ABSTRACT
A competitive direct enzyme-linked immunosorbent assay (cd-ELISA) was developed for the rapid detection of deoxynivalenol (DON) in food and feedstuff. Polyclonal antibodies against DON were generated by immunizing rabbits with 3-HS-DON-BSA conjugates. In this assay, the sensitivity (measured as IC50) and limit of detection (measured as IC15) were 0.03 and 0.003 mg kg−1, respectively. A total of eight sample types (barley, wheat, oat, maize, rice, flour, milk and feedstuff) were chosen to evaluate the cd-ELISA assay performance. The sample could be directly detected after extraction and dilution with double-distilled water. The limit of detection in the samples was in the range of 0.15–0.48 mg kg−1. In the end, the spike and recovery results in both cd-ELISA and high-performance liquid chromatography (HPLC) were compared for assay validation. The recovery results ranged between 70% and 100%. A good correlation (R2 = 0.9613) between ELISA and HPLC was obtained.
1. Introduction
Deoxynivalenol (DON) is a major secondary metabolite generated by the Fusarium genus and is a well-known trichothecene mycotoxin that is generally found in field crops, such as wheat, corn and barley (; Cahill et al., Citation1999). Toxicity studies have shown that exposure to DON causes vomiting, diarrhea, nausea, headache, immunosuppression, and loss of appetite and body weight (Pestka & Smolinski, Citation2005; Sobrova et al., Citation2010). Therefore, it is critical to develop a rapid detection method to monitor DON residue in various foods.
In recent decades, immunological approaches have been developed for the detection of DON. In addition, many novel analyses have been developed to meet the demand for high efficiency. Fluorescence polarization immunoassay (Valenzano et al., Citation2014) and chemiluminescence enzyme immunoassay (Li et al., Citation2015; Zhang, Zhou, & Zhou, Citation2015) have been successfully applied for the rapid detection of DON and have higher sensitivity than the traditional enzyme-linked immunosorbent assay (ELISA). Magnetic nanoparticles replacing a microplate as the immobile phase have further improved the sensitivity of the chemiluminescence enzymatic immunoassay for DON (Yu et al., Citation2016). Surface plasmon resonance immunoassay (SPA biosensor) for the detection of DON (Meneely et al., Citation2010) has the advantages of high efficiency and low sample volume. DON-mimic nanobody was isolated from a naive phage display nanobody library and successfully used in an immunoassay for DON (Qiu et al., Citation2015). A highly responsive label-colloidal quantum dot enrobed into a silica shell (QD@SiO2) was used to optimize a highly sensitive immunoassay for the simultaneous detection of three analytes, including DON (Beloglazova et al., Citation2016). Due to their simplicity and portability, many immunochromatographic strip tests have been developed for the rapid visual detection of DON (Huang et al., Citation2012; Liu, Zanardi, Powers, & Suman, Citation2012; Xu, Huang, & He, Citation2010). A gold nanoparticle-based ultrasensitive paper sensor (immunochromatographic strip test) was newly developed for the simultaneous detection of 20 mycotoxins, with a visual limit of detection for DON of 2.5 mu g kg−1 that could be greatly improved to 0.06 mu g kg−1 using a hand-held strip scan reader (Kong et al., Citation2016).
However, these methods also have their limitations. The fluorescence polarization immunoassay, chemiluminescence enzyme immunoassay and biosensor technique usually suffer from performance instability and cannot be used on site. Immunochromatographic strip tests are semi-quantitative and not suitable for high-throughput quantification. Although the QD@SiO2-based and nanobody-based immunoassays are novel, their quantitative stability is not sufficient for practical use. Compared with the methods above, ELISA is superior due to its stability, in addition to simplicity, specificity, sensitivity and high throughput (Ran et al., Citation2013). Several ELISAs for DON have been developed based on polyclonal and monoclonal antibodies (Lupo et al., Citation2010; Maragos & Mccormick, Citation2000; Sanders et al., Citation2014). Some commercial ELISA kits have been optimized and utilized in the global market. However, most kits are limited in their evaluation of matrix effects, effective sample pretreatment (Meneely, Ricci, van Egmond, & Elliott, Citation2011) or high cross-reactivity against nivalenol and fusarenon X (Tangni, Motte, Callebaut, & Pussemier, Citation2010), restricting their practical use. In this study, to overcome this disadvantage, a direct competitive ELISA for DON was developed and validated using eight types of agricultural products and feedstuffs.
2. Materials and methods
2.1. Reagents
DON, 3-acetyldeoxynivalenol (3-AcDON), 15-acetyldeoxynivalenol (15-AcDON), T-2 toxin, zearalenone (ZEN), ochratoxin A (OTA), albumin from chicken egg white (OVA), horseradish peroxidase (HRP), Freund’s complete and incomplete adjutants, l-butaneboronic acid, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) were obtained from Sigma Chemical (St. Louis, MO, USA). Bovine serum albumin (BSA) and tetrahydrofuran (THF) were obtained from Merck (Darmstadt, Germany). Protein A-Sepharose 4B was obtained from Amersham Biosciences (Uppsala, Sweden).
2.2. Apparatus
Polystyrene 96-well plates were purchased from Nunc (Roskilde, Denmark). A microplate washer was purchased from Bio-Rad (Hercules, USA). A Multiskan spectrometer was purchased from Thermo Labsystems (Vantaa, Finland). A chromatograph for high-performance liquid chromatography (HPLC) was purchased from Shimadzu (Tokyo, Japan). A coffee grinder was purchased from IKA (Staufen, Germany). A Milli-Q water system was purchased from Merck Millipore (Bedford, USA).
2.3. Hapten synthesis
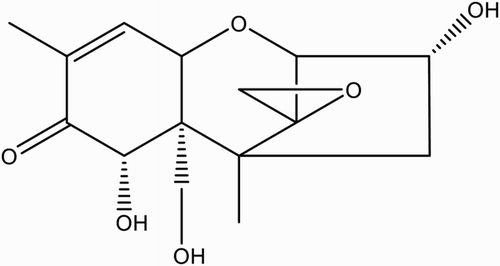
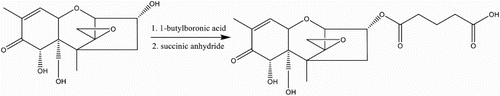
DON hapten, 3-O-hemisuccinyl-DON (3-HS-DON), was synthesized based on the method described by Chu and co-workers (; Xu, Zhang, & Chu, Citation1988). Briefly, 17 mg of l-butaneboronic acid and 5 mg of DON were dissolved in 500 μL of dry pyridine. The mixture was stirred overnight at room temperature. Then, 34 mg of succinic anhydride dissolved in 200 μL of dry pyridine was added to the mixture. After stirring for 3 h at 100°C, the mixture was dried under nitrogen stream, re-suspended in ethyl acetate and then washed three times with water. The organic phase was combined and dried under reduced pressure. The residue was chromatographed on silica (chloroform-acetone-isopropyl alcohol 8:1:1) to yield 3-HS-DON.
Figure 2. Preparation of 3-HS-DON, (1) 17 mg of l-butaneboronic acid and 5 mg of DON were dissolved in 500 μL of dry pyridine. The mixture was stirred overnight at room temperature. (2) Then, 34 mg of succinic anhydride dissolved in 200 μL of dry pyridine was added to the mixture. After stirring for 3 h at 100°C, the mixture was dried under a nitrogen stream, re-suspended in ethyl acetate and washed three times with water. The organic phase was combined and dried under reduced pressure.
2.4. Preparation of DON conjugates
3-HS-DON (2.77 mg, 0.007 mmol), NHS (2.41 mg, 0.021 mmol) and EDC (4 mg, 0.021 mmol) were dissolved in 200 μL of dry DMF. Subsequently, the mixture was stirred for 12 h at room temperature and was added dropwise into the 5 mg mL−1 BSA solution in 50 mM potassium phosphate buffer (pH 9.3). The mixture was then incubated overnight at 4°C and dialyzed against phosphate-buffered saline (PBS, 10 mmol L−1 sodium phosphate and 137 mmol L−1 NaCl, pH 7.5) for 3 days with 6 buffer changes. The 3-HS-DON-BSA conjugates were stored at −20°C. The 3-HS-DON-HRP conjugation was synthesized using the mixed anhydride method. 3-HS-DON (1.35 mg, 0.003 mmol) and EDC (1.78 mg, 0.009 mmol) were dissolved in 200 μL of dry DMF. The mixture was added dropwise to 2 mL of 5 mg mL−1 HRP in 130 mM NaHCO3 solution (pH 8.1) and stirred for 6 h at room temperature. Then, EDC (1.2 mg, 0.006 mmol) was added to the mixture and stirred overnight at 4°C. Finally, the crude conjugates were dialyzed against PBS and stored at 4°C before use.
2.5. Antibody production
Polyclonal antibodies were produced based on the method described by Lu et al. (Citation2010). Two Japanese white rabbits were immunized with 3-HS-DON-BSA conjugates. The BSA conjugates were emulsified with an equal volume of Freund’s complete adjuvant and were injected into multiple sites on the rabbits. Subsequent booster injections were administered at a 4-week interval. Blood was collected from the marginal ear vein 8-10 days after each booster injection. Polyclonal antibodies in the antisera were purified using protein A-Sepharose 4B affinity chromatography assay for competitive direct ELISA (cd-ELISA) as described below.
2.6. Competitive direct ELISA of DON
2.6.1. cd-ELISA procedure
Polyclonal antibodies were immobilized with a carbonate buffer (1 μg well−1) on a microwell plate. After washing three times with the washing solution (PBST, 0.05% Tween 20 PBS solution), the wells were blocked with 0.5% skim milk PBS solution (200 µL well−1) at 37°C for 1 h. The plates were washed another three times, and the DON calibration solutions (50 µL well−1) with a 1000x dilution of HRP conjugate stock (50 µL well−1) were incubated in their respective wells at 37°C for 40 min. After an additional three washes with PBST, the TMB substrate solution (100 µL well−1) was added to each well at 37°C for 15 min to develop the color. The enzymatic reaction was then stopped by adding 2.5 M H2SO4 (50 µL well−1). The absorbance values were measured using a microplate reader with a dual wavelength mode at 450 nm as the test and 650 nm as the reference.
2.6.2. Cross-reactivity
The specificity of the antibody was evaluated using structurally related DON analogues 3-AcDON, 15-AcDON, OTA, T-2 toxin and ZEN. Percent cross-reactivity was calculated using the equation below:IC50 is defined as the concentration of a test compound at which the corresponding absorbance is equal to half of the maximum absorbance.
2.6.3. Sample extraction and spiking method
The food samples (barley, wheat, oat, maize, rice, flour and milk) were bought from local markets. Feedstuff was kindly provided by Tianjin import and export inspection and the Quarantine Bureau. All samples were confirmed to be DON-free by HPLC.
2.6.4. Cereal (barley, wheat, oat, maize, rice, flour) and feedstuff
Cereal and feedstuff samples were homogenized using a coffee grinder and dried at 50°C for approximately 20 h before being stored at −20°C.
For barley, wheat, oat, rice, flour and feedstuff, sample mince (5 g) was spiked to final levels of 3.2, 6.4 and 9.6 mg kg−1 DON. Maize sample was spiked to final levels of 2, 4 and 6 mg kg−1 DON. The spiked samples were allowed to stand at room temperature overnight. For the extraction, DON was extracted by shaking 20 mL of double-distilled water in 5 g of spiked sample using a rotary shaker for 2 min. The sample was then centrifuged (16000 × g) at 4°C for 10 min. The clear supernatant was collected and directly diluted (40-fold for barley, wheat, oat, rice and flour; 20-fold for maize) with PBS before analysis.
2.6.5. Milk
Five milliliters of milk sample was spiked with DON at three different levels (1, 2 and 3 mg kg−1). The spiked samples were defatted by centrifugation at 4°C for 10 min. The remaining sample was 50-fold diluted with PBS for ELISA test.
2.7. HPLC analysis of DON
2.7.1. Sample preparation
The sample preparation methods are mostly identical to the methods described in Section 2.6.3. The supernatant of the solid samples and the defatted milk sample were evaporated to dryness. The residues were re-dissolved in the mobile phase [acetonitrile: water (1:9, v/v)].
2.7.2. HPLC determination of DON
The HPLC system consisted of 2 LC-10ATVP pumps and an SPD-10AVP ultraviolet detector set at 218 nm. Chromatographic separation was performed using an analytical Hypersil-ODS C18 column, 5 μm, 250 × 4.6 mm i.d. (Thermo, Beijing, China). The mobile phase was acetonitrile/water (1:9, v/v) at a flow rate of 1.0 mL min−1. The temperature of the column oven was 30°C. The Class-VP software (Shimadzu, Tokyo, Japan) was used to acquire and process the spectral and chromatographic data.
3. Results and discussion
3.1. Development of cd-ELISA
3.1.1. The design of cd-ELISA
The strategy of hapten synthesis is to preserve the main structure of DON as the potential immunodominant epitope and to utilize succinic anhydride as a linker to generate polyclonal antibodies. 3-HS-DON with three carbons was subjected to hydrolysis to yield the 3-HS-DON active ester with terminal carboxyl groups for subsequent coupling to BSA, which was used as an immunoreagent to minimize any back-folding of hapten into the carrier protein (Lu, Peterson, Gooding, & Lee, Citation2012). The antibodies raised against 3-HS-DON-BSA conjugates (denoted as Ab∝DON) were expected to recognize the main structure of DON. The sensitivity of Ab∝DON from each bleed was determined using an indirect competitive assay and was found to increase with the booster immunization. The fourth bleed of 3-HS-DON-BSA (denoted as Ab∝DON#4) gave the best sensitivity and was selected for further assay development.
3.1.2. Sensitivity and selectivity of cd-ELISA
The coating amount of antibody in the range of 0.5–2 μg well−1 and the dilution factor of 3-HS-DON-HRP (500, 1000, 2000 and 4000) were utilized in a direct competitive assay format to increase the assay sensitivity. The antibody coating amount (1 μg well−1)/enzyme conjugate dilution factor (1000) pair was selected due to the highest sensitivity (measured as IC50, IC50 = 0.030 mg kg−1) and limit of detection performance (measured as IC15, IC15 = 0.003 mg kg−1).
For the assay selectivity, the cross-reactivity for OTA, T-2, ZEN and 15-AcDON was less than 3.0%, except for 3-AcDON, which was different from DON by only a hydroxyl group at 3-carbon (). The cross-reactivity for 3-AcDON was 500%, indicating that DON and 3-AcDON may share the same immunodominance. The difference between the 15-AcDON and DON structures was the hydroxyl group at 15-carbon. Thus, the cross-reactivity for 15-AcDON was 2.5%, which was much lower than that for 3-AcDON. This occurred because the hydroxyl group at 15-carbon is critical for the immunodominance of DON.
Table 1. Cross-reactivity of DON antibody.
3.1.3. The optimization of antibody/antigen interaction time (AI-time) and enzyme/substrate reaction time (ER-time)
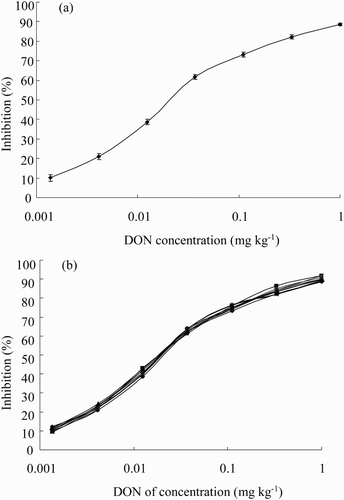
Combinations of different lengths of AI-time (1 h, 40 min) and ER-time (30 min, 15 min) were used to evaluate their effects on assay performance. Shortening both the AI-time and ER-time had little effect on the absorbance values and no effect on the assay sensitivity (data not shown). Therefore, an AI-time of 40 min and an ER-time of 15 min were chosen in the assay. Under these optimized conditions, the sensitivity (measured as IC50) and the limit of detection (measured as IC15) reached 0.030 and 0.003 mg kg−1, respectively ((a)).
3.1.4. Effects of methanol and ionic strength on cd-ELISA
To evaluate the variability of the assays due to matrix effects, the effects of the methanol and ionic strength were also evaluated.
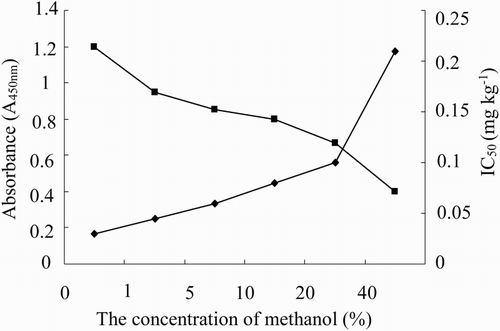
In the sample preparation, methanol and PBS were usually used as the extraction solvent and the dilution solution for DON, respectively. The high percentage of methanol and strong ionic strength may disturb the reaction of antigen and antibody and inhibit enzyme catalysis. Therefore, the effects of methanol and ionic strength on the assay were investigated to improve the assay performance, including the sensitivity and the absorbance value. As shown in , the absorbance values and sensitivity decreased as the percentage of methanol increased, indicating that methanol could inhibit the binding between antibody and DON. Therefore, the percentage of methanol should be lower than 1% to maintain assay performance.
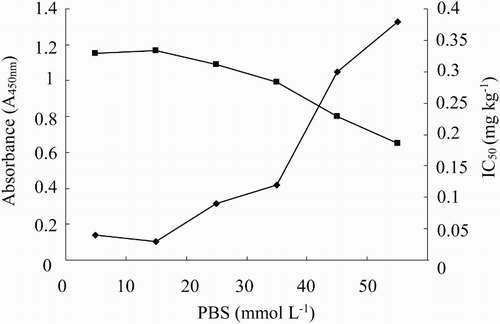
The effect of ionic strength on assay performance is shown in . The maximum absorbance value decreased with the increasing ionic strength from 0 to 50 mmol L−1. The assay with 10 mmol L−1 PBS had the highest sensitivity and a reasonable absorbance value; thus, 10 mmol L−1 PBS was selected for studying further matrix effects.
3.2. Matrix effects
The matrix effect in cd-ELISA was considered to be the result of some unwanted compounds in the samples, such as pigment, starch, protein and fat. These impurities might hinder the binding between antibody and antigen, which could consequently reduce the sensitivity of the competitive immunoassay and even cause false positives by inhibiting color development.
Barley, wheat, oat, maize, rice, feedstuff, flour and milk were chosen for the matrix interferences study. Because methanol could inhibit the antibody/antigen interaction mentioned in Section 3.1.2, double-distilled water was used to extract DON from the samples. After the extraction, the mixture was centrifuged to remove the precipitate, and the clear supernatant was further diluted with PBS before assay use. As the results in (b) show, the DON standard curves prepared both in PBS and in the prepared sample extracts overlapped, which indicated that the matrix effect could be simply achieved by diluting the sample extracts with PBS. The dilution factor of maize was 100. Barley, wheat, oat, rice, feedstuff and flour were rich in protein and minerals. The dilution factor of these samples was 160 to minimize the matrix effects. Because the milk sample contained plenty of protein and fat, a 50-fold dilution was required for the assay use. The limits of detection were 0.3 mg kg−1 for maize, 0.15 mg kg−1 for milk and 0.48 mg kg−1 for barley, wheat, oat, rice, flour and feedstuff.
3.3. Recovery studies of food samples
The analytical performance of the assay was evaluated by determining the percent recovery of DON in a spike and recovery study. The percent recovery in each sample was generally in a range of 80–98%, except for 3.2 mg kg−1 in barley, 3.2 and 6.4 mg kg−1 in wheat, and 3.2 mg kg−1 in flour (). The lower percent recovery was found at low spiking level, which may be caused by the absorption of DON in the tubes during the sample preparation.
Table 2. Recovery studies of eight samples at three levels by ELISA (n = 3).
3.4. Correlation studies between cd-ELISA and HPLC analysis
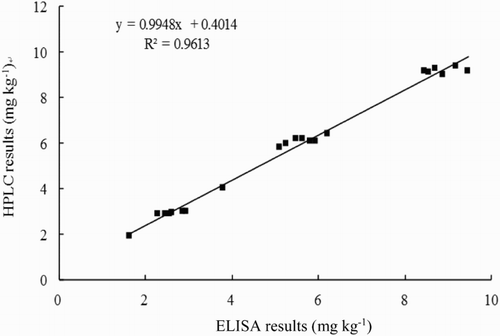
To further validate the accuracy of the assay, the correlation studies were performed on barley, wheat, oat, maize, rice, flour and feedstuff samples by comparing the results between ELISA and HPLC. The regression equation was y = 0.9948x + 0.4014, and the correlation coefficient was 0.9613, indicating a good correlation between the ELISA and HPLC data for these samples ().
4. Conclusion
A cd-ELISA was developed and optimized to detect DON in eight types of agricultural products and feedstuffs with simple sample preparation. The sensitivity and limit of detection were 0.030 and 0.003 mg kg−1, respectively. The limit of detection in the samples ranged between 0.15 and 0.48 mg kg−1, which is lower than the DON maximum residue limit of 1 mg kg−1 in China. To validate the ELISA method, the samples were analyzed using HPLC, and a good correlation (R2 = 0.9613) was obtained between ELISA and HPLC. Compared with the reported ELISAs and test kits, the advantages of stability, specificity and sensitivity, in addition to having more practical applications with a full evaluation of matrix effects, make the established cd-ELISA suitable for use as a practical assay to test for DON residue in foods and foodstuffs.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Additional information
Funding
References
- Beloglazova, N. V., Foubert, A., Gordienko, A., Tessier, M., Aubert, T., Drijvers, E., … Saeger, S. D. (2016). Sensitive QD@SiO2-based immunoassay for triplex determination of cereal-borne mycotoxins. Talanta, 160, 66–71. doi: 10.1016/j.talanta.2016.05.015
- Cahill, L. M., Kruger, S. C., McAlice, B. T., Ramsey, C. S., Prioli, R., & Kohn, B. (1999). Quantification of deoxynivalenol in wheat using an immunoaffinity column and liquid chromatography. Journal of Chromatography A, 859, 23–28. doi: 10.1016/S0021-9673(99)00846-8
- Huang, Z., Xu, Y., Li, L., Li, Y., Zhang, H., & He, Q. (2012). Development of an immunochromatographic strip test for the rapid simultaneous detection of deoxynivalenol and zearalenone in wheat and maize. Food Control, 28, 7–12. doi: 10.1016/j.foodcont.2012.04.035
- Kong, D., Liu, L., Song, S., Suryoprabowo, S., Li, A., Kuang, H., … Xu, C. L. (2016). A gold nanoparticle-based semi-quantitative and quantitative ultrasensitive paper sensor for the detection of twenty mycotoxins. Nanoscale, 8, 5245–5263. doi: 10.1039/C5NR09171C
- Li, Y., Liu, G., Fu, X., He, J., Wang, Z., Hou, J., … Zhang, S. (2015). High-sensitive chemiluminescent ELISA method investigation for the determination of deoxynivalenol in rice. Food Analytical Methods, 8, 656–660. doi: 10.1007/s12161-014-9941-4
- Liu, J., Zanardi, S., Powers, S., & Suman, M. (2012). Development and practical application in the cereal food industry of a rapid and quantitative lateral flow immunoassay for deoxynivalenol. Food Control, 26, 88–91. doi: 10.1016/j.foodcont.2012.01.005
- Lu, Y., Peterson, J. R., Gooding, J. J., & Lee, N. A. (2012). Development of sensitive direct and indirect enzyme-linked immunosorbent assays (ELISAs) for monitoring bisphenol-A in canned foods and beverages. Analytical and Bioanalytical Chemistry, 403, 1607–1618. doi: 10.1007/s00216-012-5969-8
- Lupo, A., Roebuck, C., Settimo, K., Quain, A., Kennedy, J., & Abouzied, M. (2010). Validation study of a rapid ELISA for detection of deoxynivalenol in wheat, barley, malted barley, corn, oats and rice. Journal of AOAC International, 93, 600–610.
- Lu, Y., Xu, N., Zhang, Y., Liu, B., Song, Y., & Wang, S. (2010). Development of general immunoassays for pyrethroids: A new approach for hapten synthesis using pyrethroid metabolite analogue and application to food samples. Food and Agricultural Immunology, 21, 27–45. doi: 10.1080/09540100903418867
- Maragos, C. M., & Mccormick, S. P. (2000). Monoclonal antibodies for the mycotoxins deoxynivalenol and 3-acetyl-deoxynivalenol. Food and Agricultural Immunology, 12, 181–192. doi: 10.1080/09540100050140722
- Meneely, J. P., Fodey, T., Armstrong, L., Sulyok, M., Krska, R., & Elliott, C. T. (2010). Rapid surface plasmon resonance immunoassay for the determination of deoxynivalenol in wheat, wheat products, and maize-based baby food. Journal of Agricultural and Food Chemistry, 58, 8936–8941. doi: 10.1021/jf101517s
- Meneely, J. P., Ricci, F., van Egmond, H. P., & Elliott, C. T. (2011). Current methods of analysis for the determination of trichothecene mycotoxins in food. TrAC Trends in Analytical Chemistry, 30, 192–203. doi: 10.1016/j.trac.2010.06.012
- Pestka, J. J., & Smolinski, A. T. (2005). Deoxynivalenol: Toxicology and potential effects on humans. Journal of Toxicology & Environmental Health Part B, 8, 39–69. doi: 10.1080/10937400590889458
- Qiu, Y. L., He, Q. H., Xu, Y., Bhunia, A. K., Tu, Z., Chen, B., & Liu, Y. Y. (2015). Deoxynivalenol-mimic nanobody isolated from a naive phage display nanobody library and its application in immunoassay. Analytica Chimica Acta, 887, 201–208. doi: 10.1016/j.aca.2015.06.033
- Ran, R., Wang, C. H., Han, Z., Wu, A. B., Zhang, D. B., & Shi, J. X. (2013). Determination of deoxynivalenol (DON) and its derivatives: Current status of analytical methods. Food Control, 34, 138–148. doi: 10.1016/j.foodcont.2013.04.026
- Sanders, M., Guo, Y. R., Iyer, A., Garcia, Y. R., Galvita, A., Heyerick, A., … Bracke, M. (2014). An immunogen synthesis strategy for the development of specific anti-deoxynivalenol monoclonal antibodies. Food Additives & Contaminants: Part A: Chemistry, Analysis, Control, Exposure & Risk Assessment, 31, 1751–1759.
- Sobrova, P., Adam, V., Vasatkova, A., Beklova, M., Zeman, L., & Kizek, R. (2010). Deoxynivalenol and its toxicity. Interdisciplinary Toxicology, 3, 94–99. doi: 10.2478/v10102-010-0019-x
- Tangni, E. K., Motte, J. C., Callebaut, A., & Pussemier, L. (2010). Cross-reactivity of antibodies in some commercial deoxynivalenol test kits against some fusariotoxins. Journal of Agricultural and Food Chemistry, 8, 12625–12633. doi: 10.1021/jf103025e
- Valenzano, S., Lippolis, V., Pascale, M., De Marco, A., Maragos, C. M., Suman, M., & Visconti, A. (2014). Determination of deoxynivalenol in wheat bran and whole-wheat flour by fluorescence polarization immunoassay. Food Analytical Methods, 7, 806–813. doi: 10.1007/s12161-013-9684-7
- Xu, Y., Huang, Z. B., & He, Q. H. (2010). Development of an immunochromatographic strip test for the rapid detection of deoxynivalenol in wheat and maize. Food Chemistry, 119, 834–839. doi: 10.1016/j.foodchem.2009.08.049
- Xu, Y. C., Zhang, G. S., & Chu, F. S. (1988). Enzyme-linked immunosorbent assay for deoxynivalenol in corn and wheat. Journal – Association of Official Analytical Chemists, 71, 945–949.
- Yu, S. C., Yu, F., Li, Y. P., Liu, L., Zhang, H. Q., Qu, L. B., & Wu, Y. J. (2016). Magnetic nanoparticles replacing microplate as immobile phase could greatly improve the sensitivity of chemiluminescence enzymatic immunoassay for deoxynivalenol. Food Control 60, 500–504. doi: 10.1016/j.foodcont.2015.08.012
- Zhang, R. S., Zhou, Y. J., & Zhou, M. G. (2015). A sensitive chemiluminescence enzyme immunoassay for the determination of deoxynivalenol in wheat samples. Analytical Methods, 7, 2196–2202. doi: 10.1039/C4AY03079F