal of Oral Pathology and Medicine, 42, 82–88. doi: 10.1111/j.1600-0714.2012.01204.x
- Besu, I., Jankovic, L., Magdu, I. U., Konic-Ristic, A., Raskovic, S., & Juranic, Z. (2009). Humoral immunity to cow’s milk proteins and gliadin within the etiology of recurrent aphthous ulcers? Oral Diseases, 15, 560–564. doi: 10.1111/j.1601-0825.2009.01595.x
- Ceballos, L. S., Sampelayo, M. R. S., Extremera, F. G., & Osorio, M. R. (2010). Antigenic stimulation with goat and cow milk by oral and parenteral route in Guinea pigs. Food and Agricultural Immunology, 21, 1–13. doi: 10.1080/09540100903365860
- Chiofalo, B., Dugo, P., Bonaccorsi, I. L., & Mondello, L. (2011). Comparison of major lipid components in human and donkey milk: New perspectives for a hypoallergenic diet in humans. Immunopharmacology and Immunotoxicology, 33, 633–644. doi: 10.3109/08923973.2011.555409
- Cunsolo, V., Cairone, E., Fontanini, D., Criscione, A., Muccilli, V., Salettia, R., & Fotia, S. (2009). Sequence determination of αS1-casein isoforms from donkey by mass spectrometric methods. Journal of Mass Spectrometry, 44, 1742–1753.
- El-Hatmi, H., Jrad, Z., Salhi, I., Aguibi, A., Nadri, A., & Khorchani, T. (2015). Comparison of composition and whey protein fraction of human, camel, donkey, goat and cow milk. Mljekarstvo, 65, 159–167. doi: 10.15567/mljekarstvo.2015.0302
- Ellis, M. H., Short, J. A., & Heiner, D. C. (1991). Anaphylaxis after ingestion of a recently introduced hydrolysed whey protein formula. Journal of Pediatrics, 118, 74–77. doi: 10.1016/S0022-3476(05)81849-9
- Gubic, J., Milovanovic, I., Ilicic, M., Tomic, J., Torbica, A., Saric, L., & Ilic, N. (2015). Comparison of the protein and fatty acid fraction of Balkan donkey and human milk. Mljekarstvo, 65, 168–176. doi: 10.15567/mljekarstvo.2015.0303
- Guo, H. Y., Pang, K., Zhang, X. Y., Zhao, L., Chen, S. W., Dong, M. L., & Ren, F. Z. (2007). Composition, physicochemical properties, nitrogen fraction distribution, and amino acid profile of donkey milk. Journal of Dairy Science, 90, 1635–1643. doi: 10.3168/jds.2006-600
- Jurge, S., Kuffer, R., Scully, C., & Porter, S. R. (2006). Recurrent aphthous stomatitis. Oral Diseases, 12, 1–21. doi: 10.1111/j.1601-0825.2005.01143.x
- Kugler, W., Grunenfelder, H.-P., & Broxham, E. (2008). Donkey breeds in Europe: Inventory, description, need for action, conservation: Report 2007/2008. St. Gallen: Monitoring Institute for Rare Breeds and Seeds in Europe.
- Lara-Villoslada, F., Olivares, M., & Xaus, J. (2005). The balance between caseins and whey proteins in cow’s milk determines its allergenicity. Journal of Dairy Science, 88, 1654–1660. doi: 10.3168/jds.S0022-0302(05)72837-X
- Leitner, G., Chaffer, M., Shamay, A., Shapiro, F., Merin, U., Ezra, E., … Silanikove, N. (2004). Changes in milk composition as affected by subclinical mastitis in sheep. Journal of Dairy Science, 87, 46–52. doi: 10.3168/jds.S0022-0302(04)73140-9
- Lerner, A., Rossi, T. M., Park, B., Albini, B., & Lebenthal, E. (1989). Serum antibodies to cow’smilk proteins in pediatric inflammatory bowel disease. Crohn’s disease versus ulcerative colitis. Acta Paediatrica Scandinavica, 78, 384–389. doi: 10.1111/j.1651-2227.1989.tb11097.x
- Lionetti, L., Cavaliere, G., Bergamo, P., Trinchese, G., De Filippo, C., Gifuni, G., … Mollica, M. P. (2012). Diet supplementation with donkey milk upregulates liver mitochondrial uncoupling, reduces energy efficiency and improves antioxidant and antiinflammatory defences in rats. Molecular Nutrition & Food Research, 56, 1596–1600. doi: 10.1002/mnfr.201200160
- Monti, G., Bertino, E., Muratore, M. C., Coscia, A., Cresi, F., Silvestro, L., … Conti, A. (2007). Efficacy of donkey’s milk in treating highly problematic cow’s milk allergic children: An in vivo and in vitro study. Pediatric Allergy and Immunology, 18, 258–264. doi: 10.1111/j.1399-3038.2007.00521.x
- Monti, G., Viola, S., Baro, C., Cresi, F., Tovo, P. A., Moro, G., … Bertino, E. (2012). Tolerability of donkey’s milk in 92 highly-problematic cow’s milk allergic children. Journal of Biological Regulators and Homeostatic Agents, 26(Suppl. 3), 75–82.
- Nagpal, R., Behare, P. V., Kumar, M., Mohania, D., Yadav, M., Jain, S., … Yadav, H. (2012). Milk, milk products, and disease free health: An updated overview. Critical Reviews in Food Science and Nutrition, 52, 321–333. doi: 10.1080/10408398.2010.500231
- Restani, P., Gaiaschi, A., Plebani, A., Beretta, B., Cavagni, G., Fiocchi, A., … Galli, C. L. (1999). Cross-reactivity between milk proteins from different animal species. Clinical & Experimental Allergy, 29, 997–1004. doi: 10.1046/j.1365-2222.1999.00563.x
- Scully, C. (2006). Clinical practice. Aphthous ulceration. The New England Journal of Medicine, 355, 165–172. doi: 10.1056/NEJMcp054630
- Shakeri, R., Zamani, F., Sotoudehmanesh, R., Amiri, A., Mohamadnejad, M., Davatchi, F., & Shahram, F. (2009). Gluten sensitive enteropathy in patients with recurrent aphthous stomatitis. BMC Gastroenterology, 9, 44. doi:10.1186/1471-230X-9-44
- Taylor, K. B., Truelove, S. C., & Wright, R. (1964). Serologic reactions to gluten and cow’s milk proteins in gastrointestinal disease. Gastroenterology, 46, 99–108.
- Tesse, R., Paglialunga, C., Braccio, S., & Armenio, L. (2009). Adequacy and tolerance to ass’s milk in an Italian cohort of children with cow’s milk allergy. Italian Journal of Pediatrics, 35, 19. doi:10.1186/1824-7288-35-19
- Thomas, H. C., Ferguson, A., Mclennan, J. G., & Mason, D. K. (1973). Food antibodies in oral disease: A study of serum antibodies to food proteins in aphthous ulceration and other oral diseases. Journal of Clinical Pathology, 26, 371–374. doi: 10.1136/jcp.26.5.371
- Trinchese, G., Cavaliere, G., Canani, R. B., Matamoros, S., Bergamo, P., De Filippo, C., … Mollica, M. P. (2015). Human, donkey and cow milk differently affects energy efficiency and inflammatory state by modulating mitochondrial function and gut microbiota. The Journal of Nutritional Biochemistry, 26, 1136–1146. doi: 10.1016/j.jnutbio.2015.05.003
- Vincenzetti, S., Foghini, L., Pucciarelli, S., Polzonetti, V., Cammertoni, N., Beghelli, D., & Polidori, P. (2014). Hypoallergenic properties of donkey’s milk: A preliminary study. Veterinaria Italiana, 50, 99–107.
- Webber, S. A., Graham-Brown, R. A. C., Hutchinson, P. E., & Burns, D. A. (1989). Dietary manipulation in childhood atopic dermatitis. British Journal of Dermatology, 121, 91–98. doi: 10.1111/j.1365-2133.1989.tb01404.x
- Zicarelli, L. (2004). Buffalo milk: Its properties, dairy yield and mozzarella production. Veterinary Research Communications, 28, 127–135. doi: 10.1023/B:VERC.0000045390.81982.4d
