ABSTRACT
A sensitive monoclonal antibody, 3G10, derived from a mouse immunized with an immunogen of salbutamol hapten conjugated to keyhole limpet hemocyanin was developed against β-agonists, with a view to establishing a rapid gold nanoparticle immunochromatographic strip. The antibody displayed a 50% inhibitory concentration (IC50) of 1.04 ng/mL for salbutamol and simultaneously detected nine other β-agonists (clenbuterol, cimatero, brombuterol, mabuterol, terbutaline, clenpenterol, carbuterol, mapenterol, pirbuterol) with uniform cross-reactivity. The immunochromatographic strip had cut-off values of 5 ng/mL for salbutamol and clenbuterol, 20 ng/mL for cimaterol, brombuterol and mabuterol, and 50 ng/mL for terbutaline, clenpenterol, carbuterol, mapenterol and pirbuterol. The collective advantages of the newly developed strip, such as high affinity, sensitivity and rapidity, support its utility in detecting β-agonists from actual biological samples, such as urine.
Introduction
β-Agonists are a class of sympathomimetic drugs that inhibit growth of animal fat and increase lean meat yield (Johnson, Smith, & Chung, Citation2014), including salbutamol (sal), clenbuterol, cimaterol, brombuterol, mabuterol, terbutaline (Zhang et al., Citation2015), clenpenterol (González-Antuña, Rodríguez-González, Centineo, & García Alonso, Citation2014), carbuterol, mapenterol (Tao et al., Citation2014) and pirbuterol. These drugs have selective effects on animal adrenergic receptors, enhancing fat decomposition and promoting protein synthesis (Weber et al., Citation2013). However, β-agonists often trigger dizziness, chest tightness, heart palpitations, fatigue, nausea, vomiting, rapid heartbeat as well as muscle, hand and foot tremors (Brett, Dawson, & Brown, Citation2014). Residues of clenbuterol have been detected at concentrations of 0.05–0.6 μg/kg in horse and cattle tissues as well as products approved by the Codex Alimentarius Commission (CAC), European Union (EU) and Japan. Ractopamine (RCT) residues are present at concentrations of 10–450 μg/kg in pork, beef, turkey and derived products in the CAC, USA, Australia and Japan. Various food products have tested negative for β-agonists in China. China’s Ministry of Agriculture issued 176, 193, 235, 1519 and other announcements, which made it clear that the β-agonist should not be used as an additive for animal feed.
Currently, β-agonists are detected using a number of methods, including chromatography, immunosorbent assay (Gu, Liu, Song, Kuang, & Xu, Citation2015) and biosensors (Chen et al., Citation2016; Özkütük, Uğurağ, Ersöz, & Say, Citation2016). Chromatographic procedures include liquid chromatography, gas chromatography, high-performance liquid chromatography (HPLC) (Sai, Hong, Yunfeng, Huijing, & Yongning, Citation2012; Yan et al., Citation2016), ultra-performance liquid chromatography (UPLC) (Gao, Ye, & Li, Citation2015; Mauro et al., Citation2014; Wu et al., Citation2015), liquid chromatography-mass spectrometry (LC-MS) (Amelin, Korolev, & Tret’yakov, Citation2015; Diru et al., Citation2016) and capillary electrophoresis (Nguyen et al., Citation2014; Pérez-Silva et al., Citation2016). While these procedures offer the advantages of high sensitivity and specificity, their usage is limited owing to complex pre-treatment processes and expensive equipment (Yan, Liu, Xu, Kuang, & Xu, Citation2015).
Immunoassays, including enzyme-linked immunosorbent assay (ELISA) (Bui et al., Citation2016; Du et al., Citation2016; Wu et al., Citation2014), fluorescence immunoassay (FLA) (Cao et al., Citation2015; Zou et al., Citation2008) and colloidal gold immunoassay (Zhang et al., Citation2016), are based on antigen–antibody reactions. The ELISA is a simple and sensitive method (Ren, Zhang, Chen, & Yang, Citation2009), but not conducive to large-scale sample testing. More recently, immunosorbent technology linked to nanomaterials was developed to prepare immunochromatographic strips. This newly developed technology has the advantages of immunization on the one hand and detection of a large number of samples on the other. In addition, the matrix does not interfere with solid-phase assay conditions.
Since the standards of drug testing are extremely strict in China, rapid and sensitive detection methods need to be established. To meet the market demand for rapid detection, we prepared a monoclonal antibody-based gold nanoparticle immunochromatographic strip that is able to effectively detect 10 different β-2 stimulants.
Materials and methods
Chemicals
Albuterol sulfate, clenbuterol, cimaterol, brombuterol hydrochloride, mabuterol, terbutaline, clenpenterol hydrochloride, carbuterol, mapenterol, pirbuterol, rimethylamine and sodium triacetoxyborohydride [NaBH(OAc)3] were purchased from J&K Scientific (Shanghai, China). Ovalbumin (OVA; MW, 43,000 Da), keyhole limpet hemocyanin (KLH; MW, 500,0000 Da) and bovine serum albumin (BSA; MW, 67,000) were acquired from Sigma Chemical Company (St. Louis, MO, USA), and 2-methylpropyl carbonochloridate, N-hydroxysuccinimide (NHS), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), enzyme immunoassay-grade horseradish peroxidase (HRP)-labeled goat anti-mouse immunoglobulin, 3,3′,5,5′-tetramethylbenzidine (TMB), Tween-20, HRP and gelatin from Sigma–Aldrich (St. Louis, MO, USA). Polyethylene glycol solution, HAT supplement, HT supplement and 1640 cell culture were obtained from Gibco (Shanghai, China). Materials for the immunochromatographic strips were purchased from JieYi Biotech, Co., Ltd. (Shanghai, China), including glass fiber membrane for the sample pad, NC membrane for immobilizing the coating antigen, H5076 filter paper for the absorbent pad and Ahlstrom 8964 for the conjugated pad.
Solutions
The solutions used for assay included phosphate-buffered saline (PBS: 0.01 M phosphate, pH 7.4), coating buffer (CB: 0.05 M carbonate bicarbonate, pH 9.6), washing buffer (PBST: 0.05% Tween-20 (v/v) in 0.01 M PBS), blocking buffer (0.2% gelatin added in coating buffer), antibody dilution buffer (0.1% gelatin added in PBS), solution A (citric acid, H2O2, and Na2HPO4) and solution B (0.06% m/v TMB in glycol). Solutions A and B were applied at a 5:1 (v/v) ratio, which is color buffer. Urine was obtained from a local farm (Wuxi, China).
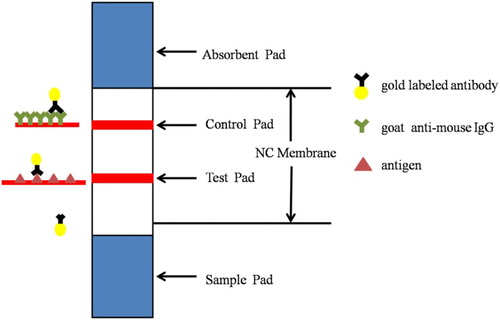
The principle of immunochromatographic strip test
The structure of a test strip is shown in . Test line (T-line) was combined with the antigen. Control line (C-line) was goat anti-mouse IgG. The sample solution reacted with gold-labeled monoclonal antibody (mAb) was added to the sample pad and then moved to the test line direction. If substance is absent, colloidal gold-labeled conjugate with antigen and test line emerges clear red. If the target substance is present in the sample, it competes with the antigen for gold-labeled mAb. Therefore, the higher the concentration of the substance to be measured, the lighter the test line color.
Antigen preparation
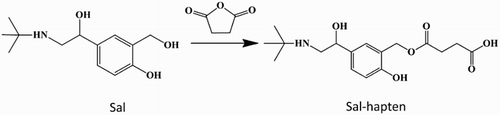
Since the majority of β-agonists have similar chemical structures, we selected salbutamol for derivatization of hapten (Liu, Kuang, Peng, Wang, & Xu, Citation2014). Salbutamol hapten (sal-hapten) was synthesized using the succinic anhydride method (). The immunogen and coating antigen were hapten salbutamol coupled with protein (KLH or OVA) (sal-KLH or sal-OVA). Firstly, 10 mg sal-hapten was dissolved in 800 μL dimethylformamide (DMF) and 1 mL of 0.1 M 2-[N-morpholino] ethanesulfonic acid, pH 4.7. Next, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and NHS (n/n/n, sal-hapten/EDC/NHS = 1:3:3) (referred to as solution I) were added and stirred overnight at room temperature in the dark. KLH or OVA was dissolved in 2 mL of 0.1 M CB, pH 9.6. The dissolved protein solution was added to solution I and stirred overnight at room temperature in the dark. Protein solutions were dialyzed with 0.01 M PBS for 2 days to remove unbound sal-hapten. UV-Visible spectrophotometry (UV) and polyacrylamide gel electrophoresis were employed to confirm the presence of antigen, which was subsequently stored at –20°C.
Monoclonal antibody preparation
BALB/c mice (6–8 weeks of age) were initially immunized via subcutaneous injection of immunogen (100 μg per mouse) mixed with Freund’s complete adjuvant. Four weeks later, mice were immunized with Freund’s incomplete adjuvant mixed with immunogen (50 μg per mouse), which was repeated once every three weeks. Mice blood was tested with indirect competitive ELISA (ic-ELISA) one week after the third immunization. Mice displaying the highest sensitivity/inhibitory activity and titer of antibody were intraperitoneally injected with sal-KLH (25 μg per mouse); subsequently, splenocytes were fused with Sp 2/0 myeloma cells. The single cell line with the highest sensitivity was screened by ELISA among several subclones. Following screening, hybridoma cells producing the required antibody were amplified in culture and injected into a mouse peritoneal ascites preparation. Antibodies were purified by combined stepwise caprylic acid and ammonium sulfate precipitation and dialyzed against PBS for 2 days. The concentrations of monoclonal antibodies were determined using UV absorbance.
Characterization of mAbs
ELISA was employed to characterize the sensitivity and affinity of mAbs. The checkerboard method was used to determine the concentrations of antibody and antigen. Coating with sal-OVA was performed in 96-well plates at 100 μL/well at 37°C for 2 h, followed by washing three times with diluted Tween-20 (Tween/water: 1/3) in 4 mL 0.01 M PBS (Solution 1). After adding 200 μL/well blocking buffer at 37°C for 2 h, plates were washed three times with Solution 1, followed by drying. Next, 50 μL/well antibody and standard diluent (v/v, 1/1) were added at 37°C for 30 min and washed three times. HRP (100 μL/well, diluted with antibody dilution buffer, 1:3,000) was added at 37°C for 30 min and subsequently removed by washing. Color buffer was added at a concentration of 100 μL/well at 37°C for 15 min and 2 M H2SO4 was added to terminate the reaction. Absorbance values were measured at 450 nm based on the enzyme marker.
Cross-reactivity
Cross-reactivities (CRs) between the monoclonal antibody and a series of structural analogs (salbutamol, clenbuterol, cimaterol, brombuterol hydrochloride, mabuterol, terbutaline, clenpenterol hydrochloride, carbuterol, mapenterol, pirbuterol) were confirmed with ic-ELISA using the formula:
Analysis of urine samples
Samples negative for β-agonists were established using HPLC. Urine was purified via centrifugation (4700 g, 10 min), spiked with different concentrations of standards, and analyzed using ic-ELISA to determine the influence of different samples on the antibody.
Preparation of mAb-labeled gold nanoparticles
Gold particles for experiments were required to have an average diameter of 30 nm. The following method was employed: 100 mL chloroauric acid (0.01 g/L) was boiled for 30 min, mixed with 2 mL trisodium citrate solution (1%) and stirred. Within 1 min, the reaction solution became wine-red. Heating was continued, along with stirring for 15 min until the reaction was complete. The solution was diluted with ultrapure water to a total volume of 100 mL and stored at 4°C (Sun, Liu, Song, Kuang, & Xu, Citation2016).
Preparation of the immunochromatographic strip
Purified mAb was mixed with 1 mL gold solution and 0.1 M K2CO3 at room temperature for 2 h. BSA (10%, 100 mL) was added to the solution, stirred for 2 h and centrifuged at 5100 g for ∼20 min. After centrifugation, the suspension was re-dissolved into a total volume of 0.1 mL and stored at 4°C until use. The immunochromatographic strip was composed of four parts: a conjugate pad, a nitrocellulose membrane (NC membrane), a sample pad and an absorbent pad. The NC membrane was pasted on the middle of the conjugate pad, the absorbent pad affixed to the top and the sample pad attached to the base. The test line and control line were sprayed on the NC membrane, combined with goat anti-mouse IgG (0.5 mg/mL) and coating antigen (0.3 μg/mL), respectively. The NC membrane was subsequently dried at 37°C for 2 h and adhered to the conjugate pad (Peng, Song, Liu, Kuang, & Xu, Citation2015; Chen et al., Citation2015). Cut the strip into 3 mm for use.
Assay of the immunochromatographic strip
The liquid sample reacted with 100 mL gold-labeled antibody (8 μg/mL) once added to the sample pad and migrated upward via capillary action. After 3–5 min, if both control and test lines appeared dark red, the sample was considered negative. If the control line was darker while the test line was lighter or had no color, the sample was positive.
Recovery
The recovery rate reflected the accuracy of the antibody. At present, many products determined residual of β-agonists in urine. Therefore, negative urine was taken after centrifugation in this experiment. Urine was spiked with different sal concentrations and analyzed by ic-ELISA.
Results and discussion
Antigen preparation
Since the drugs had relatively low molecular weights and were not immunogenic, carrier proteins (KLH, BSA and OVA) were required to impart immunogenicity. KLH coupled to hapten was used as immunogen and OVA selected as the carrier protein for coating. Immunogen and coating antigen were prepared using the EDC method. EDC is able to catalyze the carboxyl group and form an amide bond with amino condensation. NHS was added to the activated fluid to stabilize the reaction between protein and EDC. During the complete antigen synthesis process, the content of organic solvent (DMF) was limited to 30% or lower (v/v), since EDC was more active in water solution.
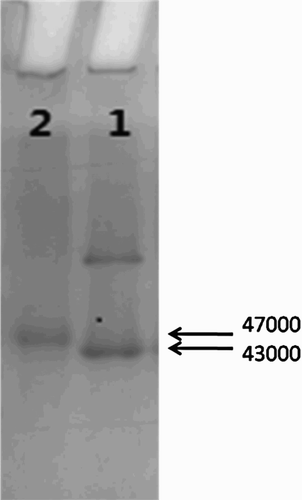
The presence of immunogen and coating antigen was confirmed using polyacrylamide gel electrophoresis (). The carrier protein, OVA, was labeled as 1 and coupled sal-hapten-OVA as 2, with a reaction ratio of 60:1. The band of sal-hapten-OVA migrated slower than OVA, owing to its higher molecular weight.
Preparation of mAbs
After five immunizations with sal-hapten-KLH, antibodies produced by the test mouse showed an IC50 of 5 μg/L for Sal and uniform cross-reactivity with other β-agonists, then Mouse splenocytes were fused with Sp 2/0 myeloma cells and cell lines sal-1A7, sal-3G10, sal-3H7 and sal-4F12 were obtained via multiple clone selection. mAbs were confirmed using ic-ELISA. Standards for different β-agonist types in negative urine were used to select a cell line displaying cross homogeneity and sensitivity to all the test drugs on the strip. Consequently, the Sal-3G10 cell line was selected for the immunochromatographic strip assay.
Cross-reactivity
The sensitivity of the monoclonal antibody was defined based on the values of cross-reactivity with structural analogs (). The values of cross-reactivity of animal cultures in the feed were less than 10%, which was considered as no cross-reaction (). The IC50 value for clenbuterol was 1.2 ng/mL and CR was 83.3%. The IC50 values for bromobuterol and mabuterol were 2 and 3 ng/mL, with CR values of 50% and 33.3%, respectively. The IC50 value for the remaining β-agonist compounds (cimaterol, terbutaline, clenpenterol, carbuterol, mapenterol, pirbuterol) was ∼5 ng/mL and the CR value was ∼20%. Our data clearly indicate uniform antibody cross-reactivity to the different β-agonists examined in this study. Meanwhile, the limit of detection of other methods is as shown in .
Table 1. Cross-reactivity of related structural analogs.
Table 2. Detection limit of sal by other methods.
Analysis of urine samples
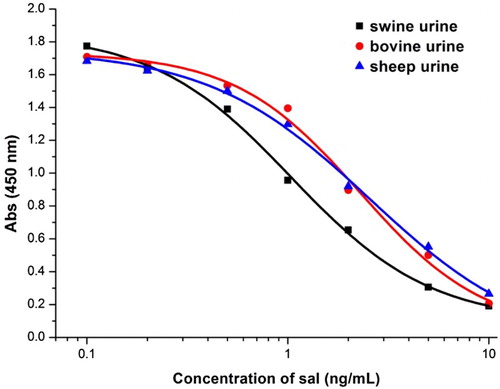
The tolerance of the monoclonal antibody to antigens was defined with the aid of ic-ELISA (). The IC50 value for sal was influenced lower in swine urine than bovine and sheep urine. In the different samples, Absmax was similar (between 1.7 and 1.9), while IC50 was lower in bovine and sheep urine. Under the same conditions, IC50 was 1.04 ng/mL in swine urine, 2.28 ng/mL in bovine urine and 2.55 ng/mL in sheep urine.
Confirmation of β-agonist detection using the immunochromatographic strip assay
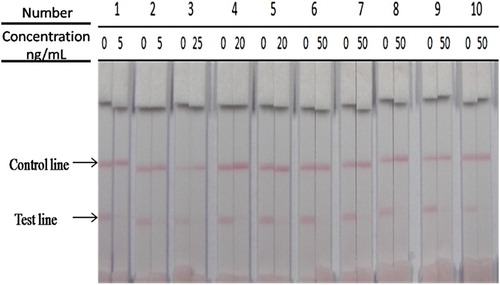
To confirm whether an immunochromatographic strip can be used to simultaneously detect salbutamol, clenbuterol, cimaterol, brombuterol, mabuterol, terbutaline, clenpenterol, carbuterol, mapenterol and pirbuterol in swine urine samples, 5 ng/mL sal and 20 or 50 ng/mL of other compounds were added to β-agonist negative urine samples. The results appeared in 5 min (). The strip cut-off values were 5 ng/mL for sal and clenbuterol, 20 ng/mL for cimaterol, brombuterol and mabuterol, and 50 ng/mL for terbutaline, clenpenterol, carbuterol, mapenterol and pirbuterol.
Figure 5. The sensitivity of the immunochromatographic assay in urine (1, sal; 2, clenbuterol; 3, cimaterol; 4, brombuterol; 5, mabuterol; 6, terbutaline; 7, clenpenterol; 8, carbuterol; 9, mapenterol; 10, pirbuterol).
Different standard concentrations of sal were added to swine urine. The test line became weaker with increasing concentrations. Urine samples containing different concentrations of sal (0, 1, 2, 5 ng/mL) were added to the sample pad. The cut-off limit was the concentration at which the test line disappeared completely (; ).
Figure 6. Analysis of sal concentration with strip (1, 0 ng/mL; 2, 1 ng/mL; 3, 2 ng/mL; 4, 5 ng/mL; the strip cut-off completely at 5 ng/mL of sal).
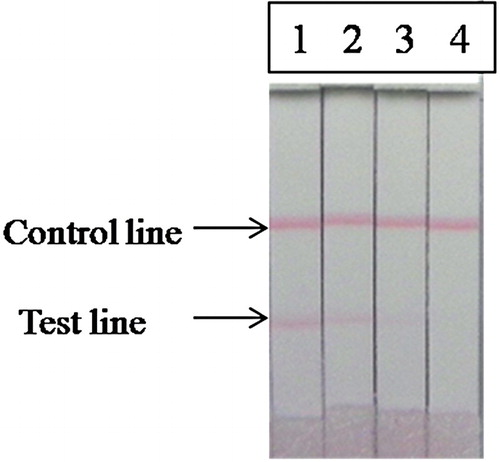
Table 3. Comparison of sal by ic-ELISA and immunochromatographic strip in the swine urine (n = 3).
In the case of sal, swine urine was spiked with different concentrations (0, 1, 2 and 5 ng/mL) and analyzed using the ic-ELISA and immunochromatographic strip assay. The results are shown in . And sal recovery was 100–120% due to the urine matrix effects. Coefficient of variation (CV) was 1.5–7%, and the test line gradient clearly facilitated antigen detection in the biological sample.
Conclusions
In this study, we generated a monoclonal antibody able to simultaneously detect 10 β-agonists generally added to animal fodder. The antibody was prepared based on sal-hapten and ic-ELISA, and used to establish an immunochromatographic strip to detect β-agonist levels in swine urine, which reflects their contents in fodder to a degree. The newly developed immunochromatographic strip, which tests the samples taken directly from animal urine without harm to the animal, provides a simple, realistic and rapid means to determine the presence of β-agonists in actual biological samples.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Rui Liu has earned her bachelor's from Jiangnan University, Wuxi, China in 2014 and then she began to study in Jiangnan University (Wuxi, China) as a graduate student in food science. Her research interests include immunoassay applications in food.
Liqiang Liu has obtained his Ph.D. in Food science in 2014 from Jiangnan University, Wuxi, China and then became a faculty in college of Food science and technology of Jiangnan University. His research interests include immunochromatographic strip design and application.
Shanshan Song holds a Master's degree in Food science in 2012 from Jiangnan University, Wuxi, China and then became a research assistant in college of Food science and technology of Jiangnan University. Her research interests include monoclonal antibody development.
Gang Cui is a professor of Food science and technology of Jiangnan University. He earned his Ph.D. in food science in 2007. His research interests include fast detection technology and food safety evaluation.
Qiankun Zheng graduated from Nanjing Agricultural University in 1996. Currently, he works as a senior engineer in Delicious food company, China. He is good at food quality control and assurance.
Hua Kuang obtained her Ph.D. from China Agricultural University in 2009 and then began to work as a faculty in college of Food science and technology of Jiangnan University. She is currently a full professor in food safety. Her research interests include biosensor development.
Chuanlai Xu is a full professor of Food science and technology of Jiangnan University. He earned his Ph.D. in food science in 2002. His research interests include fast detection technology and food safety evaluation.
Additional information
Funding
References
- Amelin, V. G., Korolev, D. S., & Tret’yakov, A. V. (2015). QuEChERS sample preparation in the simultaneous determination of diethylstilbestrol and ractopamine in food by gas-liquid chromatography. Journal of Analytical Chemistry, 70(4), 419–423. doi: 10.1134/S1061934815040024
- Brett, J., Dawson, A. H., & Brown, J. A. (2014). Clenbuterol toxicity: A NSW poisons information centre experience. The Medical Journal of Australia, 200(4), 219–221. doi: 10.5694/mja13.10982
- Bui, Q. A., Vu, T. H., Ngo, V. K., Kennedy, I. R., Lee, N. A., & Allan, R. (2016). Development of an ELISA to detect clenbuterol in swine products using a new approach for hapten design. Analytical and Bioanalytical Chemistry, 408(22), 6045–6052. doi: 10.1007/s00216-016-9750-2
- Cao, X., Li, H., Lian, L., Xu, N., Lou, D., & Wu, Y. (2015). A dual-responsive fluorescence method for the detection of clenbuterol based on BSA-protected gold nanoclusters. Analytica Chimica Acta, 871, 43–50. doi:10.1016/j.aca.2015.02.031 doi: 10.1016/j.aca.2015.02.031
- Chen, Y., Kong, D., Liu, L., Song, S., Kuang, H., & Xu, C. (2015). Development of an ELISA and immunochromatographic assay for tetracycline, oxytetracycline, and chlortetracycline residues in milk and honey based on the class-specific monoclonal antibody. Food Analytical Methods, 9(4), 905–914. doi: 10.1007/s12161-015-0262-z
- Chen, D., Yang, M., Zheng, N., Xie, N., Liu, D., Xie, C., & Yao, D. (2016). A novel aptasensor for electrochemical detection of ractopamine, clenbuterol, salbutamol, phenylethanolamine and procaterol. Biosensors & Bioelectronics, 80, 525–531. doi: 10.1016/j.bios.2016.01.025
- Diru, Y., Susu, W., Jianqiao, X., Ruifen, J., Fang, Z., & Gangfeng, O. (2016). Rapid determination of clenbuterol in pork by direct immersion solid-phase microextraction coupled with gas chromatography-mass spectrometry. Journal of Chromatographic Science, 54(2), 112–118.
- Du, H., Chu, Y., Yang, H., Zhao, K., Li, J., She, P., … Deng, A. (2016). Sensitive and specific detection of a new β-agonist brombuterol in tissue and feed samples by a competitive polyclonal antibody based ELISA. Analytical Methods, 8(17), 3578–3586. doi: 10.1039/C6AY00079G
- Gao, T., Ye, N., & Li, J. (2015). Determination of ractopamine and clenbuterol in beef by graphene oxide hollow fiber solid-phase microextraction and high-performance liquid chromatography. Analytical Letters, 49(8), 1163–1175. doi: 10.1080/00032719.2015.1094662
- González-Antuña, A., Rodríguez-González, P., Centineo, G., & García Alonso, J. I. (2014). Simultaneous determination of seven β2-agonists in human and bovine urine by isotope dilution liquid chromatography–tandem mass spectrometry using compound-specific minimally 13C-labelled analogues. Journal of Chromatography A, 1372, 63–71. doi: 10.1016/j.chroma.2014.10.065
- Gu, H., Liu, L., Song, S., Kuang, H., & Xu, C. (2015). Development of an immunochromatographic strip assay for ractopamine detection using an ultrasensitive monoclonal antibody. Food and Agricultural Immunology, 27(4), 471–483. doi: 10.1080/09540105.2015.1126808
- Johnson, B. J., Smith, S. B., & Chung, K. Y. (2014). Historical overview of the effect of beta-adrenergic agonists on beef cattle production. Asian-Australasian Journal of Animal Sciences, 27(5), 757–766. doi: 10.5713/ajas.2012.12524
- Liu, L., Kuang, H., Peng, C., Wang, L., & Xu, C. (2014). Structure-specific hapten design for the screening of highly sensitive and specific monoclonal antibody to salbutamol. Analytical Methods, 6(12), 4228–4233. doi: 10.1039/c4ay00062e
- Mauro, D., Ciardullo, S., Civitareale, C., Fiori, M., Pastorelli, A. A., Stacchini, P., & Palleschi, G. (2014). Development and validation of a multi-residue method for determination of 18 β-agonists in bovine urine by UPLC–MS/MS. Microchemical Journal, 115, 70–77. doi: 10.1016/j.microc.2014.02.012
- Nguyen, T. A. H., Pham, T. N. M., Doan, T. T., Ta, T. T., Sáiz, J., Nguyen, T. Q. H., & Mai, T. D. (2014). Simple semi-automated portable capillary electrophoresis instrument with contactless conductivity detection for the determination of β-agonists in pharmaceutical and pig-feed samples. Journal of Chromatography A, 1360, 305–311. doi: 10.1016/j.chroma.2014.07.074
- Peng, S., Song, S., Liu, L., Kuang, H., & Xu, C. (2015). Rapid enzyme-linked immunosorbent assay and immunochromatographic strip for detecting ribavirin in chicken muscles. Food and Agricultural Immunology, 27(4), 449–459. doi: 10.1080/09540105.2015.1104657
- Pérez-Silva, I., Ramírez-Silva, M. T., Galán-Vidal, C. A., Álvarez-Romero, G. A., Rodríguez, J. A., & Páez-Hernández, M. E. (2016). Evaluation of the use of solvent impregnated resins in the analysis of salbutamol in human urine followed by capillary electrophoresis. Reactive & Functional Polymers, 105, 89–94. doi: 10.1016/j.reactfunctpolym.2016.05.018
- Ren, X., Zhang, F., Chen, F., & Yang, T. (2009). Development of a sensitive monoclonal antibody-based ELISA for the detection of clenbuterol in animal tissues. Food and Agricultural Immunology, 20(4), 333–344. doi: 10.1080/09540100903365852
- Sai, F., Hong, M., Yunfeng, Z., Huijing, C., & Yongning, W. (2012). Simultaneous detection of residues of 25 beta(2)-agonists and 23 beta-blockers in animal foods by high-performance liquid chromatography coupled with linear ion trap mass spectrometry. Journal of Agricultural and Food Chemistry, 60(8), 1898–1905. doi: 10.1021/jf2039058
- Sun, C., Liu, L., Song, S., Kuang, H., & Xu, C. (2016). Development of a highly sensitive ELISA and immunochromatographic strip to detect pentachlorophenol. Food and Agricultural Immunology, 27(5), 689–699. doi: 10.1080/09540105.2016.1148668
- Tao, Y., Zhu, F., Chen, D., Xie, S., Yuanhu, P., Wang, X., & Yuan, Z. (2014). Evaluation of matrix solid-phase dispersion extraction for 11 β-agonists in swine feed by liquid chromatography with electrospray ionization tandem mass spectrometry. Journal of Separation Science, 37(18), 2574–2582. doi: 10.1002/jssc.201400402
- Weber, M. J., Dikeman, M. E., Jaeger, J. R., Unruh, J. A., Murray, L., & Houser, T. A. (2013). Effects of feeding a single or sequence of beta-adrenergic agonists on cull cow meat quality. Meat Science, 93(2), 275–281. doi: 10.1016/j.meatsci.2012.09.004
- Wu, Y., Bi, Y., Bingga, G., Li, X., Zhang, S., Li, J., & Xia, X. (2015). Metabolomic analysis of swine urine treated with beta2-agonists by ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Journal of Chromatography A, 1400, 74–81. doi: 10.1016/j.chroma.2015.04.050
- Wu, Y., Xu, F., Jiang, H., Tao, X., Zhu, K., Liu, W., & Ding, S. (2014). Determination of salbutamol, clenbuterol, and brombuterol in urine by a highly sensitive chemiluminescence enzyme immunoassay. Analytical Letters, 47(16), 2761–2773. doi: 10.1080/00032719.2014.917422
- Yan, H., Liu, L., Xu, N., Kuang, H., & Xu, C. (2015). Development of an immunoassay for carbendazim based on a class-selective monoclonal antibody. Food and Agricultural Immunology, 26(5), 659–670. doi: 10.1080/09540105.2015.1007446
- Yan, K., Zhang, H., Hui, W., Zhu, H., Li, X., Zhong, F., & Chen, C. (2016). Rapid screening of toxic salbutamol, ractopamine, and clenbuterol in pork sample by high-performance liquid chromatography-UV method. Journal of Food and Drug Analysis, 24(2), 277–283. doi: 10.1016/j.jfda.2015.12.002
- Zhang, G., Chen, M., Liu, D., Xiong, Y., Feng, R., Zhong, P., & Lai, W. (2016). Quantitative detection of β2-adrenergic agonists using fluorescence quenching by immunochromatographic assay. Analytical Methods, 8(3), 627–631. doi: 10.1039/C5AY02585K
- Zhang, Z., Yan, H., Cui, F., Yun, H., Chang, X., Li, J., & Hu, Q. (2015). Analysis of multiple β-agonist and β-blocker residues in porcine muscle using improved QuEChERS method and UHPLC-LTQ Orbitrap mass spectrometry. Food Analytical Methods, 9(4), 915–924. doi: 10.1007/s12161-015-0238-z
- Özkütük, E. B., Uğurağ, D., Ersöz, A., & Say, R. (2016). Determination of clenbuterol by multiwalled carbon nanotube potentiometric sensors. Analytical Letters, 49(6), 778–789. doi: 10.1080/00032719.2015.1079213
- Zou, M., Gao, H., Li, J., Xu, F., Wang, L., & Jiang, J. (2008). Rapid determination of hazardous compounds in food based on a competitive fluorescence microsphere immunoassay. Analytical Biochemistry, 374(2), 318–324. doi: 10.1016/j.ab.2007.10.024