ABSTRACT
To discuss the molecular mechanism of immunoenhancing activities of Hyriopsis cumingii polysaccharides (HCPS), influences of HCPS on mice immunosignaling molecules (IL-2, IL-10, IFN-γ, NO, cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP)) and T lymphocyte differentiation (to CD4+ and CD8+ T cells) were evaluated by cell model in vitro and/or cyclophosphamide-induced immunosuppression animal model in vivo. Results showed that gastric gavage of crude HCPS could promote the production of IL-2, IFN-γ, inducible nitric oxide synthase (iNOS), cGMP, CD4 and CD8 in a dose-dependent manner. While, crude HCPS by gastric gavage exhibited suppressive effects on the production of IL-10 and cAMP in a dose-dependent manner. Crude HCPS and its purified fractions (HCPS-1, HCPS-2 and HCPS-3) could strengthen peritoneal macrophage expressing iNOS in vitro in a dose-dependent manner. HCPS-stimulated immunostrengthening functions was mediated, at least in part, by immunosignaling molecules of IL-2, IL-10, IFN-γ, NO, cAMP and cGMP, and T lymphocyte differentiation (to CD4+ and CD8+ T cells).
1. Introduction
Immunostimulatory activities of polysaccharides, such as increasing index of immune organ, activating immunocyte, promoting production of cytokine and antibody, have been reported widely (Fang & Chen, Citation2013; Hao & Zhao, Citation2016; Lee et al., Citation2013; Sansone, Sansone, Shiga, & Nascimento, Citation2016; Sun et al., Citation2015). About the molecular mechanism of immunoenhancing activities of polysaccharides, it has been discussed in some papers. Regulation of immunosignaling molecules, such as interleukin-2 (IL-2), IL-10, interferon-γ (IFN-γ), nitric oxide (NO), cyclic adenosine monophosphate (cAMP), cyclic guanosine monophosphate (cGMP) and so on, is one of the possible immunomodulatory mechanisms (Bai, Ma, Wang, & Yu, Citation1997; Kumar & Tiku, Citation2016; Liu et al., Citation2016; Sun, Gao, Xiong, Huang, & Xu, Citation2014; Zhang, Ding, et al., Citation2014). It has been reported that cluster of differentiation 4+ (CD4+) and CD8+ T cells are key links of immunoregulation of organism (Zhang & Bevan, Citation2011). Promotion of T lymphocyte differentiation (to CD4+ and CD8+ T cells) by upregulating the expression of CD4+ and CD8+ is also a possible immunoenhancing mechanism of polysaccharides (Feng et al., Citation2015; Wang, Huang, Sun, & Pan, Citation2015).
H. cumingii, a member of freshwater pearl mussels, is widely cultivated in China because it can produce high quality pearls. In addition, it is also one of the animals which are used as food and medicine simultaneously in traditional Chinese medicine. In previous studies, we optimized the extraction of H. cumingii polysaccharides (HCPS), and purified the crude HCPS. Three purified fractions of HCPS (HCPS-1, HCPS-2 and HCPS-3) were obtained (Qiao et al., Citation2009). Furthermore, we evaluated the immunostimulatory activities of crude HCPS, HCPS-1, HCPS-2 and HCPS-3. It was found that HCPS exhibited some immunoenhancing activities (Qiao et al., Citation2010). The immunostimulatory activities of HCPS have been reported (Dai, Zhang, Zhang, & Wang, Citation2009; Qiao et al., Citation2010), but little attention was paid on their molecular mechanism. In this paper, we report the influences of HCPS on mice immunosignaling molecules (IL-2, IL-10, IFN-γ, NO, cAMP and cGMP) and T lymphocyte differentiation (to CD4+ and CD8+ T cells) to discuss the possible molecular mechanism of immunoenhancing activities of HCPS.
2. Materials and methods
2.1. Materials and reagents
Crude HCPS and its purified fractions of HCPS-1, HCPS-2 and HCPS-3 were prepared from H. cumingii according to our previous method (Qiao et al., Citation2009). Kunming mice (8-weeks-old), grade of specific pathogen frees with body weight (BW) of 20 ± 2 g, were purchased from the Experimental Animal Center of Anhui Medical University (Hefei, China). All procedures involving animals were conducted in strict accordance with the Chinese legislation on the use and care of laboratory animals.
Fetal bovine serum (FBS), RPMI-1640 medium, agarose, DNA marker and reagent kits (Column Animal total RNA Purification Kit, M-MuLV First Strand cDNA Synthesis Kit and SGExcel FastSYBR Mixture Kit) for real-time fluorescence quantitative PCR (RTFQ-PCR) were obtained from Sangon Biotech (Shanghai) Co. Ltd., China. Enzyme-linked immunosorbent assay (ELISA) kits of IL-2, IL-10, IFN-γ, inducible nitric oxide synthase (iNOS), CD4, CD8, cAMP and cGMP were purchased from Cloud-Clone Corp., China. Cyclophosphamide (CPA) was purchased from Jiangsu Hengrui Medcine Co., China. Cell plate was purchased from Corning Inc., China. All other reagents were of analytical grade. Preparation of 6% starch medium: beef extract 0.3 g, peptone 1.0 g and NaCl 0.5 g were added into 100 mL distilled water, then 6.0 g soluble starch was appended and sterilized by autoclaving.
2.2. Determination of HCPS acting on immunosignaling molecule in vitro
To determine the influences of HCPS on immunosignaling molecule in vitro, contents of iNOS in peritoneal macrophages were measured. Preparation and treatment of mouse peritoneal macrophage was carried out according to the reported method with minor modification (Dai et al., Citation2009; Qiao et al., Citation2010). In brief, sterile 6% starch medium (50 mL/kg BW) was injected intraperitoneally into Kunming mice. Three days later, the mice were killed by cervical dislocation and peritoneal macrophages were harvested and adjusted to a density of 2 × 106 cell/mL in the RPMI 1640 medium supplemented with FBS (10%). Then, the peritoneal macrophages suspension (2 × 106 cell/mL) was seeded into a 24-well flat-bottom plate (800 μL/well) and the cells were allowed to adhere to the bottom of the plate at 37°C in a humidified 5% CO2 incubator (3111, Thermo Fisher Scientific, USA) for 3 h. Non-adherent cells were removed by washing three times with RPMI 1640 medium. After 400 μL of RPMI 1640 medium with 10% FBS was added into each well, the RPMI 1640 medium with 10% FBS (400 μL/well, control group) or test sample (400 μL/well, sample group, crude or purified HCPS at a concentration of 100, 200 or 400 μg/mL) was appended to respective well. The cell plate was incubated in a 5% CO2 incubator at 37°C for 48 h, then, the cultured peritoneal macrophages was collected by vigorous pipetting and stored at −80°C for analysis.
Levels of iNOS in lysates of peritoneal macrophages were measured with commercial ELISA kits according to their instruction manual and absorbance of each well was determined by an ELISA microplate reader (iMark, Bio-RAD, Japan). The relative content of sample group to control group was calculated by the following equation: Relative content = C1/C0, where C1 is the concentration of sample group and C0 is the concentration of control group.
2.3. Determination of crude HCPS acting on immunosignaling molecules and T lymphocyte differentiation in vivo
2.3.1. Experimental design and sample collection
Immunosuppressed mice were induced by hypodermic injection of CPA in order to investigate the influences of crude HCPS on immunosignaling molecules and T lymphocyte differentiation in vivo. Animal grouping, experimental design and sample collection were done according to the reported methods with some modifications (Dai et al., Citation2009; Qiao et al., Citation2010). Briefly, female Kunming mice were housed in an air-conditioned animal room with constant temperature (25 ± 1°C) and 70–80% relative humidity, then the mice were free to access to food and water and kept on a 12-h light/dark cycles during the experiments. After adapting to their environment for 1 week, these mice were randomly divided into five groups (10 for each) for experiment. Mice in Group I (control group) were treated with 0.9% NaCl by gastric gavage (25 mL/kg BW) once daily for 10 days and by hypodermic injection (15 mL/kg BW) on days 1, 3, 5, 7 and 9. Mice in Group II (model group) were administrated with gastric gavage of 0.9% NaCl (25 mL/kg BW) once a day for 10 days and with hypodermic injection of 0.5% CPA (15 mL/kg BW) on days 1, 3, 5, 7 and 9. Mice in Group III (crude HCPS of low dose), Group IV (crude HCPS of medium dose) and Group V (crude HCPS of high dose) were treated with crude HCPS at dose of 200, 400 and 800 mg/kg BW by gastric gavage (25 mL/kg BW), respectively once daily for 10 days and with 0.5% CPA by hypodermic injection (15 mL/kg BW) on days 1, 3, 5, 7 and 9. At the eleventh day, the mice were weighed and killed by decapitation after overnight fasting. Blood sample was harvested immediately in centrifuge tube, then, spleen sample was excised, weighed and collected. After 2 h, the blood sample was centrifuged at 4000 rpm for 10 min to afford serums sample. The spleen sample and serum sample were stored at −80°C for analysis.
2.3.2. Content assay of IL-2, IL-10, IFN-γ, iNOS, CD4, CD8, cAMP and cGMP in spleen and/or serum by ELISA
Contents of IL-2, IL-10, IFN-γ, iNOS, CD4, CD8, cAMP and cGMP in spleen and/or serum were measured by the method of ELISA. The frozen spleen was homogenized in ice-cold 0.9% NaCl solution (0.1 g tissue/mL solution) to afford spleen suspension, then the suspension was centrifuged at 4000 rpm for 10 min, and the supernatant was collected for ELISA. The frozen serum was defrosted at room temperature for ELISA. Commercial ELISA kits, used according to their instruction manual, were applied in the present work and absorbance of each well was determined by an ELISA microplate reader (iMark, Bio-RAD, Japan). The relative content of sample group to control group was calculated by the following equation: Relative content = C1/C0, where C1 is the concentration of sample group and C0 is the concentration of control group.
2.3.3. Assay of mRNA content of IL-2, IL-10, IFN-γ, CD4 and CD8 in spleen by RTFQ-PCR
Determination of mRNA content of IL-2, IL-10, IFN-γ, CD4 and CD8 in spleen was carried out by RTFQ-PCR according to the reported method with slight modification (Liao & Lin, Citation2013; Zhang et al., Citation2013). Toll RNA was extracted by Column Animal total RNA Purification Kit, then, cDNA was synthesized by M-MuLV First Strand cDNA Synthesis Kit in peltier thermal cycler (PTC-200, Bio-RAD, USA). Finally, RTFQ-PCR was done by SGExcel FastSYBR Mixture Kit in real-time thermal cycler (5100, Thermo Fisher Scientific, USA). Primers of IL-2, IL-10, IFN-γ, CD4 and CD8 were designed and synthesized by Sangon Biotech (Shanghai) Co. Ltd., China, and the prime sequences were presented in . Mouse β-actin, a stably expressed house-keeping gene, was selected as an endogenous reference gene. Primer of the mouse β-actin (order no.: B661302) was purchased from Sangon Biotech (Shanghai) Co. Ltd., China.
Table 1. Prime sequences of IL-2, IL-10, IFN-γ, CD4 and CD8 used in RTFQ-PCR.
RTFQ-PCR product of endogenous reference gene and target gene were observed by agarose gel electrophoresis. The relative mRNA contents of IL-2, IL-10, IFN-γ, CD4 and CD8 in spleen were determined by the method of comparing Ct value, where the Ct value is the threshold cycle number of target or reference gene (Liao & Lin, Citation2013). The relative mRNA content of sample group to control group was calculated by the following equation: Relative mRNA content = 2X and X = (T0−T1)−(R0−R1), where T0 is the Ct value of target gene in control group, T1 is the Ct value of target gene in sample group, R0 is the Ct value of endogenous reference gene in control group and R1 is the Ct value of endogenous reference gene in sample group.
2.4. Statistical analysis
The data were reported as mean ± standard deviation (SD) and evaluated by one-way analysis of variance (ANOVA) followed by the Duncan’s multiple-range tests. Difference was considered to be statistically significant if P < .05. All statistical analyses were carried out by using SPSS for Windows, Version11.5 (SPSS, Chicago, IL).
3. Results and discussion
3.1. Influences of HCPS on immunosignaling molecules
3.1.1. Effects of HCPS on cytokines of IL-2, IL-10 and IFN-γ
To evaluate the effects of crude HCPS on the production of IL-2, IL-10 and IFN-γ in vivo, the level and the mRNA content of IL-2, IL-10 and IFN-γ was investigated by ELISA and RTFQ-PCR, respectively. displayed the electrophoresis spectra of DNA marker and RTFQ-PCR product of β-actin, IL-2, IL-10 and IFN-γ. Two fractions (about 175 bp and 275 bp) were amplified in endogenous reference gene of β-actin, the fraction length of IL-2, IL-10 and IFN-γ was about 120 bp, 200 bp and 180 bp, respectively. The actions of crude HCPS in vivo on the gene expression and the mRNA synthesis of IL-2, IL-10 and IFN-γ in spleen and serum were showed in , and . Significant decreases (P < .05) of relative level and mRNA content of IL-2 and IFN-γ were observed after treatment of CPA in Group II (model group) compared with Group I (control group with relative content of 1.0). In group III (crude HCPS of low dose), there were increases of relative level and mRNA content of IL-2 and IFN-γ than that in Group II. From group III to group V (crude HCPS of high dose), the relative level and mRNA content of IL-2 and IFN-γ increased significantly (P < .05) with the dose increase of crude HCPS. While, the variation tendency of crude HCPS acting on IL-10 was contrary to that on IL-2 and IFN-γ. These results demonstrated that hypodermic injection of CPA could suppress the production of IL-2 and IFN-γ, and promote the production of IL-10 significantly. Gastric gavage of crude HCPS could improve the production of IL-2 and IFN-γ, and suppress the production of IL-10 in a dose-dependent manner. The crude HCPS was able to act against the suppression of IL-2 and IFN-γ, and the promotion of IL-10 induced by CPA in vivo.
Figure 1. Electrophoresis spectra of DNA marker and RTFQ-PCR products of endogenous reference gene (β-actin) and target genes (IL-2, CD4, CD8, IL-10 and IFN-γ). Channel M: DNA marker; Channel 1: IL-2 (about 120 bp); Channel 2: CD4 (about 115 bp); Channel 3: CD8 (about 130 bp); Channel 4: β-actin (about 275 bp and 175 bp); Channel 5: IL-10 (about 200 bp); Channel 6: IFN-γ (about 180 bp).
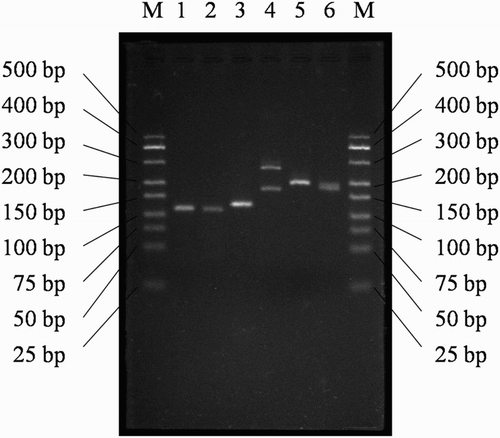
Table 2. Effects of crude HCPS on the content of IL-2, IL-10, IFN-γ, iNOS, CD4, CD8 and cAMP in spleen in vivo by ELISA.
Table 3. Effects of crude HCPS on the mRNA synthesis of IL-2, IL-10, IFN-γ, CD4 and CD8 in spleen in vivo by RTFQ-PCR.
Table 4. Effects of crude HCPS on the content of IL-10 and cGMP in serum in vivo by ELISA.
It has been reported that IL-2 was secreted mainly by activated T cells and had many immunopotentiating effects, such as proliferation of T cells, B-cells, NK cells and monocytes, augmentation of cytotoxicites of T cells and NK cells and in vivo generation of lymphokine-activated killer (LAK) cells, which exhibit high cytolytic activities against autologous tumor cells. In addition to these, IL-2 could also enhance secretion and activity of cytokines and antibody, induce apoptosis of activated T cells to prevent autoimmunity (Gaffen & Liu, Citation2004; Sun et al., Citation2014). Although initially defined as an agent with direct antiviral activity, IFN-γ has a multitude of biological functions including regulation of several aspects of the immune responses, stimulation of antigen presentation via up-regulating major histocompatibility complex (MHC) molecules, promotion of leukocyte-endothelium interactions, stimulation of inflammatory mediator production in target cells and recruitment of cells to the site of injury through enhancing expression of chemokines and adhesion molecules (Schroder, Hertzog, Ravasi, & Hume, Citation2004). As an anti-inflammatory cytokine, IL-10 is an immunosuppressive cytokine that regulates immune responses by inhibiting the ability of APCs to present antigens to T cells. IL-10 could suppress the antigen presentation ability of DCs, monocytes and macrophages, all of which are required for optimal pathogen clearance (Mittal & Roche, Citation2015). During inflammation, IL-10 may act in a negative feedback mechanism to prevent deleterious effects from excessive macrophage activation (Yamassaki et al., Citation2015). This result, crude HCPS by gastric gavage could improve the production of IL-2 and IFN-γ, and inhibit the production of IL-10 in a dose-dependent manner, implied that: When HCPS executed their immunostimulatory activities, IL-2 and IFN-γ might be induced and act as the immune regulators modulating innate and adaptive immune responses, IL-10 might be suppressed and act in a negative feedback mechanism to protect host from excessive tissue damage.
3.1.2. Effects of HCPS on gas signaling molecule of NO
NO, synthesized from L-arginine by NOS, could mediate a variety of biological functions as an intracellular gas signaling molecule (Kong et al., Citation2014). iNOS is a main enzyme responsible for the NO production (Jiang, Okimura, Yamaguchi, & Oda, Citation2011; Zhang & Dai, Citation2011). In this paper, effects of HCPS on the production of iNOS were analyzed. The promoting actions of crude HCPS in vivo on the gene expression of iNOS in spleen were showed in . From , results were obtained that CPA by hypodermic injection could suppress the gene expression of iNOS significantly (P < .05), and crude HCPS by gastric gavage was able to enhance the gene expression of iNOS in a dose-dependent manner. The crude HCPS could act against the suppression of iNOS induced by CPA in vivo. presented the promoting effects of crude HCPS and its purified fractions (HCPS-1, HCPS-2 and HCPS-3) in vitro on the gene expression of iNOS in peritoneal macrophage. In group II (50 μg/mL), there was no significantly difference (P > .05) in the ability of improving gene expression of iNOS among crude HCPS, HCPS-1, HCPS-2 and HCPS-3. But, the promoting effects of HCPS increased significantly (P < .05) with the increase of sample concentration ranging from 50 to 200 μg/mL, and at the concentration of 200 μg/mL, the differences in the capability of strengthening gene expression of iNOS among crude HCPS, HCPS-1, HCPS-2 and HCPS-3 were significant (P < .05). About the improving effects on gene expression of iNOS, it was HCPS-3, crude HCPS, HCPS-2 and HCPS-1 in orderly from strongest to weakest. This result demonstrated that crude HCPS and its purified fractions (HCPS-1, HCPS-2 and HCPS-3) could promote peritoneal macrophage in vitro producing iNOS in a dose-dependent manner.
Figure 2. Effects of HCPS on the gene expression of iNOS in peritoneal macrophage in vitro by ELISA. Group I: control group; Group II: low dose of HCPS (50 μg/mL); Group III: medium dose of HCPS (100 μg/mL); Group IV: high dose of HCPS (200 μg/mL). Different letter (a, b, c, d, e, f) indicted there was significant difference (P < .05) between groups. Same letter suggested difference between groups was not significant (P > .05).
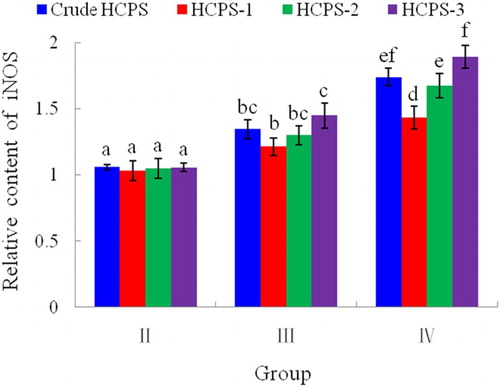
NO holds many biological functions, such as, vasodilatation, neurotransmission, immunoresponse and plateletaggregation (Lee et al., Citation2015). NO also participates in the cytolytic function of macrophages (Lee & Jeon, Citation2003). iNOS-derived NO plays an important role in numerous physiological and pathophysiological conditions (Wen et al., Citation2014). The augmentation of iNOS activity could result in the enhancement of NO production in activated macrophages. The present result, HCPS could promote the gene expressing of iNOS in a dose-dependent manner, implied that HCPS might induce NO production by up-regulating the expression of iNOS when HCPS exerted their immunomodulatory functions. However, it has been stated that NO has dual effects. Low concentration of NO has been shown to have protective effects, while high concentration to be cytotoxic (Chen, Zhang, Shen, & Wang, Citation2010). About the reasonable amount of iNOS induced by HCPS, which produce reasonable NO concentration for protecting effects, it needs further research.
3.1.3. Effects of HCPS on second messengers of cAMP and cGMP
It is widely accepted that cAMP and cGMP are two of the second messengers, which play important roles in cellular signal transduction. To evaluate the effects of crude HCPS on the second messenger in vivo, the levels of cAMP in spleen and cGMP in serum were measured by ELISA. From and , it was harvested that the variation tendency of crude HCPS acting on cAMP and cGMP in vitro was similar to that on IL-10 and IL-2, respectively. Hypodermic injection of CPA could promote the production of cAMP and suppress the production of cGMP significantly (P < .05), and gastric gavage of crude HCPS could inhibit the production of cAMP and improve the production of cGMP in a dose-dependent manner. The crude HCPS was able to recruit the promotion of cAMP and the suppression of cGMP induced by CPA in vivo. In generally, cAMP exerts suppressive effects on the functions of inflammatory and immunocompetent cells (Horrigan, Kelly, & Connor, Citation2006), cGMP could promote the proliferation of lymphocyte and performs positive immunoregulatory activities (Bai et al., Citation1997). It has been reported that cAMP and cGMP was activator of protein kinase A(PKA) and protein kinase C (PKC), respectively. The cGMP and cAMP signaling pathways were linked, and many cellular functions were bidirectional regulated by both cAMP and cGMP (Zhang, Nie, et al., Citation2014). The present results suggested that suppressing the cAMP cascade and enhancing the cGMP cascade, subsequently effecting the expressing of downstream immunosignaling molecules might be one of the immunomodulatory mechanisms of crude HCPS.
3.2. Influences of HCPS on T lymphocyte differentiation
T cells and their subpopulations (CD4+ and CD8+ T cells) play a crucial role in immunomodulation, eradicating viruses, preventing viral persistence and inducing the death of tumor cells (Feng et al., Citation2015; Shen et al., Citation2013). According to their specific surface molecule, T lymphocytes could differentiate into two different subsets, CD4+ and CD8+ T cell with CD4 and CD8 surface marker, respectively (Shen et al., Citation2013). In this work, effects of crude HCPS on CD4 and CD8 in spleen were analyzed in vivo by ELISA and RTFQ-PCR. The electrophoresis spectrum of RTFQ-PCR product of CD4 and CD8 were showed in , and the fraction length of CD4 and CD8 was about 115 bp and 130 bp, respectively. and presented the improving effects of crude HCPS in vivo on their gene expression and mRNA synthesis, respectively. The variation tendency of crude HCPS in vivo acting on CD4 and CD8 in spleen was similar to that on IL-2, hypodermic injection of CPA could suppress the production of CD4 and CD8 significantly (P < .05), and gastric gavage of crude HCPS could improve the production of CD4 and CD8 in a dose-dependent manner. The crude HCPS was able to retard the suppression of CD4 and CD8 induced by CPA in vivo.
It is well-known that CD4+ and CD8+ are T helper (Th) and T cytotoxic (Tc) lymphocytes, respectively (Wang et al., Citation2015; Yu et al., Citation2014), which are two common T lymphocytes important for adaptive immunity (Yu et al., Citation2014). Main function of CD4+ T lymphocytes is the secretion of cytokines, thereby inducing and enhancing immune response (Zhang, Yang, et al., Citation2014). And CD8+ T cells mediate pathogen clearance, which could recognize and kill the infected host cells (Fan et al., Citation2016). If the proportion of CD4+ and CD8+ T cells subpopulations increase, the immune responses will also be promoted (Feng et al., Citation2015). This work, crude HCPS could improve the production of CD4 and CD8 in a dose-dependent manner, indicated that promoting differentiation of T lymphocytes to CD4+ and CD8+ T cells, which could enhance immune response, by enhancing amount of CD4 and CD8 molecules might be another one of the immunomodulatory mechanisms of crude HCPS.
4. Conclusion
In the present work, influences of HCPS on mice immunosignaling molecules (IL-2, IL-10, IFN-γ, NO, cAMP and cGMP) and T lymphocyte differentiation (to CD4+ and CD8+ T cells) were evaluated by cell model in vitro and/or CPA-induced immunosuppression animal model in vivo to discuss the molecular mechanism of immunoenhancing activities of HCPS. Levels of IL-2, IL-10, IFN-γ, iNOS, cAMP, cGMP, CD4 and CD8 in spleen, serum and/or peritoneal macrophage were measured by ELISA, and mRNA contents of IL-2, IL-10, IFN-γ, CD4 and CD8 in spleen were carried out by RTFQ-PCR. The results demonstrated that gastric gavage of crude HCPS could promote the mRNA synthesis of IL-2, IFN-γ, CD4 and CD8, and the production of IL-2, IFN-γ, iNOS, cGMP, CD4 and CD8 in a dose-dependent manner. While, crude HCPS by gastric gavage exhibited suppressive effects on the mRNA synthesis of IL-10, and the production of IL-10 and cAMP in a dose-dependent manner. Crude HCPS and its purified fractions (HCPS-1, HCPS-2 and HCPS-3) could strengthen peritoneal macrophage expressing iNOS in vitro in a dose-dependent manner. Take together, when HCPS executed their immunostimulatory activities, IL-2 and IFN-γ might be induced and act as the immune regulators modulating innate and adaptive immune responses, IL-10 might be suppressed and act in a negative feedback mechanism to protect host from excessive tissue damage. NO might be induced by up-regulating the expression of iNOS. cAMP cascade and cGMP cascade might be inhibited and enhanced, respectively, subsequently effecting the expressing of downstream immunosignaling molecules. Differentiation of T lymphocytes (to CD4+ and CD8+ T cells) might be promoted by enhancing the expression of CD4 and CD8. So, it was implied that HCPS-stimulated immunostrengthening functions was mediated, at least in part, by immunosignaling molecules of IL-2, IL-10, IFN-γ, NO, cAMP and cGMP, and T lymphocyte differentiation (to CD4+ and CD8+ T cells). Further works, about the expression of other signaling molecules involved in immune response, and how do immunosignaling molecules affect each other when administrated with HCPS, are in progress.
Conflict of interest
The authors declare that there is no conflict of interest.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Deliang Qiao got his bachelor's from Zhejiang Ocean University (Zhoushan, China) and got his Ph.D. in Nanjing Agricultural University (Nanjing, China). His research interests are food nutrition and food chemistry.
Chuanbao Wei got his bachelor's from Anhui University (Hefei, China) and got his Ph.D. in Zhejiang University (Hangzhou, China). His research interests are food chemistry and molecular nutrition.
Naidong Chen got his bachelor's from Anhui Normal University (Wuhu, China) and got his Ph.D. in China Pharmaceutical University (Nanjing, China). His research interests are pharmacology and medicinal chemistry.
Yunjiang Min got his bachelor's from Anhui Normal University (Wuhu, China) and got his Ph.D. in Anhui University (Hefei, China). His research interests are food chemistry and molecular nutrition.
Haijun Xu got his bachelor's from Zhejiang University (Hangzhou, China) and got his Ph.D. in University of Chinese Academy of Sciences (Beijing, China). His research interests are food nutrition and immunology.
Rui Chen got his bachelor's from Wuhan Institute of Technology (Wuhan, China) and now studying for Ph.D. in Wuhan Institute of Technology (Wuhan, China). His research interests are food biotechnology.
Additional information
Funding
References
- Bai, R., Ma, D., Wang, J., & Yu, H. (1997). Effect of letinan on the content of cGMP and cAMP in serum, thymus and spleen of mice. Journal of Xi’an Medical University, 18(1), 58–59, 69 (in Chinese). Retrieved from http://www.cnki.net/KCMS/detail/detail.aspx?QueryID=5&CurRec=5&dbcode=CJFQ&dbname=CJFD9697&filename=XAYX199701020&urlid=&yx=&v=Mjk0NDNVUkx5ZVplUnZGeWpsVWIvUFBTelNkckt4RjliTXJvOUhaSVI4ZVgxTHV4WVM3RGgxVDNxVHJXTTFGckM=
- Chen, W., Zhang, W., Shen, W., & Wang, K. (2010). Effects of the acid polysaccharide fraction isolated from a cultivated Cordyceps sinensis on macrophages in vitro. Cellular Immunology, 262, 69–74. doi:10.1016/j.cellimm.2010.01.001 doi: 10.1016/j.cellimm.2010.01.001
- Dai, Z., Zhang, H., Zhang, Y., & Wang, H. (2009). Chemical properties and immunostimulatory activity of a water-soluble polysaccharide from the calm of Hyriopsis cumingii Lea. Carbohydrate Polymers, 77(2), 365–369. doi:10.1016/j.carbpol.2009.01.003 doi: 10.1016/j.carbpol.2009.01.003
- Fang, X., & Chen, X. (2013). Structure elucidation and immunological activity of a novel pectic polysaccharide from the stems of Avicennia marina. European Food Research and Technology, 236, 243–248. doi:10.1007/s00217-012-1877-6 doi: 10.1007/s00217-012-1877-6
- Fan, Y., Ma, X., Ma, L., Zhang, J., Zhang, W., & Song, X. (2016). Antioxidative and immunological activities of ophiopogon polysaccharide liposome from the root of Ophiopogon japonicus. Carbohydrate Polymers, 135, 110–120. doi:10.1016/j.carbpol.2015.08.089 doi: 10.1016/j.carbpol.2015.08.089
- Feng, H., Fan, J., Qiu, H., Wang, Z., Yan, Z., Yuan, L., … Liu, J. (2015). Chuanminshen violaceum polysaccharides improve the immune responses of foot-and-mouth disease vaccine in mice. International Journal of Biological Macromolecules, 78, 405–416. doi:10.1016/j.ijbiomac.2015.04.044 doi: 10.1016/j.ijbiomac.2015.04.044
- Gaffen, S. L., & Liu, K. D. (2004). Overview of interleukin-2 function, production and clinical applications. Cytokine, 28, 109–123. doi:10.1016/j.cyto.2004.06.010 doi: 10.1016/j.cyto.2004.06.010
- Hao, L. X., & Zhao, X. H. (2016). Immunomodulatory potentials of the water-soluble yam (Dioscorea opposita Thunb) polysaccharides for the normal and cyclophosphamide-suppressed mice. Food and Agricultural Immunology, 27, 667–677. doi:10.1080/09540105.2016.1148666 doi: 10.1080/09540105.2016.1148666
- Horrigan, L. A., Kelly, J. P., & Connor, T. J. (2006). Immunomodulatory effects of caffeine: Friend or foe? Pharmacology & Therapeutics, 111, 877–892. doi:10.1016/j.pharmthera.2006.02.002 doi: 10.1016/j.pharmthera.2006.02.002
- Jiang, Z., Okimura, T., Yamaguchi, K., & Oda, T. (2011). The potent activity of sulfated polysaccharide, ascophyllan, isolated from Ascophyllum nodosum to induce nitric oxide and cytokine production from mouse macrophage RAW264.7 cells: Comparison between ascophyllan and fucoidan. Nitric Oxide, 25, 407–415. doi:10.1016/j.niox.2011.10.001 doi: 10.1016/j.niox.2011.10.001
- Kong, F., Li, F. E., He, Z., Jiang, Y., Hao, R., Sun, X., & Tong, H. (2014). Anti-tumor and macrophage activation induced by alkali-extracted polysaccharide from Pleurotus ostreatus. International Journal of Biological Macromolecules, 69, 561–566. doi:10.1016/j.ijbiomac.2014.05.045 doi: 10.1016/j.ijbiomac.2014.05.045
- Kumar, S., & Tiku, A. B. (2016). Immunomodulatory potential of acemannan (polysaccharide from Aloe vera) against radiation induced mortality in Swiss albino mice. Food and Agricultural Immunology, 27, 72–86. doi:10.1080/09540105.2015.1079594 doi: 10.1080/09540105.2015.1079594
- Lee, K. Y., & Jeon, Y. J. (2003). Polysaccharide isolated from Poria cocos sclerotium induces NF-κB/Rel activation and iNOS expression in murine macrophages. International Immunopharmacology, 3, 1353–1362. doi:10.1016/S1567-5769(03)00113-9 doi: 10.1016/S1567-5769(03)00113-9
- Lee, S. G., Jung, J. Y., Shin, J. S., Shin, K. S., Cho, C. W., Rhee, Y. K., … Lee, K. T. (2015). Immunostimulatory polysaccharide isolated from the leaves of Diospyros kaki Thumb modulate macrophage via TLR2. International Journal of Biological Macromolecules, 79, 971–982. doi:10.1016/j.ijbiomac.2015.06.023 doi: 10.1016/j.ijbiomac.2015.06.023
- Lee, S. J., Rim, H. K., Jung, J. Y., An, H. J., Shin, J. S., Cho, C. W., … Lee, K. T. (2013). Immunostimulatory activity of polysaccharides from Cheonggukjang. Food and Chemical Toxicology, 59, 476–484. doi:10.1016/j.fct.2013.06.045 doi: 10.1016/j.fct.2013.06.045
- Liao, C. H., & Lin, J. Y. (2013). Purified active lotus plumule (Nelumbo nucifera Gaertn) polysaccharides exert anti-inflammatory activity through decreasing toll-like receptor-2 and -4 expressions using mouse primary splenocytes. Journal of Ethnopharmacology, 147, 164–173. doi:10.1016/j.jep.2013.02.028 doi: 10.1016/j.jep.2013.02.028
- Liu, Z., Xing, J., Huang, Y., Bo, R., Zheng, S., Luo, L., … Wang, D. (2016). Activation effect of Ganoderma lucidum polysaccharides liposomes on murine peritoneal macrophages. International Journal of Biological Macromolecules, 82, 973–978. doi:10.1016/j.ijbiomac.2015.10.088 doi: 10.1016/j.ijbiomac.2015.10.088
- Mittal, S. K., & Roche, P. A. (2015). Suppression of antigen presentation by IL-10. Current Opinion in Immunology, 34, 22–27. doi:10.1016/j.coi.2014.12.009 doi: 10.1016/j.coi.2014.12.009
- Qiao, D., Hu, B., Gan, D., Sun, Y., Ye, H., & Zeng, X. (2009). Extraction optimized by using response surface methodology, purification and preliminary characterization of polysaccharides from Hyriopsis cumingii. Carbohydrate Polymers, 76(3), 422–429. doi:10.1016/j.carbpol.2008.11.004 doi: 10.1016/j.carbpol.2008.11.004
- Qiao, D., Luo, J., Ke, C., Sun, Y., Ye, H., & Zeng, X. (2010). Immunostimulatory activities of the polysaccharides from Hyriopsis cumingii. International Journal of Biological Macromolecules, 47(5), 676–680. doi:10.1016/j.ijbiomac.2010.08.014 doi: 10.1016/j.ijbiomac.2010.08.014
- Sansone, M., Sansone, A. C. M. B., Shiga, T. M., & Nascimento, J. R.O. (2016). The water-soluble non-starch polysaccharides from bananas display immunomodulatory properties on cultured macrophages. Food Research International, 87, 125–133. doi:10.1016/j.foodres.2016.07.003 doi: 10.1016/j.foodres.2016.07.003
- Schroder, K., Hertzog, P. J., Ravasi, T., & Hume, D. A. (2004). Interferon-?: An overview of signals, mechanisms and functions. Journal of Leukocyte Biology, 75, 163–189. doi:10.1189/jlb.0603252. doi: 10.1189/jlb.0603252
- Shen, H., Tang, G., Zeng, G., Yang, Y., Cai, X., Li, D., … Zhou, N. (2013). Purification and characterization of an antitumor polysaccharide from Portulaca oleracea L. Carbohydrate Polymers, 93, 395–400. doi:10.1016/j.carbpol.2012.11.107 doi: 10.1016/j.carbpol.2012.11.107
- Sun, X., Gao, R. L., Xiong, Y. K., Huang, Q. C., & Xu, M. (2014). Antitumor and immunomodulatory effects of a water-soluble polysaccharide from Lilii Bulbus in mice. Carbohydrate Polymers, 102, 543–549. doi:10.1016/j.carbpol.2013.12.002 doi: 10.1016/j.carbpol.2013.12.002
- Sun, H., Zhang, J., Chen, F., Chen, X., Zhou, Z., & Wang, H. (2015). Activation of RAW264.7 macrophages by the polysaccharide from the roots of Actinidia eriantha and its molecular mechanisms. Carbohydrate Polymers, 121, 388–402. doi:10.1016/j.carbpol.2014.12.023 doi: 10.1016/j.carbpol.2014.12.023
- Wang, Y., Huang, M., Sun, R., & Pan, L. (2015). Extraction, characterization of a Ginseng fruits polysaccharide and its immune modulating activities in rats with Lewis lung carcinoma. Carbohydrate Polymers, 127, 215–221. doi:10.1016/j.carbpol.2015.03.070 doi: 10.1016/j.carbpol.2015.03.070
- Wen, Z. S., Liu, L. J., OuYang, X. K., Qu, Y. L., Chen, Y., & Ding, G. F. (2014). Protective effect of polysaccharides from Sargassum horneri against oxidative stress in RAW264.7 cells. International Journal of Biological Macromolecules, 68, 98–106. doi:10.1016/j.ijbiomac.2014.04.037 doi: 10.1016/j.ijbiomac.2014.04.037
- Yamassaki, F. T., Lenzi, R. M., Campestrini, L. H., Bovo, F., Seyfried, M., Soldera-Silva, A., … Maurer, J. B. B. (2015). Effect of the native polysaccharide of cashew-nut tree gum exudate on murine peritoneal macrophage modulatory activities. Carbohydrate Polymers, 125, 241–248. doi:10.1016/j.carbpol.2015.02.041 doi: 10.1016/j.carbpol.2015.02.041
- Yu, Q., Nie, S. P., Wang, J. Q., Liu, X. Z., Yin, P. F., Huang, D. F., … Xie, M. Y. (2014). Chemoprotective effects of Ganoderma atrum polysaccharide in cyclophosphamide-induced mice. International Journal of Biological Macromolecules, 64, 395–401. doi:10.1016/j.ijbiomac.2013.12.029 doi: 10.1016/j.ijbiomac.2013.12.029
- Zhang, N., & Bevan, M. J. (2011). CD8+ t cells: Foot soldiers of the immune system. Immunity, 35, 161–168. doi:10.1016/j.immuni.2011.07.010 doi: 10.1016/j.immuni.2011.07.010
- Zhang, C. X., & Dai, Z. R. (2011). Immunomodulatory activities on macrophage of a polysaccharide from Sipunculus nudus L. Food and Chemical Toxicology, 49, 2961–2967. doi:10.1016/j.fct.2011.07.044 doi: 10.1016/j.fct.2011.07.044
- Zhang, P., Ding, R., Jiang, S., Ji, L., Pan, M., Liu, L., … Ji, H. (2014). The adjuvanticity of Ganoderma lucidum polysaccharide for Newcastle disease vaccine. International Journal of Biological Macromolecules, 65, 431–435. doi:10.1016/j.ijbiomac.2014.01.067 doi: 10.1016/j.ijbiomac.2014.01.067
- Zhang, S., Nie, S., Huang, D., Huang, J., Feng, Y., & Xie, M. (2014). Ganoderma atrum polysaccharide evokes antitumor activity via cAMP-PKA mediated apoptotic pathway and down-regulation of Ca2+/PKC signal pathway. Food and Chemical Toxicology, 68, 239–246. doi:10.1016/j.fct.2014.03.020 doi: 10.1016/j.fct.2014.03.020
- Zhang, X., Wang, J., Xu, Z., Li, Z., Feng, S., & Lu, H. (2013). The impact of rhubarb polysaccharides on toll-like receptor 4-mediated activation of macrophages. International Immunopharmacology, 17, 1116–1119. doi:10.1016/j.intimp.2013.10.015 doi: 10.1016/j.intimp.2013.10.015
- Zhang, Y., Yang, S., Zhao, X., Yang, Y., Li, B., Zhu, F., & Zhu, R. (2014). Immune enhancement of Taishan Robinia pseudoacacia polysaccharide on recombinant Proteus mirabilis OmpA in chickens. International Immunopharmacology, 22, 236–241. doi:10.1016/j.intimp.2014.06.036 doi: 10.1016/j.intimp.2014.06.036
