ABSTRACT
Illegal additives of tadalafil and related analogues in functional foods threaten public health. To generate broad-specific antibodies against both tadalafil and its analogues, tadalafil was activated through an oximation reaction for synthesis of conjugates, leaving the common structure of tadalafil analogues exposed as a major antigenic site. The desired antisera showed satisfied specificities to tadalafil and its major analogues with immunochromatographic (IC50) values ranging from 16.1 to 29.5 ng mL−1 in a referring indirect competitive enzyme-linked immunosorbent assay. The optimized IC assay showed detection thresholds ranging in 10∼20 μg g–1 for tadalafil and major analogues when detected fortified herbal samples in a dilution ratio of 1:103. Sixty herbal food supplements were screened and five were found to be positive using IC strip. It was confirmed by UPLC-PDA-MS/MS that positive samples contain target illegal additives in levels of 10∼20 mg g−1 (1%∼2%). In this range of containing percentage, sensitivity of the IC strip is adequate to screen tadalafil and its potential analogues in herbal products.
Introduction
Phosphodiesterase-5 (PDE-5) inhibitors can selectively inhibit the PDE-5 enzyme thus raising the cyclic guanosine monophosphate levels and enhancing relaxation of the penile corpus cavernosum and therefore have the potential to improve penile erectile function (Fleshner et al., Citation2005; Venhuis, Blok-Tip, & de Kaste, Citation2008). Sildenafil (Viagra, Pfizer), vardenafil hydrochloride (Levitra, Bayer) and tadalafil (Cialis, Lilly) are PDE-5 inhibitors approved by both Europe and US-FDA for the treatment of erectile dysfunction (ED) (). With the commercial success of these drugs, it is frequently exposed that undeclared PDE-5 inhibitors are adulterated onto natural aphrodisiacs for promoting therapeutic effect of ED in recent years (Gilard et al., Citation2015; Sugita & Miyakawa, Citation2010; Venhuis et al., Citation2008). To evade being identified by official institutions, more and more analogues of above licensed PDE-5 drugs were synthesized as illicit adulterant by deliberate modifications in their molecular structures (Gratz, Gamble, & Flurer, Citation2006; Singh et al., Citation2009). Analogues of sildenafil are most common ones being identified in food supplements (Gratz, Flurer, & Wolnik, Citation2004; Lebel, Gagnon, Furtos, & Waldron, Citation2014). It also cannot be ignored that novel analogues of tadalafil kept emerging in food supplements as adulterants in an endless stream (Singh et al., Citation2009; Venhuis & de Kaste, Citation2012). These analogues are optical isomers of tadalafil with deliberate modification in their molecular structure (Patel et al., Citation2014; Poon, Lam, Lai, Chan, & Mak, Citation2007; Schramek, Wollein, & Eisenreich, Citation2014). As described in , related analogues vary from tadalafil at the N-atom site in the pyrazine ring which is the non-essential part for pharmacological activity, containing the common pharmacological necessary structure remain activity of sexual performance enhancement (Hadwiger et al., Citation2010; Hasegawa et al., Citation2008; Hasegawa et al., Citation2009; Huang, Lee, Lin, Li, et al., Citation2016; Huang, Lee, Lin, Tsai, & Cheng, Citation2016; Kern, Nickum, Flurer, Toomey, & Litzau, Citation2015; Lee, Kim, et al., Citation2013; Lee, Kim, Mandava, et al., Citation2015; Lee, Kim, Noh, et al., Citation2015; Lee, Mandava, Baek, & Lee, Citation2016; Lee et al., Citation2016; Toomey, Litzau, & Flurer, Citation2012; Ulloa et al., Citation2015; Xu, Kee, Ge, Low, & Koh, Citation2016; Zhang, Yu, Wu, & Li, Citation2014; Zou, Hou, Low, & Koh, Citation2006). The analytical characters of the modified analogues are quite different from any known one that causes far more difficulty in screening such illicit additives in food supplements (Bortolini, Pivato, Bogialli, & Pastore, Citation2015; Gilard et al., Citation2015; Jeong et al., Citation2016; Singh et al., Citation2009; Ulloa et al., Citation2015; Zou, Hou, Oh, Low, & Koh, Citation2006).
Figure 1. Structural descriptions of tadalafil and its analogues, which differ from each other in the pyrazine ring, while remain the essential part for pharmacological activity.
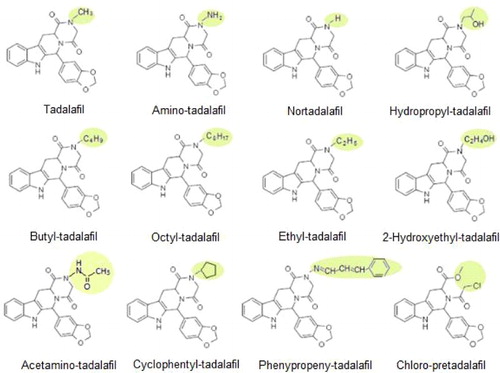
Table 1. Cross-reactivities (%) of related drugs in the ic-ELISA system.
Illegal tadalafil and its analogues adulterated in herbal aphrodisiacs could present a serious threat to public health. First, PDE-5 inhibitors have pharmacological adverse effects such as facial flushing, headache, muscle aches, dyspepsia and visual disturbances (Gilard et al., Citation2015; Sugita & Miyakawa, Citation2010; Venhuis et al., Citation2008), possibility of hearing loss and blindness (Singh et al., Citation2009; Skalicka-Wozniak, Georgiev, & Orhan, Citation2016; Venhuis & de Kaste, Citation2012). In Citation2007, cases about sudden decreases or loss of hearing after using sildenafil, vardenafil and tadalafil were reported in website of US-FDA (http://www.fda.gov/medwatch/report.htm.2007). Secondly, drastically lower blood pressure may be caused by the interaction between PDE-5 inhibitors and nitrate drugs (Patel et al., Citation2014; Poon et al., Citation2007; Singh et al., Citation2009; Taylor, Baldo, Storey, Cartledge, & Eardley, Citation2009). It would be in lethal risk for a patient to take nitrate in combination with an unwitting PDE-5 inhibitor from “herbal product” (Gratz et al., Citation2006; Schramek et al., Citation2014). It presents a higher health risk for a consumer to take an unknown analogue of tadalafil from functional foods, because no safety and toxicity profiles of such drugs have been known (Gratz et al., Citation2006; Kern et al., Citation2015). It is urgent to develop reliable assays to screen illegal tadalafil and its analogues as illegal additives in herbal products.
Because the chromatographic character and molecular mass of a novel tadalafil analogue are quite different from those known ones, it is difficult to identify a novel analogue of tadalafil in complex matrices by using HPLC or even LC-MS (Gratz et al., Citation2006; Patel et al., Citation2014). Now, only methods of LC-MS/MS (Gratz et al., Citation2004; Lee, Lee, et al., Citation2013; Zhu, Xiao, Chen, & Yao, Citation2005) or LC-DAD-QTOF (Bortolini et al., Citation2015) can be competent for identification of potential analogues of PDE-5 inhibitors in a single procedure. Although being accurate and reliable, LC-MS/MS is time-consuming and costly, relies upon expensive instruments and sophisticated operators. By referring to the successful precedents of immunochromatographic (IC) assay for food monitoring (Kong, Liu, Song, Kuang, & Xu, Citation2016; Mukunzi et al., Citation2016) and former experience (Guo, Liu, Lan, Chen, & Xiao, Citation2016), attempts have been made to generate polyclonal antisera being specific to the pharmacological necessary group of tadalafil, then to develop a rapid IC strip for detection of not only tadalafil but also its potential analogues as illegal additives in a simplified and sensitive way.
Materials and methods
Chemicals and materials
Standard reference material: aminotadalafil (T-101), tadalafil (T-103), chloropretadalafil (T-104), N-octylnortadalafil (T-105), N-butyltadalafil (T-106) and nortadalafil (T-107) were received from thin-layer chromatography (TLC) PharmaChem Inc. (Ontario, Canada). Samples of functional foods for evaluation were purchased from supermarkets (Guangzhou, China). Carboxymethoxylaminehemihydrochloride (CMO), bovine serum albumin (BSA), ovalbumin (OVA), Tween-20, 1,2-ethylenediamine, carbodiimide, glutaraldehyde, complete and incomplete Freund’s adjuvant, 3,3′,5,5′-tetramethyl-benzidine were obtained from Sigma Chemical Co. (St. Louis, MO, USA); Peroxidase-labeled goat anti-rabbit IgG(H+L) (HRP-IgG) were purchased from Jackson Co., Ltd. (Wuhan, China). The Millipore 135 nitrocellulose (NC) membrane, the colloidal gold (40 nm in diameter), the sample pad, the conjugation pad and the absorbent pad were obtained from Jiening Bio. Co. (Shanghai, China). All other reagents used were of analytical grade.
Buffer solutions and stock of standards
Phosphate buffer saline (PBS) (10 mM sodium phosphate,137 mM NaCl, 2.7 mM KCl, pH 7.4), coating buffer (CB, 50 mM sodium carbonate/bicarbonate buffer pH 9.5), washing buffer (10 mM PBS containing 0.05% Tween-20, pH 7.4), blocking buffer (10 mM PBS containing 0.3% gelatin, pH 7.4), extraction buffer (5 mM Tris-HCl containing 60% methanol and 0.1% Tween-20, pH 5.0), assay buffer (10 mM PBS containing 0.05% Tween-20, and 0.2% BSA, pH7.4), probe dilution buffer (10 mM PBS containing 5% sucrose, 0.2% BSA, 0.3% PVP, 1% mycose and 0.05% sodium azide) were used. Stock solutions of standards were prepared at concentrations of 0.1 mg mL−1 in extraction buffer and stored at 4°C.
Instrumentation
The UltroSpec 4300 Pro UV–visible Spectrophotometer (Pharmacia Co., USA) was used to measure the absorbance of the protein solution. Enzyme-linked immunosorbent assay (ELISA) plates were obtained from Costar (Cambridge, MA, USA). The absorbance was measured at 450 nm on a micro-ELISA Reader (Labsystems Multiskan MCC/340, Finland). The equipment used spraying and cutting strips was purchased from BioDot (Irvine, CA, USA). The BioDot system consisted of two BioJets Quanti 3000 and one Air jet Quanti 3000 attached on a BioDot XYZ-3000 (Irvine, CA, USA) dispensing platform. The guillotine cutter (model CM 4000) was supplied by BioDot (Irvine, CA, USA). A Sigma 2K15 centrifuge from Sigma-Aldrich (St. Louis, MO, USA) was used for centrifugation of colloidal gold-Ab conjugates. Identification of IC results was performed on an ACQUITY UPLC system (XEVO TQ; Waters, USA) based on a BEH-C18 column (2.1 mm × 100 mm i.d., 1.7 µm; Waters, USA) equipped with a diode array detector (model 2996; Waters, USA) and a triple quadrupole tandem mass spectrometry (XEVO TQ-S; Waters, USA).
Preparation of immunogens and testing antigens
Derivation of tadalafil
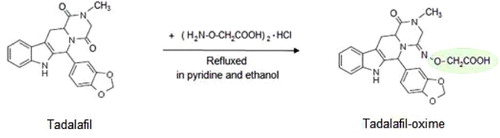
Tadalafil was employed as representative hapten for generation desired antibodies for detection of this type of compounds. To introduce a reactive group to tadalafil for conjugating with BSA or OVA, derivation of tadalafil was carried out through an oximation reaction: 5 mg tadalafil and 10 mg CMO were dissolved in a solution containing 3.0 mL pyridine and 2.0 mL ethanol in a micro round bottom flask. The reaction mixture was refluxed for 5 h gently under continuous magnetic stirring. Then, the reaction mixture was concentrated to final volume of 0.5 mL by using a rotary evaporator. The remaining mixture and tadalafil standard were spotted in two individual dots onto a TLC plate, respectively. The TLC plate was developed in a reagent of chloroform/hexane/methanol (5:4:1) containing 0.5% formic acid, and detected by UV light with a wavelength of 254 nm. The tadalafil-oxime was collected from correspondent spot on the TLC plate under the reference of tadalafil standard and re-dissolved in chloroform. After centrifuged at 5000 rpm for 5 min to remove silica gel residues, the tadalafil-oxime was air dried for solvent-displacement. The derived hapten re-dissolved in 0.01M carbonate buffer (pH 9.5) was identified by UV–visible spectroscopy before conjugation.
Preparation of tadalafil conjugates
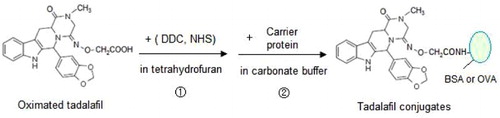
Active ester method was used to couple the tadalafil-oxime to carrier proteins (BSA and OVA). The dehydrated tadalafil-oxime was dissolved in anhydrous tetrahydrofuran with 10 mg DCC and 10 mg NHS being added for synthesis of active ester under shaking. Active ester solution was added to BSA in carbonate buffer to react at room temperature for 30 min by gentle stirring. The mixture was centrifuged and the supernatant collected and dialyzed using 0.01 M PBS. Preparation of the OVA-tadalafil conjugate was similar to BSA-tadalafil. After being identified by the UV Spectrometer, all conjugates were stored at −20°C for further use.
Generation and purification of polyclonal antibodies
Three female rabbits (1.5 kg) were immunized subcutaneously to generate specific antisera. Immunogens (200 μg) were emulsified with Freund’s complete adjuvant for the primary immunization, and followed by three boosters with 100 μg of the same immunogen while in Freund’s incomplete adjuvant at three-week intervals. Working polyclonal antibodies were purified from collected sera using a caprylic acid and ammonium sulfate method (Huang et al., Citation2012).
Characterization of polyclonal antibodies by indirect competitive enzyme-linked immunosorbent assay (ic-ELISA)
Procedure of ic-ELISA
To evaluate the titer and affinity of the antisera, a referring ic-ELISA was optimized by a checkerboard experiment described as previously (Guo et al.,Citation2016). The absorption value was read at 450 nm. A linear standard curve was prepared by plotting concentration of the tadalafil standard versus the binding ratio (B/B0) for quantitative analysis. The formula for calculation of the binding rate (Bi/B0) is as follow:Here, Ai is an absorption value in ELISA under inhibition of a testing target, A0 is the initial absorption value without inhibition.
Evaluation of specificity
The specificity of the antisera for the target analytes was evaluated by detecting cross-reactivities between tadalafil and related compounds by the referring ic-ELISA. Tadalafil and major analogues (aminotadalafil, chloropretadalafil, N-octylnortadalafil, N-butyltadalafil and nortadalafil) were employed for the evaluation. Each testing compound was detected by the ic-ELISA in gradient concentrations (0, 0.3, 1, 3, 10, 30 and 100 ng mL−1), respectively. The inhibition competitive curves with ratios of Bi/B0 referring to the standard dose-responses were linear fitted by RIDAWIN software.
Then each IC50 value for individual compound was obtained on the curve. Cross-reactivities were calculated with the formula:
Development of IC assay
Preparation of antibody-gold nanoparticle probe
Colloidal golds (40 nm) were labeled with a series of concentrations of working antisera to obtain optimum color and sensitivity. The preparation of antibody-gold nanoparticle probes was carried out according to former reports (Huang et al., Citation2012). Firstly, various concentrations of antisera solutions (0.05, 0.10, 0.15 and 0.20 mg mL−1) were diluted in 0.05M boric acid-borax buffer (pH 7.6), respectively. Then 10 μL of each antisera solution was added dropwisely to 2.0 mL of gold nanoparticle solution (in 0.05 M K2CO3, pH7.6) and stirred gently for 25 min. To stabilize the antibody-gold nanoparticle probe, 50 μL of 10% BSA solution was added to the mixture with a further stirring for 15 min. Each mixture was centrifuged at 12,000 rpm for 30 min at 4°C, then the supernatant was discarded. Each gold nanoparticle was suspended in 3.0 mL of probe dilution buffer. These working antibodies-colloidal gold probes were stored at 4°C for later use.
Preparation of IC strip
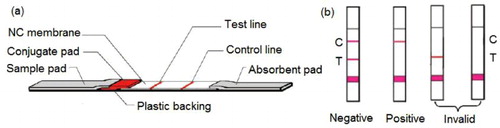
An IC strip consists of sample pad, conjugate pad, NC membrane, absorbent pad and backing card. NC membrane is most important for an IC strip. After evaluation, Millipore 135 NC membrane was selected out of several candidates for development of the current IC strip.
Following single factor experiments, a checkerboard experiment was employed to optimize the combination of testing antigen and colloidal gold labeled probes referring to previous report (Huang et al., Citation2012). Gradient concentrations of testing antigens (0.1, 0.2, 0.4 and 0.6 mg mL−1) were dissolved in 0.05 M PBS (pH 7.4) containing 10% methanol. Each antigen solution was sprayed onto the NC membrane at 1.0 μL per 1.0 cm as the test line (T line). A goat anti-rabbit second antibody (0.1 mg mL−1) was sprayed onto the NC membrane at the same density of above as a control line (C line), in a distance of 4.0 mm above the T line. All sprayed NC membranes were vacuum-dried at 37°C for 2 h. The conjugate pad was activated with the probe dilution buffer and vacuum-dried at 37°C overnight. The series of antibodies-colloidal gold probes, which were labeled with gradient concentrations of antibodies, were jetted on pretreated conjugate pads using a BioDot XYZ Platform. All conjugate pads were then vacuum-dried at 25°C for 3 h.
The next step was to assemble the whole IC strip according to the checkerboard testing plan. Series of NC membrane sprayed with different concentration of testing antigens were adhered to a backing card. Series of conjugate pads jetted with different gold probes were then secured to the backing card by overlapping 2 mm with the NC membrane. Similarly, the sample pad was also pasted on the backing card by overlying 2 mm with conjugate pad. The absorbent pad was pasted on the top of the membrane sheet. The whole assembled sheets were cut into strips (4.0 mm × 75 mm) by a cutting machine. These assembled strips were ready for assay and kept in 4°C refrigerator in a sealed plastic bag until use.
Validation of the IC assay
Sample preparation
Sample preparation is a most important approach for analytical sensitivity and overcoming the matrix effect. In order to ensure the reliability of the IC assay, sample preparation was designed according to former experience (Guo et al., Citation2016): an aliquot 0.2 ml (0.2 g) of the sample was extracted in a vial using 10 mL extraction buffer by hand shaking. After filtered through rapid qualitative filter paper, the supernatant was diluted with the assay buffer in a 1:20 proportion for quantitative IC assay. Under this program, the dilution ratio of a sample is 1:103 for detection after preparation.
Methanol is employed for extraction of tadalafil type compounds from herbal samples due to its hydrophobicity, but too high a concentration of methanol is a negative influence for the immunological reaction which can lead to non-specific results in the IC assay. Gradient concentrations of methanol were applied in the extraction buffer to evaluate the influence of methanol for the IC assay by observing the color intensity in the testing lines.
Evaluate of detection threshold
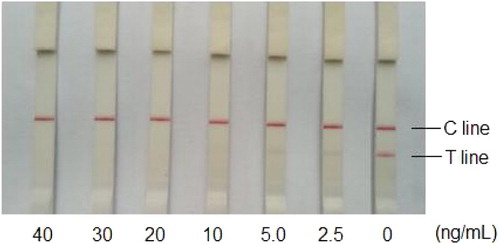
The semi-quantitative performance of the IC assay was evaluated with threshold levels for the detection of target analytes. To validate threshold levels of the IC assay for tadalafil and major analogues, the certificated standards of tadalafil, aminotadalafil, nortadalafil, N-octylnortadalafil, N-butyltadalafil and chloropretadalafil were fortified in herbal samples in gradient concentrations (0, 1.25, 2.5, 5.0, 10, 20 and 40 μg g–1, respectively). Parallel measurements (n = 4) of the IC strip were carried out for each sample fortified with individual certificated compound in series concentrations; the lowest fortified concentration that causes complete invisibility on a T line of all four testing strips is confirmed as the threshold level for this target analyte.
Determination of samples by the IC assay
Sixty herbal products from market were screened by the current IC assay. Aliquot 0.2 mL (0.2 g) of the sample was extracted in a vial using 10 mL extraction buffer by hand shaking. After filtered through rapid qualitative filter paper, the supernatant was diluted with the assay buffer in 1/10 proportion for quantitative IC assay. Results would be identified by referring ic-ELISA, then by UPLC-DAD-MS/MS to evaluate the performance of the IC assay for determination of analogues of tadalafil.
UPLC-DAD-MS/MS analysis
According to previous report (Xiang, Zhang, Zhu, & Zheng, Citation2010), separation was performed on an ACQUITY UPLC system with a BEH-C18 column (2.1 mm × 100 mm i.d., 1.7 µm; Waters, USA) with 0.1% formic acid methanol (A) and 0.1% formic acid water (B) as the mobile phase by gradient elution at a flow rate of 0.2 mL min−1. Detection was carried out by a diode array detector and an ACQUITY TQD tandem mass spectrometer (Waters, USA) operated in the electrospray ionization (ESI) positive-ion mode, respectively.
Results and discussion
Design of derived hapten
To generate antibodies specific to both tadalafil and its analogues, it is key to design appropriate complete antigens with the common group of these compounds as a major antigenic site to stimulate desired immune response. As described in tadalafil and its analogues vary from each other in the pyrazine ring, while share a common molecular structure for pharmacological activity. Thus, it is suitable to employed tadalafil as representative hapten by using the pyrazine ring as the reacting site for coupling of complete antigens. An oximation reaction () was carried out to derive the tadalafil through adding a carboxyl group at carbonyl group of pyrazine ring. Based on such a derived hapten, the common structure of tadalafil and analogues can be remained as a desired antigenic site, which exposed in surface of further conjugated immunogen. Thus, it can be feasible to raise broad-specific antibodies against both tadalafil and analogues through immunization ().
Identification of derived hapten and conjugations
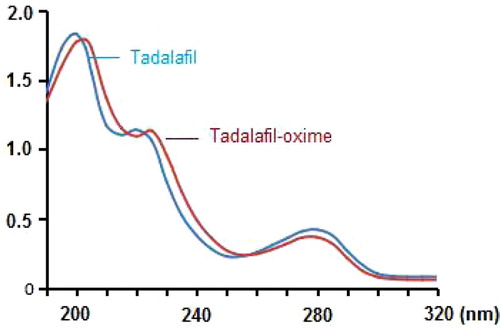
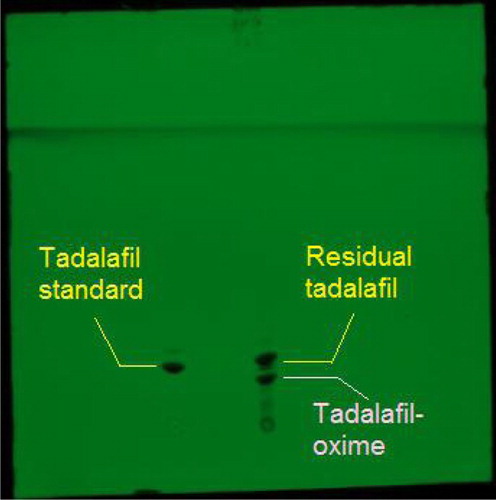
Derived hapten of tadalafil-oxime was isolated from the oxime mixture by a TLC plate. Two major spots indicated in the plate were deduced as residual tadalafil and tadalafil-oxime under the reference of tadalafil standard (). Compared to the lipophilic tadalafil, the collected tadalafil-oxime can easily dissolve in carbonate buffer due to the existence of carboxy group improving its molecular polarity. The tadalafil-oxime solution was identified by UV–visible spectrum scanning. As described in , the shape of UV absorption spectrum of tadalafil-oxime seems very similar to that of original tadalafil, while with an optical red shift in ultraviolet regions. The fact verified that tadalafil-oxime remains the major groups of tadalafil with a chemical modification in its structure.
Figure 4. TLC chromatogram for the isolation of tadalafil-oxime the oxime product under the reference of tadalafil standard.
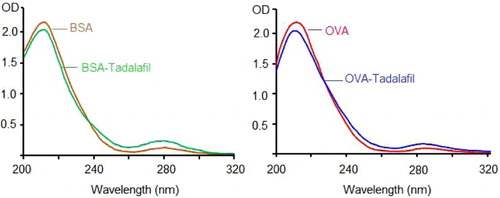
In the consequent identification of conjugations, each UV absorption spectrum of immunogen and testing antigen indicates quite difference from its related carrier (). This result indicated that both conjugations were successful, benefited from the carboxy group in derived hapten of tadalafil-oxime.
Specificity evaluation of the antibody
After primary immunization following three boosters with immunogen for 3 times, the specific antibodies titer against tadalafil from rabbits sera were higher than 5.0 × 105 in the referring ELISA. The IC50 value of each certificated standard was shown in , the working antibody is highly selective to tadalafil and major analogues, with IC50 values ranging from 16.1 to 29.5 ng mL−1 in ic-ELISA. The results indicated that the polyclonal antibodies have sufficient specificity to tadalafil and its analogues due to the antibodies’ capability of recognizing the common structure of this class of drugs.
Optimization of the IC strip
The structure and working principle of an IC strip is demonstrated in . Besides the quality of gold pad, it is another key factor for the performance of the assay to combine testing antigen in test line on NC membrane. According to the competitive immunoassay principle, a lower concentration of testing antigen or antibody-gold probe resulted in better cut-off limits of the assay. While a too low concentration of antigen or probe could lead to weak color development on the test line, causing difficulty in determination of positive and negative results by naked eye. Thus, a checkerboard experiment was carried out to optimize combination of testing antigen and probe to balance the sensitivity and color development of the IC strip. As a result, the optimum concentration of testing antigen for spraying in NC membrane was 0.4 mg mL−1, and the optimum concentration of specific antibodies for colloidal gold labeling was 0.2 mg mL−1, respectively. Under the optimum conditions, the pink color which shown on the T line is clear for negative control and should fade completely for positive solutions of tadalafil with concentration being greater than 10 ng mL−1 ().
Sample preparation
Sample preparation is an important procedure for ensuring analytical sensitivity and overcoming matrix effect. Methanol is a common reagent for extraction of tadalafil and its analogues from herbal samples due to similar performance of hydrophobic property, but too high level concentration of methanol could suppress immunological reaction leading to non-specific results in IC assay. Tolerance evaluation of methanol in the IC strips showed that no different color intensity in testing lines was observed from all strips when methanol concentration is less than 15% (v/v) (data not shown). Although a high concentration of methanol (60%,v/v) is employed in extraction buffer to enhance extraction of tadalafil and its analogues described as above, the interference of methanol could be ignored when extracts were diluted in 1/10 proportion by assay buffer for final IC assay.
Validation of the IC assay
Aim of the current assay is to screen both tadalafil and its analogues in a satisfied sensitivity, which is validated by threshold level, conforming as the lowest certificating concentration that lead to a complete invisibility on the T line. For a validation of threshold level for related analogues, certificated standards were detected using the optimized IC strip in gradient concentrations according to 2.8. As shown in , threshold levels for screening of tadalafil and major analogues ranged from 10 to 20 ng g−1. Considering the percentage level of related adulterants in herbal samples usually exceed mg g−1 (mg mL−1), the sensitivity of the current IC assay is sufficient for screening tadalafil and related analogues in a dilution ratio of 1/103 for sample extraction.
Table 2. Threshold validation of the IC assay for tadalafil and major analogues of tadalafil by detecting of fortified samples.
Verification of IC assay by UPLC-PDA-MS/MS
In total 60 herbal products collected from market were detected by the current IC assay and referring ELISA for screening tadalafil and its analogues. A following verification of the results was carried out by UPLC-PDA-MS/MS.
Each positive sample in IC analysis was identified containing an individual tadalafil analogue by diode array detector after UPLC separation, whose UV absorbance spectrum are quite resemble to tadalafil but with different retention times. A further analysis by a TQD tandem mass spectrometer through comparison of parent ion and daughter ion of the target compound verified that three positive samples contain tadalafil and two positive samples contain animo-tadalafil, respectively (). The result showed good agreement between the IC strips and UPLC-PDA-MS/MS.
Table 3. Comparison of IC analysis, ic-ELISA and UPLC-DAD-MS/MS in analysis of 60 herbal products for screening of analogues of tadalafil.
Conclusions
In order to screen tadalafil and related analogues in functional foods simultaneously, it is most important to generate antibodies being broad-specific to this class of drugs. As a desired antigen design, tadalafil was activated through an oximation reaction for conjugation, leaving the common structure of tadalafil analogues exposed as a major antigenic site. The referring ic-ELISA based on raised antisera showed satisfied specificities to tadalafil and its major analogues with IC50 values ranging from 16.1 to 29.5 ng mL−1. The IC assay showed detection thresholds ranging in 5∼20 μg g–1 for tadalafil and major analogues when detected fortified herbal samples in a dilution ratio of 1:103. The IC strip was in good agreement with UPLC-PDA-MS/MS when detected herbal products containing this class of compounds. Thus, the current IC assay is a suitable tool for screening tadalafil and its analogues as illegal additives in herbal products.
Acknowledgements
The authors are grateful to Shaoguan Institute for Food and Drug Control for technical assistance of UPLC-PDA-MS/MS in validation of the current IC assay.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Jie-Biao Guo got his bachelor from East China University of Science and Technology , Shanghai, China in 1994. After working for antibody drugs in enterprise for years, he came back to study in Nanchang University (Nanchang, China) and got his Master and doctoral Degree in food science. His research interests are development of immunoassays for illegal compounds in food.
Wang-Pei Liu got his bachelor from Guangdong Pharmaceutical University, Guangzhou, China in 2000. He was trained in National Institute of Food and Drug Control for 1 year. His research interests are development of analytic method of UPLC-MS/MS.
Hua-Long Chen got his bachelor from Guangdong Pharmaceutical University, Guangzhou, China in 2001. He was trained in National Institute of Food and Drug Control. His research interests are development of analytic method for Food control.
Min-Ying Zhang got her bachelor from Shaoguan College, Shaoguan, China in 2016. Her research interests are development of immunoassays for Food safety control.
Xian-Quan Lan got his bachelor from Guangdong Pharmaceutical University, Guangzhou, China in 2001. He came back to campus and got his Master degree from Sun Yat-sen University, Guangzhou, China in 2013. His research interests are development of analytic method for Food control.
Additional information
Funding
References
- Bortolini, C., Pivato, A., Bogialli, S., & Pastore, P. (2015). “One-shot” analysis of PDE-5 inhibitors and analogues in counterfeit herbal natural products using an LC-DAD-QTOF system. Analytical and Bioanalytical Chemistry, 407, 6207–6216. doi: 10.1007/s00216-015-8801-4
- Fleshner, N., Harve, M., Adomat, H., Wood, C., Eberding, A., Hersey, K., & Guns, E. (2005). Evidence for contamination of herbal erectile dysfunction products with phosphodiesterase-5 inhibitors. The Journal of Urology, 174, 636–641. doi: 10.1097/01.ju.0000165187.31941.cd
- Food and Drug Administration’s reporting system for an adverse event or sentinel event. Retrieved October 18, 2007, November 14, 2007, from http://www.fda.gov/medwatch/report.htm.2007
- Gilard, V., Balayssac, S., Tinaugus, A., Martins, N., Martino, R., & Malet-Martino, M. (2015). Detection, identification and quantification by 1H NMR of adulterants in 150 herbal dietary supplements marketed for improving sexual performance. Journal of Pharmaceutical and Biomedical Analysis, 102, 476–493. doi: 10.1016/j.jpba.2014.10.011
- Gratz, S. R., Flurer, C. L., & Wolnik, K. A. (2004). Analysis of undeclared synthetic phosphor-diesterase-5 inhibitors in dietary supplements and herbal matrices by LC-ESI-MS and LC-UV. Journal of Pharmaceutical and Biomedical Analysis, 36, 525–533. doi: 10.1016/j.jpba.2004.07.004
- Gratz, S. R., Gamble, B. M., & Flurer, R. A. (2006). Accurate mass measurement using Fourier transform ion cyclotron resonance mass spectrometry for structure elucidation of designer drug analogs of tadalafil, vardenafil and tadalafil in herbal and pharmaceutical matrices. Rapid Communications in Mass Spectrometry, 20, 2317–2327. doi: 10.1002/rcm.2594
- Guo, J. B., Liu, W. P., Lan, X. Q., Chen, H. L., & Xiao, Z. J. (2016). Development and evaluation of an immunochromatographic strip for rapid screening of sildenafil-type compounds as illegal additives in functional foods. Food Additives & Contaminants: Part A, 33, 1095–1104. doi: 10.1080/19440049.2016.1203072
- Hadwiger, M. E., Trehy, M. L., Ye, W., Moore, T., Allgire, J., & Westenberger, B. (2010). Identification of amino-tadalafil and rimonabant in electronic cigarette products using high pressure liquid chromatography with diode array and tandem mass spectrometric detection. Journal of Chromatography A, 1217, 7547–7555. doi: 10.1016/j.chroma.2010.10.018
- Hasegawa, T., Saijo, M., Ishii, T., Nagata, T., Haishima, Y., Kawahara, N., & Goda, Y. (2008). Structural elucidation of a tadalafil analogue found in a dietary supplement. Journal of the Food Hygienic Society of Japan, 49, 311–315. doi: 10.3358/shokueishi.49.311
- Hasegawa, T., Takahashi, K., Saijo, M., Ishii, T., Nagata, T., Kurihara, M., … Kawahara, N. (2009). Isolation and structural elucidation of cyclopentynafil and N-octylnortadalafil found in a dietary supplement. Chemical & Pharmaceutical Bulletin (Tokyo), 57, 185–189. doi: 10.1248/cpb.57.185
- Huang, Y. C., Lee, H. C., Lin, Y. L., Li, C. Y., Tsai, C. F., & Cheng, H. F. (2016). Separation and identification of a novel tadalafil analogue adulterant in a dietary supplement. Food Additives & Contaminants, Part A., 33, 179–185.
- Huang, Y. C., Lee, H. C., Lin, Y. L., Tsai, C. F., & Cheng, H. F. (2016). Identification of a new tadalafil analogue, dipropylaminopretadalafil, in a dietary supplement. Food Additives & Contaminants, Part A, 33, 953–958. doi: 10.1080/19440049.2016.1184530
- Huang, Z. B., Xu, Y., Li, L. S., Li, Y.-P., Zhang, H., & He, Q.-H. (2012). Development of an immunochromatographic strip for rapid simultaneous detection of deoxynivalenol and zearalenone in wheat and maize. Food Control, 28, 7–12. doi: 10.1016/j.foodcont.2012.04.035
- Jeong, J. H., Lee, J. H., Kim, H. J., Park, H. J., Hwang, I. S., Han, K. M., … Kim, W. S. (2016). LC-ESI-MS/MS analysis of phosphodiesterase-5 inhibitors and their analogues in foods and dietary supplements in Korea. Food Additives & Contaminants, Part B, 9, 1–8. doi: 10.1080/19393210.2014.968220
- Kern, S. E., Nickum, E. A., Flurer, R. A., Toomey, V. M., & Litzau, J. J. (2015). Isolation and structural characterization of a new tadalafil analog (2-hydroxyethylnortadalafil) found in a dietary supplement. Journal of Pharmaceutical and Biomedical Analysis, 103, 99–103. doi: 10.1016/j.jpba.2014.10.021
- Kong, D. Z., Liu, L. Q., Song, S. S., Kuang, H., & Xu, C. L. (2016). Development of ic-ELISA and lateral-flow immunochromatographic assay strip for the detection of folic acid in energy drinks and milk samples. Food and Agricultural Immunology, 27, 841–854. doi: 10.1080/09540105.2016.1183600
- Lebel, P., Gagnon, J., Furtos, A., & Waldron, K. C. (2014). A rapid quantitative liquid chromatography-mass spectrometry screening method for 71 active and 11 natural erectile dysfunction ingredients present in potentially adulterated or counterfeit products. Journal of Chromatography A, 1343, 143–151. doi: 10.1016/j.chroma.2014.03.078
- Lee, E. S., Kim, K. W., Lee, J. H., Han, K. M., Cho, S., Hwang, I., … Kim, J. (2013). Identification of a new tadalafil analogue found in a dietary supplement. Food Additives & Contaminants, Part A, 30, 621–626. doi: 10.1080/19440049.2013.766766
- Lee, J. H., Kim, H. J., Mandava, S., Hwang, J., Park, H. J., Cho, S., … Lee, J. (2015). Identification of a new tadalafil analogue in an adulterated dietary supplement: Trans-Bisprehomotadalafil. Journal of Pharmaceutical and Biomedical Analysis, 115, 352–358. doi: 10.1016/j.jpba.2015.07.023
- Lee, J. H., Kim, H. J., Noh, E., Kim, J. Y., Cho, S. H., Do, J.-A., … Kim, W. S. (2015). Identification and screening of a tadalafil analogue found in adulterated herbal products. Journal of Pharmaceutical and Biomedical Analysis, 103, 80–84. doi: 10.1016/j.jpba.2014.11.006
- Lee, E.-S., Lee, J. H., Han, K. M., Kim, J. W., Hwang, I. S., Cho, S., … Kim, J. (2013). Simultaneous determination of 38 phosphodiestrase-5 inhibitors in illicit erectile dysfunction products by liquid chromatography-electrospray ionization-tandem mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis, 83, 171–178. doi: 10.1016/j.jpba.2013.05.009
- Lee, J. H., Mandava, S., Baek, S. Y., & Lee, Y.-M. (2016). Identification and structural elucidation of three new tadalafil analogues found in a dietary supplement. Journal of Pharmaceutical and Biomedical Analysis, 123, 1–9. doi: 10.1016/j.jpba.2015.10.039
- Lee, J. H., Park, H. N., Ganganna, B., Jeong, J. H., Park, S. K., Lee, J., & Baek, S. Y. (2016). Isolation and structural elucidation of a new tadalafil analogue in health supplements: Bisprenortadalafil. Food Additives & Contaminants, Part A, 33, 945–952. doi: 10.1080/19440049.2016.1179134
- Mukunzi, D., Tochi, B. N., Isanga, J., Liu, L. L., Kuang, H., & Xu, C. L. (2016). Development of an immunochromatographic assay for hexestrol and diethylstilbestrol residues in milk. Food and Agricultural Immunology, 27, 855–869. doi: 10.1080/09540105.2016.1183601
- Patel, D. N., Li, L., Kee, C. L., Ge, X., Low, M. Y., & Koh, H. L. (2014). Screening of synthetic PDE-5 inhibitors and their analogues as adulterants: Analytical techniques and challenges. Journal of Pharmaceutical and Biomedical Analysis, 87, 176–190. doi: 10.1016/j.jpba.2013.04.037
- Poon, W. T., Lam, Y. H., Lai, C. K., Chan, A. Y., & Mak, W. L. (2007). Analogues of erectile dysfunction drugs: An under-recognised threat. Hong Kong Medical Journal, 13, 359–363.
- Schramek, N., Wollein, U., & Eisenreich, W. (2014). Identification of new synthetic PDE-5 inhibitors analogues found as minor components in a dietary supplement. Journal of Pharmaceutical and Biomedical Analysis, 96, 45–53. doi: 10.1016/j.jpba.2014.03.023
- Singh, S., Prasad, B., Savaliya, A. A., Shah, R. P., Gohil, V. M., & Kaur, A. (2009). Strategies for characterizing tadalafil, vardenafil, tadalafil and their analogues in herbal dietary supplements, and detecting counterfeit products containing these drugs. Trends in Analytical Chemistry, 28, 13–28. doi: 10.1016/j.trac.2008.09.004
- Skalicka-Wozniak, K., Georgiev, M. I., & Orhan, I. E. (2016). Adulteration of herbal sexual enhancers and slimmers: The wish for better sexual well-being and perfect body can be risky. Food and Chemical Toxicology. Corrected Proof Available online 20 June 2016.
- Sugita, M., & Miyakawa, M. (2010). Economic analysis of use of counterfeit drugs: Health impairment risk of counterfeit phosphodiesterase type 5 inhibitor taken as an example. Environmental Health and Preventive Medicine, 15, 244–251. doi: 10.1007/s12199-010-0134-5
- Taylor, J., Baldo, O. B., Storey, A., Cartledge, J., & Eardley, I. (2009). Differences in side-effect duration and related bother levels between phosphodiesterase type 5 inhibitors. British Journal of Urology International, 103, 1392–1395. doi: 10.1111/j.1464-410X.2008.08328.x
- Toomey, V. M., Litzau, J. J., & Flurer, C. L. (2012). Isolation and structural characterization of two tadalafil analogs found in dietary supplements. Journal of Pharmaceutical and Biomedical Analysis, 59, 50–57. doi: 10.1016/j.jpba.2011.09.038
- Ulloa, J., Sambrotta, L., Redko, F., Mazza, O. N., Garrido, G., Becher, E. F., & Muschietti, L. (2015). Detection of a tadalafil analogue as an adulterant in a dietary supplement for erectile dysfunction. The Journal of Sexual Medicine, 12, 152–157. doi: 10.1111/jsm.12759
- Venhuis, B. J., Blok-Tip, L., & de Kaste, D. (2008). Designer drugs in herbal aphrodisiacs. Forensic Science International, 177, e25–e27. doi: 10.1016/j.forsciint.2007.11.007
- Venhuis, B. J., & de Kaste, D. (2012). Towards a decade of detecting new analogues of sildenafil, tadalafil and vardenafil in food supplements: A history, analytical aspects and health risks. Journal of Pharmaceutical and Biomedical Analysis, 69, 196–208. doi: 10.1016/j.jpba.2012.02.014
- Xiang, Z. M., Zhang, L., Zhu, M., & Zheng, C. (2010). Identification of tadalafil analog illegally added in dietary supplement using HPLC and LC-MS/MS. Chinese Journal of Modern Applied Pharmacy, 27, 167–171.
- Xu, Y., Kee, C.-L., Ge, X., Low, M.-Y., & Koh, H.-L. (2016). Isolation and characterization of a tadalafil analogue, N-cyclopentyl nortadalafil in health supplement. Journal of Pharmaceutical and Biomedical Analysis, 118, 235–241. doi: 10.1016/j.jpba.2015.08.005
- Zhang, G., Yu, Y., Wu, X., & Li, J. (2014). Separation and structural elucidation of a new tadalafil analogue diethylaminopretadalafil included as an adulterant in a dietary supplement. Journal of Pharmaceutical and Biomedical Analysis, 94, 210–214. doi: 10.1016/j.jpba.2014.01.043
- Zhu, X. L., Xiao, S., Chen, B., & Yao, S. Z. (2005). Simultaneous determination of tadalafil, vardenafil and tadalafil as forbidden components in natural dietary supplements for male sexual potency by high performance liquid chromatography-electrospray ionization mass spectrometry. Journal of Chromatography A, 1066, 89–95. doi: 10.1016/j.chroma.2005.01.038
- Zou, P., Hou, P., Low, M. Y., & Koh, H. L. (2006). Structural elucidation of a tadalafil analogue found as an adulterant of a herbal product. Food Additives and Contaminants, 23, 446–451. doi: 10.1080/02652030500479690
- Zou, P., Hou, P., Oh, S. S., Low, M. Y., & Koh, H. L. (2006). Electrospray tandem mass spectrometric investigations of tadalafil and its analogue. Rapid Communications in Mass Spectrometry, 3488–3490. doi: 10.1002/rcm.2752