ABSTRACT
An anti-hydrocortisone (HDS) monoclonal antibody, 2G8, based on a HDS succinic anhydride derivative hapten, was prepared and used to develop an indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) and an immunochromatographic assay for the detection of HDS in milk samples. The half inhibitory concentration (IC50) of the antibody was 0.095 ng/mL, its limit of detection was 0.013 ng/mL, its linear range of detection was 0.026–0.356 ng/mL, and its cross-reactivity with HDS analogs was <5%. In spiked samples and a recovery test, the recovery rates ranged from 92% to 98.5%, indicating the suitability of this ic-ELISA for the analysis of HDS in milk. The immunochromatographic strip had a cutoff value of 2 ng/mL in milk and could be used for the semiquantitative analysis of HDS. When milk samples were added to the sample pad of the strip, a bright test line indicated <0.2 ng/mL HDS, a weak test line indicated 0.2–2 ng/mL HDS, and no test line indicated ≥2 ng/mL HDS. Analysis of HDS in milk samples showed that results acquired by the immunochromatographic assay agreed well with results acquired by ic-ELISA. Thus, the ic-ELISA and strip assay developed in this study rapidly and sensitively detect HDS residues in milk samples.
Introduction
Hydrocortisone (HDS) is included in many widely used medications because it has anti-anaphylaxis (Goadby & Smith, Citation1964) and anti-inflammatory properties (Rautava, Walker, & Lu, Citation2016). It is also known as “cortisol,” which is secreted by the adrenal cortex and plays vital roles in various human physiological processes (Elder & Dimitri, Citation2015), or the biomarker “stress hormone” (Gao et al., Citation2010). HDS is commonly used to treat inflammatory skin diseases (Greive & Barnes, Citation2015) and is also administered to stock animals to promote growth. It is used to suppress the immune response (Pyka, Babuska-Roczniak, & Bochenska, Citation2011) and promote weight gain in animals by reducing energy consumption (Quillet et al., Citation2014). However, its overuse causes residues to accumulate in animal products, such as milk and pork, which can endanger human health (Chen et al., Citation2011). Therefore, there is an urgent need to develop methods to monitor HDS residues in animal products.
Various studies have reported different HDS detection methods, including gas chromatography–mass spectrometry (El-Farhan et al., Citation2013; Magnisali, Dracopoulou, Mataragas, Dacou-Voutetakis, & Moutsatsou, Citation2008; Wood et al., Citation2008), liquid chromatography–mass spectrometry (LC–MS) (Hasegawa, Kubo, Shinozaki, Nowatari, & Ishii, Citation2010; Herrero, Borrull, Marce, & Pocurull, Citation2013; Zhao, Yue, Wu, & Lai, Citation2014), LC–tandem MS (LC–MS/MS) (Nam, Kwon, Lee, & Lee, Citation2012; Shi, Chen, Chen, & Yu, Citation2015; Wu et al., Citation2010), high-performance LC (HPLC) (Ronowicz et al., Citation2014; Saracino et al., Citation2014; Szeitz, Manji, Riggs, Thamboo, & Javer, Citation2014), radioimmunoassay methods (Nigel & Cook, Citation1997), and enzyme immunoassay (Sarkar et al., Citation2007; Tintos, Miguez, Mancera, & Soengas, Citation2006; Yadav et al., Citation2013). Although these instrumental methods are highly sensitive and specific, and allow the simultaneous analysis of several hormone residues, they require expensive instrumentation, a lot of time, professional expertise, and extensive sample cleanup. Radioimmunoassay methods also entail several problems, including the disposal of radioisotope waste and the short shelf-life of markers. Immunoassays have several advantages over complex instrumental methods in screening samples, including high throughput and rapid turnaround times. However, there are few reports of the use of immunochromatographic strips for the detection of HDS in biological matrices. Therefore, in this study, we produced a high-affinity anti-HDS monoclonal antibody (mAb) with a new immunogen and developed an immunochromatographic strip based on this mAb to analyze HDS in milk samples.
Materials and methods
Chemicals and apparatus
HDS, fludrocortisone, dexamethasone, betamethasone, progesterone, estrone, estradiol, and estriol were purchased from J&K Scientific Ltd. (Beijing, China). Keyhole limpet hemocyanin (KLH), ovalbumin (OVA), succinic anhydride, N,N′-carbonyldiimidazole (CDI), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), N-hydroxysuccinimide (NHS), Freund’s complete adjuvant, Freund’s incomplete adjuvant, and 3,3′,5,5′-tetramethylbenzidine (TMB) were obtained from Sigma–Aldrich (St Louis, MO, USA). RPMI-1640 cell culture medium, hypoxanthine–aminopterin–thymidine supplement, hypoxanthine–thymidine supplement, and fetal calf serum were purchased from Gibco BRL (Paisley, UK). All the reagents used in this study were of analytical or HPLC grade.
The instruments used in this work were obtained from the following suppliers: UV/Vis scanner (Bokin instruments, Tsushima, Japan), Waters Maldi Synapt Q-Tof MS (Waters, Shanghai, China), vortex machine (Shanghai Huxi Analysis Instrument Factory Co., Ltd, Shanghai, China), Multiskan MKS microplate reader (Thermo Labsystems Company, Beijing, China), membrane dispenser (Xinqidian Gene-Technology Co. Ltd, Beijing, China), and water bath (Shanghai Instrument Group Co., Ltd, Supply & Sales Co., Shanghai, China).
Buffers and solutions
The following buffers were used in the enzyme-linked immunosorbent assay (ELISA): (1) assay buffer was 0.01 M phosphate-buffered saline (PBS, pH 7.4); (2) coating buffer was 0.05 M carbonate–bicarbonate buffer (CBS, pH 9.6); (3) blocking buffer consisted of CBS and 0.2% gelatin (m/v); (4) washing buffer was PBS containing 0.05% Tween 20 (v/v); (5) antibody dilution buffer was PBS containing 0.1% gelatin (w/v); (6) substrate buffer was 0.01% TMB (w/v), 0.3% H2O2 (v/v), and phosphate buffer; and (7) stop reagent was 2 M H2SO4.
Hapten (HDS-HS) synthesis
In this study, a new HDS hapten was synthesized with the succinic anhydride method (Li et al., Citation2014). As shown in , HDS has three hydroxyls at different positions. This method was used to transform the hydroxyls into carboxyl groups, which were used to conjugate proteins. The 21-hydroxyl reacted first with succinic anhydride because it is more active than 11α-hydroxyl or 17β-hydroxyl. The steps are described below.
Table 1. Cross-reaction results of mAb 2G8.
A mixture of 100 mg of HDS and 30 mg of succinic anhydride in 10 mL of anhydrous pyridine was prepared and refluxed at 60°C for 12 h. After the reaction product was dried with rotary evaporation, the residue was cooled to room temperature and dissolved in 2 mL of methanol mixed with 4 mL of distilled water. The mixture was then extracted three times with 10 mL of ethyl acetate. A white precipitate was generated after 10% HCl (v/v) was added to the organic layer. The precipitate was collected, washed three times with distilled water, and dried at 37°C in a drying oven. The hapten was obtained as a white solid.
An LC–MS analysis confirmed that the derivative met our requirements.
Preparation and identification of antigen
The HDS-succinic anhydride (HS)–KLH conjugate was used as the immunogen, and was prepared with the active ester method, as previously reported (Kong et al., Citation2015), with some modifications. HDS-HS (2 mg) was dissolved in 0.3 mL of dimethyl formamide (DMF), and 2.5 mg of EDC and 1.5 mg of NHS were added to the mixture. The reaction solution was stirred for 6 h, and was then slowly added to 7.5 mg of KLH (MW: 5,000,000 Da) in 3 mL of CBS. The solution was incubated overnight at room temperature with continuous stirring. The HDS-HS–KLH conjugate was dialyzed against PBS for 2 days with three changes of dialysis solution per day, and was stored at −20°C until use.
HDS was conjugated to OVA (HDS–OVA) with CDI and used as the heterogeneous coating antigen. A mixture of 2.4 mg of HDS and 2.1 mg of CDI was dissolved in 0.3 mL of anhydrous DMF and stirred for 1 h at 37°C. The reaction solution was then added to 10 mg of OVA (MW 45,000 Da) in 3 mL of CBS, and stirred for an additional 12 h at room temperature. The solution was finally dialyzed against PBS for 2 days and stored at −20°C.
The final conjugates were characterized with UV/Vis spectroscopy.
Monoclonal antibody preparation
Ten female BALB/c mice (6–8 weeks old) were immunized subcutaneously with HDS-HS–KLH, as described previously (Chen et al., Citation2016). An indirect competitive ELISA (ic-ELISA) (Suryoprabowo, Liu, Peng, Kuang, & Xu, Citation2015) was used to determine the antibody production in each mouse.
A mAb specific for HDS was produced from hybridoma 2G8, which was developed with polyethylene glycol (PEG)-induced cell fusion technology (Zhang, Du, Liu, Xia, & Sun, Citation2013). Myeloma cells were fused with the spleen cells collected from the BALB/c mouse whose antiserum had the lowest 50% inhibitory concentration (IC50) and the highest titer, using the standard procedure (Liu, Hung, Lu, Chou, & Yu, Citation2014). The supernatant from the hybridoma was detected with an ic-ELISA. We subcloned the positive hybridoma showing the expected inhibition. After three rounds of subcloning with the limiting dilution method, we isolated the target cell strain producing the antibody that best inhibited HDS. The strain was expanded, cultured, and injected intraperitoneally into 8–10-week-old BALB/c mice. The antibody was purified from the mouse ascites with the caprylic acid–ammonium sulfate precipitation method (Kuang et al., Citation2013) and dialyzed against PBS at 4°C for 3 days.
The sensitivity of this mAb was evaluated from its 50% inhibition (IC50) and the limit of detection (LOD, IC10). The IC50 value and LOD were determined with a standard curve. The specificity of the mAb was determined as its cross-reactivity (CR):
CR was tested by the addition of a series of related analogs, including fludrocortisone, dexamethasone, betamethasone, progesterone, estrone, estradiol, and estriol.
Synthesis of gold nanoparticles
Gold nanoparticles were prepared with a previously reported method (Sun et al., Citation2014), with some modifications. Briefly, 100 mL of chlorauric acid solution (HAuCl4, 0.01%, w/v) was added to an Erlenmeyer flask and brought to boiling point with vigorous stirring. Next, 5 mL of freshly prepared 1% (w/v) trisodium citrate solution was quickly added with stirring. The solution changed from colorless to black and then quickly changed to wine red during this reaction. The solution was boiled for another 15 min, then cooled to room temperature, and stored at 4°C until use.
The average diameter of the gold nanoparticles was 17 ± 2 nm, measured with UV/Vis spectrometry, and confirmed with transmission electron microscopy. The colloidal gold (CG) particles were ready for use in the subsequent experiment.
Labeling the mAb with CG particles
The purified mAb was labeled with CG particles to generate 2G8–CG, as previously described (Wang et al., Citation2016). The pH of the CG solution was adjusted to 8.8 with 0.1 M K2CO3. Then 8 μg of purified mAb 2G8 was added to 1 mL of CG solution with continuous stirring, and the mixture was stirred for 50 min at room temperature. An aqueous solution of BSA (10% w/v; 50 μL) was added to block the free CG and the solution incubated for 2 h at room temperature. After centrifugation at 8000 rpm for 30 min at 4°C, the supernatant was removed and the soft sediment was collected and washed three times with gold-labeled resuspension buffer (20 mM Tris [pH 8.2], 0.1% PEG, 0.1% Tween, 5% sucrose, 5% trehalose, 0.2% BSA, and 5% Brij). Finally, the 2G8–CG conjugate was resuspended in 1 mL of the resuspension buffer and stored at 4°C until use.
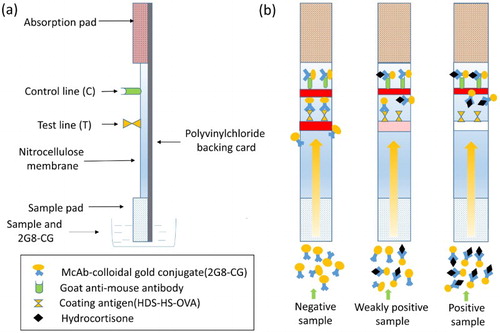
Preparation of the immunochromatographic strip
A schematic description of the immunochromatographic strip is shown in . A polyvinylchloride (PVC) backing pad forms the bottom layer, and above it is placed a sample pad, a nitrocellulose (NC) membrane containing the test and control lines, and an absorption pad. The sample pad was treated with 0.01 M PBS containing 1% BSA, 1% sucrose, and 0.2% Tween 20, and dried at room temperature for 4 h. The coating antigen (0.5 mg/mL) and a goat anti-mouse antibody (1 mg/mL) were applied to the NC membrane as the test and control lines, respectively, with a membrane dispenser at 1 μL/cm, and dried at 37°C. The sample pad, the NC pad, the absorption pad, and the PVC backing card were assembled into strips.
Test procedure and principle of the immunochromatographic strip assay
First, 150 μL of standard HDS solution or sample extract and 50 μL of 2G8–CG were mixed in the micro-well. After incubation for 5 min at room temperature, the mixture was applied to the sample pad, and allowed to flow to the absorption pad by capillary action. The test results were obtained visually in 5 min.
The principle of the assay is based on a competitive exclusion reaction. As shown in , when HDS is present in a sample, it competes with the coating antigen immobilized on the test line to combine with a fixed quantity of 2G8–CG. As the amount of HDS in the sample increases, the color of the test line becomes weaker, until it ultimately disappears. Whether or not HDS is present in the sample, 2G8–CG will react with the goat anti-mouse antibody immobilized in the control line, and the control line will become red. The color of the test line is detected visually. The result is negative, weakly positive, or positive, and the color intensity of the test line is inversely proportional to the concentration of HDS in the sample.
Sample analysis
Milk samples were obtained from local markets and the lack of HDS in them was confirmed with LC–MS. The milk samples required no processing before testing, and were spiked with a series of HDS concentrations. Each spiked and unspiked sample was analyzed three times in this experiment.
Results and discussion
Characterization of the hapten and antigen
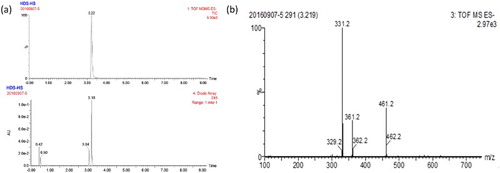
We used the succinic anhydride method to modify HDS to produce a HDS succinic anhydride derivative. The derivative was analyzed with LC–MS, and the major product formed had a retention time of 3.2 min and the main UV peak also appeared at 3.2 min ((a)), indicating that the yield of HDS-HS was high. As shown in (b), MS chromatography detected a fragment with a molecular weight of 461 Da, which is consistent with that of HDS-HS. Thus, the derivatization of HDS to HDS-HS was successful.
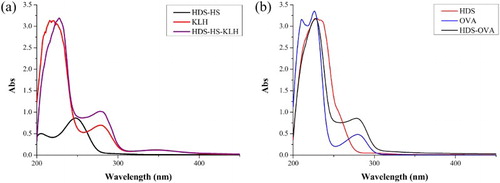
HDS-HS was conjugated to KLH with the active ester method and HDS was conjugated to OVA with CDI. Their UV absorption spectra are shown in . As shown in (a), the hapten HDS-HS had an absorption peak at ∼250 nm and KLH had two simultaneous absorption peaks at 280 and 350 nm. At the same concentration, the conjugate had a higher absorption at 280 nm than KLH, and the UV peak shifted to the unconjugated HDS-HS at 250 nm. Similarly, as shown in (b), HDS–OVA had a higher absorption at ∼280 nm than OVA at the same concentration and showed a moderate increase in the range of 240–300 nm, whereas HDS had an obvious absorption peak at ∼240 nm. These phenomena confirm that the antigen and coating antigen were successfully linked to the protein.
Sensitivity and specificity of mAb 2G8
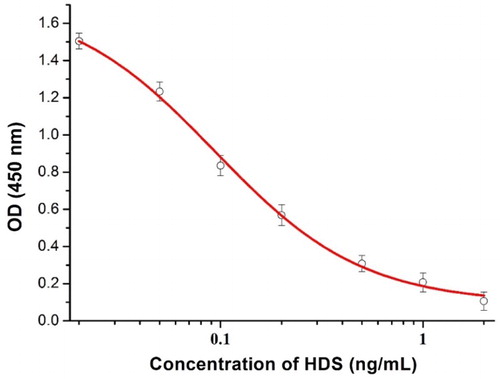
An ic-ELISA was used to measure the sensitivity and specificity of mAb 2G8. A standard curve () was constructed under optimized conditions. As shown in , the IC50 of 2G8 was calculated to be 0.095 ng/mL, the LOD was 0.013 ng/mL, and the linear range of detection (IC20–IC80) was 0.0258–0.356 ng/mL. The specificity of 2G8 was assessed as its CR. As shown in , the CR of 2G8 to all the analogs tested was <5%, indicating that the mAb is highly specific for HDS and could be used for subsequent experiments.
Analytical characteristics of the immunochromatographic strip for HDS in milk
In this experiment, the LOD (ie the HDS concentration at which the color of the test line first becomes lighter than that of the blank sample) and the cutoff value (ie the threshold HDS concentration at which the test line becomes colorless) of the immunochromatographic strip were used to evaluate its sensitivity.
Raw milk samples were obtained from local markets and analyzed with LC–MS to confirm that no HDS was present. A series of milk samples spiked with HDS (0, 0.2, 0.5, 1, and 2 ng/mL) was prepared to test the cutoff values. The results were obtained after 5 min (). The color of test line began to change when the concentration of HDS was 0.2 ng/mL, and the test line became colorless when the concentration was 2 ng/mL. Therefore, the LOD was 0.2 ng/mL and the cutoff value was 2 ng/mL. The sample was considered to be negative (−) when the red color of the test line was similar to that of the blank sample; the sample was considered to be weakly positive (±) when the red color of the test line was weaker than that of the blank sample; and the sample was considered to be positive (+) when the test line was colorless.
Figure 5. Image of detection HDS by immunochromatographic strip in milk. A series of milk samples spiked HDS were subjected to the immunochromatographic strip test. 1 = 0 ng/mL, 2 = 0.2 ng/mL, 3 = 0.5 ng/mL, 4 = 1 ng/mL, and 5 = 2 ng/mL. Cutoff value was 2 ng/mL.

All samples were detected without pretreatment, and the detection results were acquired within 10 min. Therefore, the entire process is rapid and highly efficient. We could also determine the content of HDS in the milk from the test results. A negative result occurred when the concentration of HDS was <0.2 ng/mL; a weakly positive result occurred when the concentration of HDS was 0.2–2 ng/mL; and a positive result occurred when the concentration of HDS was >2 ng/mL.
Recovery test in milk
In the recovery test, milk samples were spiked with HDS at final concentrations of 0.1–2.7 ng/mL. The results are shown in . With the ic-ELISA, the recovery rates ranged from 92% to 98.5% for HDS and the coefficients of variation (CV) ranged from 5.65% to 6.59%. We then used the immunochromatographic strip to test the same samples, and the results for the strip assay showed strong agreement with those for the ic-ELISA. These data confirm the suitability of both the ic-ELISA and the strip assay for the analysis of HDS in milk. Therefore, mAb 2G8 sensitively and efficiently detects HDS in real samples.
Table 2. Recovery of HDS in milk by ic-ELISA and strip assay (n = 3).
Analysis of HDS in milk samples by ic-ELISA and immunochromatographic strip assay
The strip assay and ic-ELISA were further applied to examine real samples. The samples consist of six random milk samples taken from local farms and one spiked sample (2.5 ng/mL). As shown in , HDS levels of No. 1 and No. 3 were separately 0.031 and 0.042 ng/mL, and other random samples were not detectable according to ic-ELISA results. However, because of the high LOD, the strip test results showed a negative outcome. In the spiked sample (S), the test result indicated that the sample was HDS-positive. Results acquired by the strip assay were in good agreement with those acquired by ic-ELISA.
Table 3. ic-ELISA and strip analysis of HDS in milk samples (n = 3).
Conclusion
In this study, a new hapten of HDS was synthesized and an mAb 2G8 that is highly specific and has the greatest sensitivity for HDS so far reported was successfully produced with this hapten. An ic-ELISA and an immunochromatographic assay showed excellent stability and sensitivity in the analysis of milk samples. Moreover, the strip assay produced results in 5–10 min. Therefore, this antibody can be used to detect HDS in milk samples and the strip assay is a more sensitive and faster detection method than instrumental analytical methods.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Zhongxing Wang got his bachelor from Zhaozhuang University, Zhaozhuang, China in 2014 and then he began to study in Jiangnan University (Wuxi, China) for his Master Degree and Ph.D in food science. His research interests are immunoassay development for veterinary drugs.
Zhengjun Xie is a full professor of Food science and technology of Jiangnan University. He got his Ph.D in food science in 2009. His research interests are fast detection technology and food safety evaluation.
Gang Cui is a full professor of Food science and technology of Jiangnan University. He got his Ph.D in food science in 2007. His research interests are fast detection technology, food safety evaluation and biosensor development.
Liqiang Liu got his Ph.D in Food science in 2014 from Jiangnan University, Wuxi, China and then became a faculty in college of Food science and technology of Jiangnan University. His research interests are immunochromatographic strip design and application.
Shanshan Song got her Master degree in Food science in 2012 from Jiangnan University, Wuxi, China and then became a research assistant in college of Food science and technology of Jiangnan University. Her research interests are monoclonal antibody development.
Hua Kuang got her Ph.D from China Agricultural University in 2009 and then began to work as a faculty in college of Food science and technology of Jiangnan University. She is currently a full professor in food safety. Her research interests are biosensor development.
Chuanlai Xu is a full professor of Food science and technology of Jiangnan University. He got his Ph.D in food science in 2002. His research interests are fast detection technology and food safety evaluation.
Additional information
Funding
References
- Chen, D. M., Tao, Y. F., Liu, Z. Y., Zhang, H. H., Liu, Z. L., Wang, Y. L., … Yuan, Z. H. (2011). Development of a liquid chromatography-tandem mass spectrometry with pressurized liquid extraction for determination of glucocorticoid residues in edible tissues. Journal of Chromatography B – Analytical Technologies in the Biomedical and Life Sciences, 879(2), 174–180. doi: 10.1016/j.jchromb.2010.11.039
- Chen, Y., Kong, D., Liu, L., Song, S., Kuang, H., & Xu, C. (2016). Development of an ELISA and immunochromatographic assay for tetracycline, oxytetracycline, and chlortetracycline residues in milk and honey based on the class-specific monoclonal antibody. Food Analytical Methods, 9(4), 905–914. doi: 10.1007/s12161-015-0262-z
- El-Farhan, N., Pickett, A., Ducroq, D., Bailey, C., Mitchem, K., Morgan, N., … Rees, D. A. (2013). Method-specific serum cortisol responses to the adrenocorticotrophin test: Comparison of gas chromatography-mass spectrometry and five automated immunoassays. Clinical Endocrinology, 78(5), 673–680. doi: 10.1111/cen.12039
- Elder, C. J., & Dimitri, P. (2015). Hydrocortisone for adrenal insufficiency. Archives of Disease in Childhood-Education and Practice Edition, 100(5), 272–276. doi: 10.1136/archdischild-2014-307325
- Gao, W., Xie, Q., Jin, J., Qiao, T., Wang, H., Chen, L., … Lu, Z. (2010). HPLC-FLU detection of cortisol distribution in human hair. Clinical Biochemistry, 43(7–8), 677–682. doi: 10.1016/j.clinbiochem.2010.01.014
- Goadby, P., & Smith, W. G. (1964). Observations on the anti-anaphylactic activity of hydrocortisone and related steroids. Journal of Pharmacy and Pharmacology, 16, 108–114. doi: 10.1111/j.2042-7158.1964.tb07428.x
- Greive, K. A., & Barnes, T. M. (2015). Increased bioavailability of hydrocortisone dissolved in a cream base. Australasian Journal of Dermatology, 56(2), e30–e34. doi: 10.1111/ajd.12128
- Hasegawa, T., Kubo, H., Shinozaki, K., Nowatari, M., & Ishii, M. (2010). Micro determination of cortisol and cortisone in umbilical cord blood by chemiluminescent high-performance liquid chromatography. Biomedical Chromatography, 24(6), 613–619.
- Herrero, P., Borrull, F., Marce, R. M., & Pocurull, E. (2013). Pressurised liquid extraction and ultra-high performance liquid chromatography-tandem mass spectrometry to determine endogenous and synthetic glucocorticoids in sewage sludge. Talanta, 103, 186–193. doi: 10.1016/j.talanta.2012.10.030
- Kong, N., Song, S., Peng, J., Liu, L., Kuang, H., & Xu, C. (2015). Sensitive, fast, and specific immunoassays for methyltestosterone detection. Sensors (Basel), 15(5), 10059–10073. doi: 10.3390/s150510059
- Kuang, H., Xing, C., Hao, C., Liu, L., Wang, L., & Xu, C. (2013). Rapid and highly sensitive detection of lead ions in drinking water based on a strip immunosensor. Sensors (Basel), 13(4), 4214–4224. doi: 10.3390/s130404214
- Li, Y., Luo, X., Yang, S., Cao, X., Wang, Z., Shi, W., & Zhang, S. (2014). High specific monoclonal antibody production and development of an ELISA method for monitoring T-2 toxin in rice. Journal of Agricultural and Food Chemistry, 62(7), 1492–1497. doi: 10.1021/jf404818r
- Liu, B. H., Hung, C. T., Lu, C. C., Chou, H. N., & Yu, F. Y. (2014). Production of monoclonal antibody for okadaic acid and its utilization in an ultrasensitive enzyme-linked immunosorbent assay and one-step immunochromatographic strip. Journal of Agricultural and Food Chemistry, 62(6), 1254–1260. doi: 10.1021/jf404827s
- Magnisali, P., Dracopoulou, M., Mataragas, M., Dacou-Voutetakis, A., & Moutsatsou, P. (2008). Routine method for the simultaneous quantification of 17alpha-hydroxyprogesterone, testosterone, dehydroepiandrosterone, androstenedione, cortisol, and pregnenolone in human serum of neonates using gas chromatography-mass spectrometry. Journal of Chromatography A, 1206(2), 166–177. doi: 10.1016/j.chroma.2008.07.057
- Nam, Y. S., Kwon, I. K., Lee, Y., & Lee, K. B. (2012). Quantitative monitoring of corticosteroids in cosmetic products manufactured in Korea using LC-MS/MS. Forensic Science International, 220(1–3), e23–e28. doi: 10.1016/j.forsciint.2011.12.011
- Nigel, J., & Cook, A. L. S. (1997). Radioimmunoassay for cortisol in pig saliva and serum. Journal of Agricultural and Food Chemistry, 45, 395–399. doi: 10.1021/jf960619d
- Pyka, A., Babuska-Roczniak, M., & Bochenska, P. (2011). Determination of hydrocortisone in pharmaceutical drug by TLC with densitometric detection in UV. Journal of Liquid Chromatography & Related Technologies, 34(9), 753–769. doi: 10.1080/10826076.2011.563891
- Quillet, E., Krieg, F., Dechamp, N., Hervet, C., Berard, A., Le Roy, P., … Pottinger, T. G. (2014). Quantitative trait loci for magnitude of the plasma cortisol response to confinement in rainbow trout. Animal Genetics, 45(2), 223–234. doi: 10.1111/age.12126
- Rautava, S., Walker, W. A., & Lu, L. (2016). Hydrocortisone-induced anti-inflammatory effects in immature human enterocytes depend on the timing of exposure. American Journal of Physiology – Gastrointestinal and Liver Physiology, 310(11), G920–G929. doi: 10.1152/ajpgi.00457.2015
- Ronowicz, J., Kupcewicz, B., Palkowski, L., Bilski, P., Siodmiak, T., Marszall, M. P., & Krysinski, J. (2014). Simultaneous determination of ciprofloxacin hydrochloride and hydrocortisone in ear drops by high performance liquid chromatography. Chemical Papers, 68(7), 861–870. doi: 10.2478/s11696-013-0526-2
- Saracino, M. A., Iacono, C., Somaini, L., Gerra, G., Ghedini, N., & Raggi, M. A. (2014). Multi-matrix assay of cortisol, cortisone and corticosterone using a combined MEPS-HPLC procedure. Journal of Pharmaceutical and Biomedical Analysis, 88, 643–648. doi: 10.1016/j.jpba.2013.10.008
- Sarkar, M., Das, B. C., Bora, B. D., Kumar, V., Mohan, K., Meyer, H. H., & Prakash, B. S. (2007). Application of sensitive enzymeimmunoassay for determination of cortisol in blood plasma of yaks (Poephagus grunniens L.). General and Comparative Endocrinology, 154(1–3), 85–90. doi: 10.1016/j.ygcen.2007.05.035
- Shi, C.-X., Chen, Z.-P., Chen, Y., & Yu, R.-Q. (2015). Quantitative analysis of hormones in cosmetics by LC-MS/MS combined with an advanced calibration model. Analytical Methods, 7(16), 6804–6809. doi: 10.1039/C5AY01437A
- Sun, Y., Hu, X., Zhang, Y., Yang, J., Wang, F., Wang, Y., … Zhang, G. (2014). Development of an immunochromatographic strip test for the rapid detection of zearalenone in corn. Journal of Agricultural and Food Chemistry, 62(46), 11116–11121. doi: 10.1021/jf503092j
- Suryoprabowo, S., Liu, L., Peng, J., Kuang, H., & Xu, C. (2015). Antibody for the development of specific immunoassays to detect nadifloxacin in chicken muscles. Food and Agricultural Immunology, 26(3), 317–324. doi: 10.1080/09540105.2014.914469
- Szeitz, A., Manji, J., Riggs, K. W., Thamboo, A., & Javer, A. R. (2014). Validated assay for the simultaneous determination of cortisol and budesonide in human plasma using ultra high performance liquid chromatography-tandem mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis, 90, 198–206. doi: 10.1016/j.jpba.2013.12.006
- Tintos, A., Miguez, J. M., Mancera, J. M., & Soengas, J. L. (2006). Development of a microtitre plate indirect ELISA for measuring cortisol in teleosts, and evaluation of stress responses in rainbow trout and gilthead sea bream. Journal of Fish Biology, 68, 251–263. doi: 10.1111/j.0022-1112.2006.00898.x
- Wang, Z., Zou, S., Xing, C., Song, S., Liu, L., & Xu, C. (2016). Preparation of a monoclonal antibody against testosterone and its use in development of an immunochromatographic assay. Food and Agricultural Immunology, 27(4), 547–558. doi: 10.1080/09540105.2015.1137276
- Wood, L., Ducroq, D. H., Fraser, H. L., Gillingwater, S., Evans, C., Pickett, A. J., … Turkes, A. (2008). Measurement of urinary free cortisol by tandem mass spectrometry and comparison with results obtained by gas chromatography-mass spectrometry and two commercial immunoassays. Annals of Clinical Biochemistry, 45(Pt 4), 380–388. doi: 10.1258/acb.2007.007119
- Wu, S., Li, W., Mujamdar, T., Smith, T., Bryant, M., & Tse, F. L. (2010). Supported liquid extraction in combination with LC-MS/MS for high-throughput quantitative analysis of hydrocortisone in mouse serum. Biomedical Chromatography, 24(6), 632–638.
- Yadav, R., Mohan, K., Kumar, V., Sarkar, M., Nitu, K., Meyer, H. H., & Prakash, B. S. (2013). Development and validation of a sensitive enzyme immunoassay (EIA) for blood plasma cortisol in female cattle, buffaloes, and goats. Domestic Animal Endocrinology, 45(2), 72–78. doi: 10.1016/j.domaniend.2013.05.003
- Zhang, H. Y., Du, X. Y., Liu, Q., Xia, C., & Sun, L. W. (2013). Detection of progesterone in bovine milk using an electrochemical immunosensor. International Journal of Dairy Technology, 66(4), 461–467.
- Zhao, C., Yue, Z., Wu, H., & Lai, F. (2014). Simultaneous determination of fourteen steroid hormone residues in beef samples by liquid chromatography-tandem mass spectrometry. Analytical Methods, 6(19), 8030–8038. doi: 10.1039/C4AY01513D