ABSTRACT
Mao-tofu was fermented for 6 days with a Mucor strain and extracted with pH 6.5 water and 40% ethanol to generate respective two extracts. Immune potentials of Mao-tofu and two extracts were assessed, via assessing the changes of several immune indices related to immune organs, cells, and molecules in normal mice. Compared with the unfermented tofu, Mao-tofu had somewhat higher immune activities towards the mice in all cases, indicating improved immune potential by the Mucor-mediated fermentation. More important, two extracts (especially water extract) had much higher immune activities towards the mice than Mao-tofu (P < .05). Two extracts were in dose-dependent manner to improve immune status of the mice, via increasing spleen and thymus indices, percentages of T-helper (CD4+) and T-cytotoxic (CD8+) lymphocytes in plasma, serum contents of two complements (C3 and C4) and Th1 cytokines (IL-2 and IFN-γ), but decreasing serum contents of Th2 cytokines (IL-4, IL-5, and IL-10).
GRAPHICAL ABSTRACT
1. Introduction
The immune system contains immune organs, cells, and molecules that perform immune functions in the body (Chandra, Citation1997). Immune organs include thymus, lymph nodes, and spleen. Immune cells mainly refer to lymphocytes, mononuclear phagocytes, and granulocytes. Immune molecules mainly compose of immunoglobulins, complements, and these specific and non-specific tutoring factors involved in immune responses of the body. Immune system is vital in the prevention and control of diseases and infections. The functions of immune system can be adversely affected by many factors such as body stress, unhealthy life-style practices, pathogens, and foreign antigens, and can also be activated by other factors such as optimistic feeling, healthy life-style, and nutritionally balanced diet. To ensure body health, modulating immune functions of the body via the daily dietary approach is essential and practical (Abdullah, Abdulghani, Ismail, & Abidin, Citation2017). Many foods contain natural components with immune potentials (Chirumbolo, Citation2012; Schmitz & Chevaux, Citation2000). Growing evidences show that there are a lot of immune modulators in natural foods, such as polysaccharides (Hao & Zhao, Citation2016) and immuno-modulatory peptides (Santiago-López, Hernández-Mendoza, Vallejo-Cordoba, Mata-Haro, & González-Córdova, Citation2016).
Food fermentation is a microorganism-mediated process, during which some new bioactive products can be yielded from raw food materials (Paredes-López & Harry, Citation1988). More important, this process may produce some bioactive components with healthcare functions (Parvez, Malik, Ah Kang, & Kim, Citation2006). Fermented foods have been well-studied for their healthcare properties in the past years (Hong et al., Citation2017). Food fermentation results in improved nutritional value and higher contents of several bioactive compounds such as isoflavone aglycones (Silva, Celeghini, & Chang, Citation2011), bioactive peptides (Lin, Liu, Liu, & Yu, Citation2017; Sanjukta & Rai, Citation2016), and especially the formation of gamma-aminobutyric acid from glutamic acid (Ko, Lin, & Guo, Citation2013). Consequentially, fermented soybean products have better healthcare properties than their unfermented counterparts (Yasuda, Citation2010), including anti-hypertension (Rhyu, Kim, & Han, Citation2002), anti-oxidation (Hu et al., Citation2004; Yang, Mau, Ko, & Huang, Citation2000), anti-diabetes (Lim et al., Citation2012), anti-obesity, and improved lipid metabolism (Cha et al., Citation2013; Lee et al., Citation2013). Besides these mentioned biological activities, immune potentials of fermented products can also be improved (Karasawa, Sugiura, Kojima, Uzuhashi, & Otani, Citation2013; Shi, Yang, Hu, & Zhang, Citation2014); for example, red alga receives enhanced immune activity after fermentation (Lin, Hwang, Lin, & Tsai, Citation2014). In general, fermented foods are considered with better healthcare properties than the unfermented ones.
Mao-tofu is one of the fermented soybean products found in several provinces of Central China. In general, Mao-tofu is produced by a short-time fermentation of tofu using a strain of Mucor (Zhao & Zheng, Citation2009). The results show that the Mucor-mediated fermentation brings desired protein degradation and texture improvement for Mao-tofu (Zhao & Zheng, Citation2009). At the same time, soluble extracts of Mao-tofu using water- and ethanol-based solvents exhibit enhanced in vitro anti-oxidant and anti-hypertensive activities, which are governed by both fermentation times of Mao-tofu and the used extracting solvents (Hang & Zhao, Citation2011, Citation2012). Mao-tofu is thus proved as one of the fermented soybean products with desired healthcare function. Immune potentials of Mao-tofu as well as its soluble extracts are also important, but still not assessed so far. To provide more scientific evidence about healthcare properties of Mao-tofu, it is necessary to assess both in vitro and in vivo immune activities of Mao-tofu and its soluble extracts using model cells and animals.
Our previous study has proved in vitro immune activities of soluble Mao-tofu extracts using water of pH 6.5 and 40% ethanol (Liu & Zhao, Citation2017). In this study, Mao-tofu samples with fermentation time of 6 days were also extracted with the two solvents to generate respective soluble extracts. The normal mice were used as animal model, given Mao-tofu and the two extracts for 4 weeks, and then assessed for the changes of several immune indices including two immune organs (spleen and thymus), two immune cell subpopulations (T-lymphocyte subset CD4+ and CD8+) in plasma, as well as serum levels of three immunoglobulins (IgA, IgG, and IgM), two complements (C3 and C4), and five cytokines (IL-2, IL-4, IL-5, IL-10, and IFN-γ). The aim of this study was to provide necessary evidence about immune potential of Mao-tofu, reflected by in vivo immune-enhancing efficiencies of Mao-tofu and especially soluble extracts for the normal mice.
2. Materials and methods
2.1 Materials and reagents
Mouse T-lymphocyte subset antibody-phycoerythrin (PE) anti-mouse CD8a antibodies and fluorescein isothiocyanate (FITC) anti-mouse CD4 antibodies were bought from Miltenyi Biological Technology Co. Ltd (Bergisch Gladbach, Cologne, Germany). Mouse cytokines (IL-2, IL-4, IL-5, IL-10, and IFN-γ) in serum ELISA Kits were bought from Wuhan Boster Biological Engineering Co. Ltd (Wuhan, Hubei Province, China). Mouse immunoglobulins (IgA, IgG, and IgM) and complements (C3 and C4) in serum ELISA Kits were bought from BlueGene Biotech Co. Ltd (Shanghai, China). Phosphate-buffered solution (PBS) and red blood cell lysis buffer were bought from Solarbio Science and Technology Co. Ltd (Beijing, China). The used water was generated from the Milli-Q PLUS (Millipore Corporation, New York, NY, USA). Other chemicals used were of analytical grade.
A Mucor strain was isolated from commercial Mao-tofu from Xiuning County (Anhui Province, China). Strain isolation and purification procedures followed those used in a previous study (Zhao & Zheng, Citation2009). The Mucor was incubated onto the potato dextrose agar for 3 days to obtain spore suspension in sterilized distilled water (about 5 × 105 spores mL−1), which was then used in Mao-tofu fermentation.
2.2 Preparation and analyses of Mao-tofu and its extracts
Commercial tofu was bought from local market of Harbin City (Heilongjiang Province, China). The tofu was cut into a size of 6 × 3 × 2 cm, sterilized in an autoclave at 121°C for 15 min to inactivate the polluted microorganisms and enzymes, and then slightly dried after cooling. The sterilized tofu was coated with the spore suspension on the surface and then placed in an incubator at 18−20°C for 6 days to conduct the Mucor-mediated fermentation.
Mao-tofu samples fermented for 6 days were heated at 95°C for 5 min to stop the action of the Mucor strain or enzymes. Mao-tofu samples of 30 g were mixed with pH 6.5 water and 40% (v/v) ethanol of 100 mL, respectively, and then stirred by a high-speed homogenizer (Type DS-1, Shanghai Jingke Ltd, Shanghai, China) for 1 min. After a centrifugation at 4000 × g for 20 min, the resultant supernatants were collected, concentrated by solvent evaporation, and then freeze-dried to obtain water and ethanol extract, respectively. The two lyophilized powders were used in animal experiments. Unfermented tofu and some Mao-tofu samples were also freeze-dried, which were used as control samples in animal experiments.
Protein contents of tofu, Mao-tofu, and the two extracts were all assessed by using the classic Kjeldahl method and a conversion factor of 6.25.
2.3 Animal experiments
Female BALB/c mice (7 weeks old, 18–20 g) were provided by Beijing Vital River Experimental Animal Technical Co. Ltd (Beijing, China). Totally 90 mice were used in this study. All mice were housed in propylene cages under standard illumination condition with 12-h light–dark cycle at 23 ± 2°C with relative humidity of 54 ± 5%. All mice were fed with the recommended pellet diet from the Beijing Vital River Experimental Animal Technical Co. Ltd (Beijing, China) and purified water ad libitum. Experimental procedures were performed in accordance with the Ethical Guidelines of the Animal Care and Use Committee of Northeast Agricultural University (Harbin, China).
The mice were randomly divided into 9 groups (10 mice for each group) after an acclimatization of 7 days. Animal groups and sample doses of this study are detailedly shown in . The mice in Group I were given sterilized saline solution (0.9% NaCl) and designed as control mice. The mice in Groups II−III were given unfermented tofu and Mao-tofu at a dose level of 80 mg protein kg−1 body weight, respectively. The mice in Groups IV−VI were given water extract set at three dose levels of 40, 80, and 160 mg protein kg−1 body weight, respectively. The mice in Groups VII−IX were given ethanol extract at the same three dose levels. All evaluated samples were dispersed in the sterilized saline solution and administered daily by oral gavage. After the latest administration, all mice were anesthetized by CO2 inhalation and then sacrificed by cervical dislocation. Some tissues and whole blood were collected, and then used in immunological and hematological evaluations.
Table 1. Groups and sample doses used for the experimental mice.
2.4 Assays of body weights and immune organs
Body weights of the mice were measured every week using an electronic balance (Mettler Toledo, Greifensee, Switzerland). The weights of spleen and thymus collected from the mice were also measured by the balance. Spleen and thymus indices were expressed as the organ weights relative to the body weights (Guo et al., Citation2012).
2.5 Assays of T-lymphocyte subpopulations
The percentages of T-helper (CD4+) and T-cytotoxic (CD8+) lymphocytes in the blood were measured by a direct immunofluorescent staining with flow cytometric analysis. The fresh whole blood sample from the mouse before scarification was diluted at 1:5 using the PBS (pH 7.4). An aliquot (500 μL) of this dilution was centrifuged at 190 × g for 10 min. The sediment was re-suspended with the red blood cell lysis buffer (500 μL) for 2 min and then centrifuged at 190 × g for 10 min. This treatment was repeated 2−3 times until the residual sediment was observed to be white. The sediment was then suspended in 500 μL PBS to achieve final cell density of 1 × 106 cells mL−1, followed by the addition of phycoerythrin (PE) anti-mouse CD8a+ antibodies (10 μL) and FITC anti-mouse CD4+ antibodies (10 μL). The mixed suspension was incubated in the dark at 4°C for 15 min. The stained cells were then passed through a 300-mesh sieve and analyzed at a BD FACS Aria II flow cytometer (BD Bioscience, Franklin Lakes, NJ, USA).
2.6 Assays of hematological parameters
Hematological parameters of the mice including total white blood cell count, red blood cell count, hemoglobin, hematocrit, mean corpuscular hemoglobin, platelet count, and differential leucocyte counts were measured using the whole blood samples collected in the heparinized tubes. These analyses were conducted at an automated blood cell counter (Medonic-CA 620, Londe, Sweden).
2.7 Evaluations of serum immunoglobulins, complements, and cytokines
The whole blood was collected by eye bleeding before mouse scarification and kept at 4°C for 12 h to separate serum. Subsequently, natural stratified plasma was centrifuged at 500 × g for 10 min to obtain supernatant. The serum was appropriately diluted and measured for three immunoglobulins (IgA, IgG, and IgM), two complements (C3 and C4), and five cytokines (IL-2, IL-4, IL-5, IL-10, and IFN-γ) using the respective ELISA Kits and the protocols provided by the kit producers. The optical density was measured at 450 nm in a microplate reader (Bio Rad Laboratories, Hercules, CA, USA).
2.8 Statistical analysis
All reported data were from three independent analyses and evaluations, and expressed as means ± standard deviations (SD) performed in at least six mice (n = 6) for each group. Statistical analysis was conducted using the SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). The differences (P < .05) between the mean values of multiple groups were analyzed by one-way analysis of variance (ANOVA) with Duncan’s multiple range tests.
3. Results and discussion
3.1 Weight grains and immune organ indices of the mice responsible to Mao-tofu and two extracts
Protein contents of tofu and Mao-tofu samples were near 60% on dry basis, while those of water and ethanol extracts were about 49.7% and 42.9% on dry basis, respectively. Weight gains of the mice after experiments are shown in . The results indicate that the normal mice of different groups had some differences in body weights. The mice administrated with unfermented tofu and Mao-tofu (Groups II−III) showed slightly differences in weight grains (2.49−2.61 g) (P > .05), but obtained weight grains higher than the control mice (Group I, 1.58 g) (P < .05). Other mice administrated with the two extracts (Groups IV−IX) had much higher weight grains than the control mice or those administrated with unfermented tofu and Mao-tofu (Groups II−III) (P < .05). In general, the mice in Groups IV−VI (or Groups VII–IX) in dose-dependent manner showed increased weight gains (P < .05), resulting in weight grains of 3.13−3.83 (or 3.05−3.68) g. Spleen and thymus indices of the mice after the last administration are also reported in . Compared with the control mice, the mice administrated with unfermented tofu and Mao-tofu had somewhat higher index values (spleen indices 3.38−3.46 versus 3.18 g kg−1, or thymus indices 2.17−2.25 versus 2.03 g kg−1) (P > .05). Administration of Mao-tofu resulted in slight but insignificant increases in the two indices, in comparison with administration of unfermented tofu (P > .05). That is, administration of Mao-tofu only conferred the mice with slightly improved immune status. If the mice were administrated with the two extracts, they in dose-dependent manner showed much improved immune status. Administration of water extract resulted in spleen indices of 3.61−3.96 g kg−1 and thymus indices of 2.41−2.98 g kg−1. Administration of ethanol extract led to spleen indices of 3.55−3.78 g kg−1 and thymus indices of 2.28−2.75 g kg−1. The two extracts thus rendered much higher index values in the mice compared with unfermented tofu or Mao-tofu did (P < .05). It is thus concluded that the Mucor-mediated fermentation conferred Mao-tofu with enhanced immune potential; however, the two extracts had higher immune activities than unfermented tofu or Mao-tofu. The components in the two extracts are thus suggested making main contribution to the enhanced immune potential of Mao-tofu.
Table 2. Body weight gains and immune organ indices of the mice after an experimental period of 4 weeks.
Weight gains of the mice during the experimental period indicated that Mao-tofu and the two extracts could promote the growth and development of the normal mice. Different weight gains among these mouse groups were mainly caused by the assessed samples, which is not the main object of this study and therefore not discussed here. Both spleen and thymus are vital immune organs; in consequence, both spleen and thymus indices have been used in studies to reflect the development of immune system as well as immune status of the body (Yang, Xie, & Depierre, Citation2000). Several substances including polysaccharides and protein digests have been detected with immune potentials via affecting immune organs or weight grains of the mice. Hazelnut-hydrolyzed peptides exhibit immune-enhancing effect on the mice, as they can increase spleen and thymus indices by 38−52% (Ren et al., Citation2016). Water soluble polysaccharides from Chinese yam show immuno-enhancing activity to the normal mice, by elevating spleen and thymus indices by 22−42% (Hao & Zhao, Citation2016). Cordyceps militaris polysaccharides can improve immune function of the mice via increasing spleen and thymus indices (Liu et al., Citation2016). In addition, the polysaccharides from Chinese rice wine can also increase weight gain and immune organ indices of the mice (Shen, Mao, Chen, Meng, & Ji, Citation2014). Sharing similarity to these mentioned studies, this study proved that Mao-tofu had slight activation on the two immune organs than unfermented tofu, whilst the two extracts (especially water extract) had stronger activities than Mao-tofu to increase the two immune organ indices. The obtained results in this study demonstrated that the Mucor-mediated fermentation improved immune potential of tofu.
3.2 Plasma T-lymphocyte subpopulations of the mice responsible to Mao-tofu and two extracts
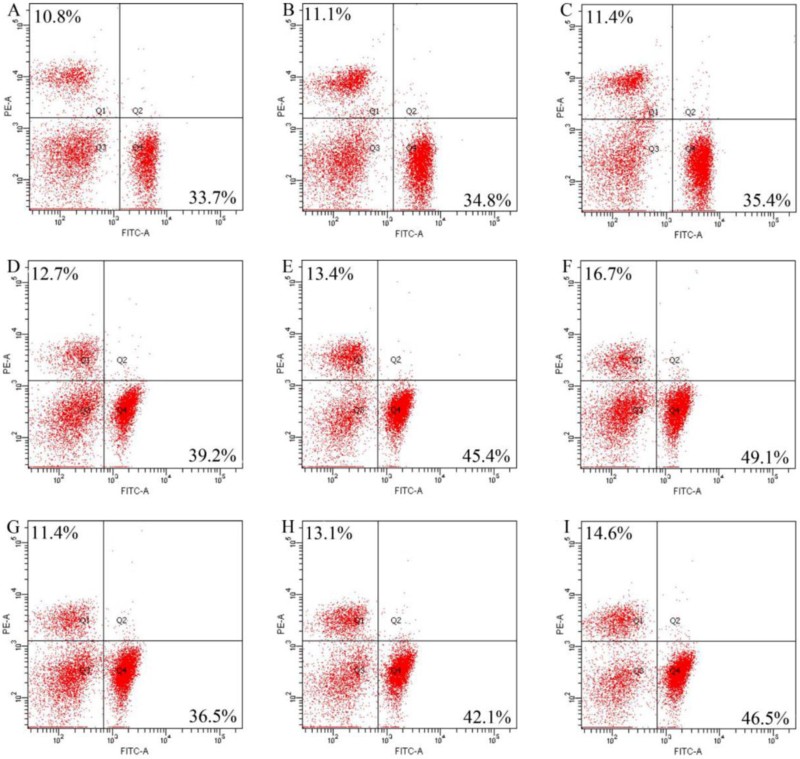
Hematological parameters of the normal mice after the experimental period of 4 weeks are reported in the Appendixes 1 and 2. In general, the mice among different groups showed some value differences in these measured indices. These differences are not discussed in this paper. Plasma samples collected from the mice were also analyzed for their cell surface antigens, to identify T-lymphocyte subpopulations. The results () demonstrated that there were significant population increases (P < .05) in both T-helper (CD4+) and T-cytotoxic (CD8+) cells in the mice administrated with the two extracts. The plasma samples from the control mice only had respective CD4+ and CD8+ cells of 33.7% and 10.8%. Administration of unfermented tofu slightly increased CD4+ and CD8+ cells to 34.8% and 11.1%, while administration of Mao-tofu enhanced CD4+ and CD8+ cells into 35.4% and 11.4%, respectively. Unfermented tofu and Mao-tofu were thus found to have similar influence on CD4+ and CD8+ subpopulations (P > .05). However, the two extracts in dose-dependent manner enhanced both CD4+ and CD8+ subpopulations. Water extract at three dose levels enhanced CD4+ and CD8+ cells into respective 39.2−49.1% and 12.7−16.7%. Ethanol extract at the three dose levels enhanced CD4+ and CD8+ into respective 36.5−46.5% and 11.4−14.6%. Compared with ethanol extract, water extract at the same dose level clearly resulted in higher CD4+ and CD8+ subpopulations. In comparison with Mao-tofu, water extract at each dose level showed higher activities while ethanol extract only at two dose levels (80 and 160 mg kg−1) exerted higher activities. It is thus briefly concluded that the Mucor-mediated fermentation resulted in Mao-tofu with improved immune potential, and using water as extracting solvent conferred the resultant extract with higher activity compared with using 40% ethanol.
Table 3. Percentages of T-lymphocyte subpopulations (CD4+ and CD8+) in the mice plasma samples after an experimental period of 4 weeks.
T-lymphocytes are the main effector cells of the immune system and play an important role in the adaptive immunity (Gertsch, Viveros-paredes, & Taylor, Citation2011; Hou, Fan, Li, Xue, & Yu, Citation2012). Mature T-lymphocytes mainly distribute in peripheral lymphoid tissues via blood circulation (Li et al., Citation2000). CD4+ and CD8+ as two major functional subpopulations of T-lymphocytes involve in humoral mediation, and their numbers and ratios are important for maintaining stable immune systems in the body (Unger et al., Citation2012). CD4+ and CD8+ subpopulations are thus assessed in many studies as important immune indices. It is known that the substances with potential immune activities are capable of increasing CD4+ cells and the ratio of CD4+ to CD8+ cells; for example, the peptides from chum salmon (Yang et al., Citation2009) and rohu egg (Chalamaiah et al., Citation2014) show immune-stimulation by increasing the percentages of CD4+ and CD8+ cells in spleen. The diet enriched with fermented milk and other substances can enhance gut-associated lymphoid tissue mass including increased numbers of CD4+ and CD8+ cells in Peyer’s patches and lamina propria (Yanagawa et al., Citation2013). The polysaccharide extract from Ganoderma lucidum can also increase the number of CD4+ and CD8+ cells in the plasma of the normal mice (Lai et al., Citation2010). In consistent with these published results, the present results indicated that Mao-tofu and especially the two extracts also could increase CD4+ and CD8+ T-lymphocytes in the plasma. Moreover, it was also observed that Mao-tofu had activity lower than the two extracts to increase CD4+ and CD8+ T-lymphocytes.
3.3 Serum immunoglobulins and complements of the mice responsible to Mao-tofu and two extracts
The contents of three immunoglobulins (IgA, IgG, and IgM) in the sera of the normal mice were influenced by the administration of these samples (). Compared with the control mice, the mice administrated with unfermented tofu and Mao-tofu had increased contents of the three immunoglobulins. Mean contents of IgA, IgG, and IgM were enhanced from 12.9 mg mL−1, 721.8, and 738.2 μg mL−1 into 13.6 mg mL−1, 778.3, and 750.1 μg mL−1 (in case of tofu), or into 14.9 mg mL−1, 782.8, and 762.4 μg mL−1 (in case of Mao-tofu). Mao-tofu was stronger than unfermented tofu to increase serum immunoglobulin contents. Administration of water extract at the three dose levels brought higher contents of the three immunoglobulins; IgG contents were detected ranging from 14.4 to 18.6 mg mL−1, whilst IgA and IgM contents were 870.5−1010.1 and 814.2−997.5 μg mL−1, respectively. However, administration of ethanol extract yielded IgG, IgA, and IgM contents of 13.8−18.2 mg mL−1, 816.5−946.7, and 786.2−872.1 μg mL−1, respectively. Based on these measured values, the two extracts were more able than Mao-tofu to increase serum immunoglobulin contents; moreover, water extract was more powerful than ethanol extract to increase serum immunoglobulin contents.
Table 4. Serum immunoglobulin and complement levels of the mice after an experimental period of 4 weeks.
Administration of these samples also showed some impacts on the contents of two complements (C3 and C4) in the sera (), implying different immune activities of these samples towards the mice. Unfermented tofu and Mao-tofu increased C3 contents from 5.1 to 5.4 and 5.7 μg mL−1, and promoted C4 contents from 2.9 to 3.1 and 3.2 μg mL−1, respectively, evidencing unfermented tofu and Mao-tofu had immune-enhancing activities via elevating C3 and C4 yields. Water extract rendered C3 contents of 6.9−8.3 μg mL−1 and was more active than ethanol extract (C3 contents of 6.3−8.2 μg mL−1). Water extract also brought C4 contents of 3.5−4.9 μg mL−1 and totally was slightly active than ethanol extract (C4 contents of 3.4−4.6 μg mL−1). In general, the two extracts were stronger than Mao-tofu to promote C3 and C4 production, while water extract conferred the sera with higher contents of C3 and C4 in comparison with ethanol extract.
Immunoglobulins play a vital role in immune functions of the body. In this study, immune activities of Mao-tofu and the two extracts were explored via assessing their effects on serum contents of three immunoglobulins and two complements. Food components with immune activities can stimulate the production of immunoglobulins; for example, the bioactive fraction from Prunus cerasus can increase both serum IgG and IgM contents in the BALB/c mice (Abid et al., Citation2012). Tryptic hydrolysates from Labeo rohita roe are able to increase serum IgA in the model mice (Chalamaiah et al., Citation2014). In complement system, complement 3 (C3) and complement 4 (C4) are usually assessed to show immune activities of the assayed components; for example, functional human serum albumin can stimulate the complement system via upregulating C3 and C4 levels (He et al., Citation2011). These mentioned studies support in vivo immune-enhancing activities of Mao-tofu and the two extracts, as these evaluated samples in different extents could increase the serum production of three immunoglobulins and two complements in the normal mice.
3.4 Serum cytokine production of the mice responsible to Mao-tofu and two extracts
The collected sera were also detected to have different cytokine levels (), indicating these samples had different capacities to impact the secretion of the five immune molecules in the mice. Using unfermented tofu and Mao-tofu could promote IFN-γ and IL-2 contents from 93.3 and 35.3 (the control mice) to 94.3−95.3 and 37.2−39.5 pg mL−1, respectively. Administration of water extract rendered IFN-γ and IL-2 contents of 99.3−118.4 and 43.5−49.3 pg mL−1, while administration of ethanol extract led to somewhat lower IFN-γ and IL-2 contents of 97.1−113.3 and 40.4−46.4 pg mL−1, respectively. Ethanol extract was thus less efficient than water extract to promote IFN-γ and IL-2 secretion in the mice. However, the two extracts were more active than Mao-tofu to increase IFN-γ and IL-2 secretion (P < .05). Using serum contents of another three cytokines (IL-4, IL-5, and IL-10) as evaluation indices, a consistent conclusion about different immune activities of these samples could also be obtained. Ethanol extract lessened respective IL-4, IL-5, and IL-10 contents to 78.3−88.7, 89.4−99.5, and 87.5−99.4 pg mL−1. However, water extract decreased respective IL-4, IL-5, and IL-10 contents to 77.7−85.5, 88.2−97.8, and 84.8−97.3 pg mL−1. Mao-tofu and unfermented tofu resulted in IL-4, IL-5, and IL-10 contents of 90.2−92.5, 101.4−102.5, and 101.7−103.1 pg mL−1, declaring that Mao-tofu and unfermented tofu were insignificantly different to decrease the secretion of IL-4, IL-5, and IL-10. These measured values demonstrated that ethanol extract was less efficient than water extract to lessen the secretion of IL-4, IL-5, and IL-10; however, the two extracts were more active than Mao-tofu to decrease the secretion of the three cytokines.
Table 5. Serum cytokine levels (pg mL−1) of the mice after an experimental period of 4 weeks.
Cytokines are the major immune regulators of the body. The complex interactions between immune cells are mainly governed by various cytokines generally divided into Th1 (T-helper 1) and Th2 (T-helper 2) subsets. Th1 cells are the primary source of IL-2, IFN-γ, lymphotoxins, and other factors, which reflect the cell-mediated immune responses (Stevens et al., Citation1988). IL-4, IL-5, and IL-10 are produced by Th2 cells which not only mediate the humoral immune responses but also promote allergic reactions by inducing the function of IgE formation (Coffman & Carty, Citation1986). Some food components show immune-enhancing activities via increasing or decreasing the secretion of several cytokines. The extract of Dioscorea alata has immune activity by upregulating the expression of Th1 cytokines (IFN-γ and IL-2) but downregulating the expression of Th2 cytokines (IL-4 and IL-10) (Dey & Chaudhuri, Citation2014). Hyriopsis cumingii polysaccharides in dose-dependent manner promote IL-2 and IFN-γ production in vivo (Qiao et al., Citation2017). Doenjang and Cheonggukjang, two fermented soybean products, can upregulate production ratio of IFN-γ to IL-4 in stimulated splenic T- and B-lymphocytes (Lee et al., Citation2017). Fermented mung bean and soybean can increase IL-2 by 1.7- and 1.9-fold, and enhance IFN-γ by 15- and 18-fold, respectively (Ali et al., Citation2015). Oral administration of Sparassis crispa is also observed to decrease IL-4 and IL-5 secretion (Yao, Kyosuke, Takashi, & Munehiko, Citation2008). These mentioned results agree that Mao-tofu and especially the two extracts had enhanced immune potentials than unfermented tofu, as they all could elevate IL-2 and IFN-γ production but decrease IL-4, IL-5, and IL-10 secretion.
This study found that the Mucor-mediated fermentation brought enhanced in vivo immune potential for Mao-tofu; moreover, two soluble extracts always had immune activities higher than Mao-tofu on protein basis. This fact implies that the two extracts made main contribution to the immune potential of Mao-tofu. The roles of extracting solvents and especially the compositions of the two extracts are thus suggested for a detailed investigation, to reveal the most important components with immune significance. More important, potential formation of other bioactive compounds with immune significance (e.g. gamma-aminobutyric acid) in Mao-tofu should be verified. If the Mucor-mediated fermentation has other beneficial effect on Mao-tofu (e.g. allergic reaction of soybean proteins) is also an interesting topic.
4. Conclusions
The results indicated that the Mucor-mediated fermentation in some extent improved immune potential of Mao-tofu. Two extracts (especially that using pH 6.5 water) had higher in vivo immune activities than Mao-tofu itself, as they in dose-dependent manner conferred the normal mice with higher values in spleen and thymus indices, plasma CD4+ and CD8+ subpopulations, serum contents of two complements (C3 and C4), serum levels of Th1 cytokines (IL-2 and IFN-γ), but lower values in serum levels of Th2 cytokines (IL-4, IL-5, and IL-10). The Mucor-mediated fermentation thus rendered Mao-tofu with desired health benefit in the view of immunological point. Mao-tofu especially its extracts thus proved to have immune-enhancing potentials higher than tofu.
Acknowledgements
The authors thank the anonymous reviewers and editors for their valuable advices.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Xin Liu is studying in Northeast Agricultural University. She will shortly complete her PhD in Food Science and Technology under the direction of Prof. X. H. Zhao. Current connection postal address for her is Key Laboratory of Dairy Science, Ministry of Education, Northeast Agricultural University, Harbin 150030, People’s Republic of China, while her E-mail is [email protected].
Xin-Huai Zhao completed his MD and PhD in Food Science and Chemistry from Northeast Agricultural University and Ocean University of China, respectively. He is working in Northeast Agricultural University for more than 30 years, and has published more than 200 research papers on different domains of food chemistry. His work mostly focuses on modification and bioactivities of food proteins/peptides, health benefits of phytochemicals, and application of edible microorganism for bioconversion and biodegradation.
ORCID
Xin-Huai Zhao http://orcid.org/0000-0001-9682-5426
References
- Abdullah, N., Abdulghani, R., Ismail, S. M., & Abidin, M. H. Z. (2017). Immune-stimulatory potential of hot water extracts of selected edible mushrooms. Food and Agricultural Immunology. doi:10.1080/09540105.2017.1293011
- Abid, S., Khajuria, A., Parvaiz, Q., Sidiq, T., Bhatia, A., Singh, S., … Dutt, P. (2012). Immunomodulatory studies of a bioactive fraction from the fruit of Prunus cerasus in BALB/c mice. International Immunopharmacology, 12, 626–634. doi: 10.1016/j.intimp.2012.02.001
- Ali, N. M., Yeap, S. K., Yusof, H. M., Beh, B. K., Ho, W. Y., Koh, S. P., … Long, K. (2015). Comparison of free amino acids, antioxidants, soluble phenolic acids, cytotoxicity and immunomodulation of fermented mung bean and soybean. Journal of the Science of Food and Agriculture, 96, 1648–1658. doi: 10.1002/jsfa.7267
- Cha, Y. S., Kim, S. R., Yang, J. A., Back, H. I., Kim, M. G., Jung, S. J., … Chae, S. W. (2013). Kochujang, fermented soybean-based red pepper paste, decreases visceral fat and improves blood lipid profiles in overweight adults. Nutrition and Metabolism, 10, 24–32. doi: 10.1186/1743-7075-10-24
- Chalamaiah, M., Hemalatha, R., Jyothirmayi, T., Diwan, P. V., Kumar, P. U., Nimgulkar, C., & Kumar, B. D. (2014). Immunomodulatory effects of protein hydrolysates from rohu (Labeo rohita) egg (roe) in BALB/c mice. Food Research International, 62, 1054–1061. doi: 10.1016/j.foodres.2014.05.050
- Chandra, R. K. (1997). Nutrition and the immune system: An introduction. American Journal of Clinical Nutrition, 66, 460–463.
- Chirumbolo, S. (2012). Plant phytochemicals as new potential drugs for immune disorders and cancer therapy: Really a promising path? Journal of the Science of Food and Agriculture, 92, 1573–1577. doi: 10.1002/jsfa.5670
- Coffman, R. L., & Carty, J. (1986). A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. Journal of Immunology, 136, 949–954.
- Dey, P., & Chaudhuri, T. K. (2014). In vitro, modulation of TH1 and TH2 cytokine expression by edible tuber of Dioscorea alata, and study of correlation patterns of the cytokine expression. Food Science and Human Wellness, 3, 1–8. doi: 10.1016/j.fshw.2014.01.001
- Gertsch, J., Viveros-paredes, J. M., & Taylor, P. (2011). Plant immunostimulants-scientific paradigm or myth? Journal of Ethnopharmacology, 136, 385–391. doi: 10.1016/j.jep.2010.06.044
- Guo, S., Shi, D., Liao, S., Su, R., Lin, Y., Pan, J., & Tang, Z. X. (2012). Influence of selenium on body weights and immune organ indexes in ducklings intoxicated with aflatoxin B1. Biological Trace Element Research, 146, 167–170. doi: 10.1007/s12011-011-9246-z
- Hang, M., & Zhao, X. H. (2011). Fermentation time and extraction solvents influenced in vitro antioxidant property of soluble extracts of Mao-tofu fermented with Mucor sp. Biotechnology, 10, 60–69. doi: 10.3923/biotech.2011.60.69
- Hang, M., & Zhao, X. H. (2012). Fermentation time and ethanol/water-based solvent system impacted in vitro ACE-inhibitory activity of the extract of Mao-tofu fermented by Mucor spp. CyTA-Journal of Food, 10, 137–143. doi: 10.1080/19476337.2011.601428
- Hao, L. X., & Zhao, X. H. (2016). Immunomodulatory potentials of the water-soluble yam (Dioscorea opposita Thunb) polysaccharides for the normal and cyclophosphamide- suppressed mice. Food and Agricultural Immunology, 27, 667–677. doi: 10.1080/09540105.2016.1148666
- He, Y., Ning, T. T., Xie, T. T., Qiu, Q. C., Zhang, L. P., Sun, Y. F., … Yang, D. C. (2011). Large-scale production of functional human serum albumin from transgenic rice seeds. Proceedings of the National Academy of Sciences, 108, 19078–19083. doi: 10.1073/pnas.1109736108
- Hong, H., Choi, J. Y., Kim, A. Y., Lee, E. M., Kim, J. H., Park, J. H., … Jeong, K. S. (2017). Anti-rheumatoid arthritic effect of fermented Adlay and Achyranthes japonica Nakai on collagen-induced arthritis in mice. Food and Agricultural Immunology, 28, 14–26. doi: 10.1080/09540105.2016.1202207
- Hou, H., Fan, Y., Li, B., Xue, C., & Yu, G. (2012). Preparation of immunomodulatory hydrolysates from Alaska pollock frame. Journal of the Science of Food and Agriculture, 92, 3029–3038. doi: 10.1002/jsfa.5719
- Hu, C. C., Hsiao, C. H., Huang, S. Y., Fu, S. H., Lai, C. C., Hong, T. M., … Lu, F. J. (2004). Antioxidant activity of fermented soybean extract. Journal of Agricultural and Food Chemistry, 52, 5735–5739. doi: 10.1021/jf035075b
- Karasawa, K., Sugiura, Y., Kojima, M., Uzuhashi, Y., & Otani, H. (2013). Fermented soybean powder with rice mold in the absence of salt stimulates the cellular immune system and suppresses the humoral immune response in mice. Journal of Nutritional Science and Vitaminology, 59, 564–569. doi: 10.3177/jnsv.59.564
- Ko, C. Y., Lin, H. T. V., & Guo, J. T. (2013). Gamma-aminobutyric acid production in black soybean milk by Lactobacillus brevis FPA 3709 and the antidepressant effect of the fermented product on a forced swimming rat model. Process Biochemistry, 48, 559–568. doi: 10.1016/j.procbio.2013.02.021
- Lai, C. Y., Hung, J. T., Lin, H. H., Yu, A. L., Chen, S. H., Tsai, Y. C., … Yu, J. (2010). Immunomodulatory and adjuvant activities of a polysaccharide extract of Ganoderma lucidum in vivo and in vitro. Vaccine, 28, 4945–4954. doi: 10.1016/j.vaccine.2010.05.037
- Lee, J. H., Paek, S. H., Shin, H. W., Lee, S. Y., Moon, B. S., Park, J. E., … Heo, Y. (2017). Effect of fermented soybean products intake on the overall immune safety and function in mice. Journal of Veterinary Science, 18, 25–32. doi: 10.4142/jvs.2017.18.1.25
- Lee, S. I., Lee, Y. K., Kim, S. D., Lee, J. E., Choi, J. K., Park, J. P., … Lee, I. A. (2013). Effect of fermented soybean curd residue (FSCR; SCR-meju) by Aspergillus oryzae on the anti-obesity and lipids improvement. Journal of Nutrition and Health, 46, 493–502. doi: 10.4163/jnh.2013.46.6.493
- Li, H., Yang, H., Zhang, X., Huang, F., Chen, Y., & Guo, Y. (2000). Distribution and phenotypic analysis of porcine T lymphocytes in peripheral blood and lymphoid tissues. Journal of Agricultural Biotechnology, 8, 37–40.
- Lim, K. H., Han, J. H., Lee, J. Y., Park, Y. S., Yong, S. C., Kang, K. D., … Kim, J. H. (2012). Assessment of antidiabetogenic potential of fermented soybean extracts in streptozotocin-induced diabetic rat. Food and Chemical Toxicology, 50, 3941–3948. doi: 10.1016/j.fct.2012.08.036
- Lin, H. T. V., Hwang, P. A., Lin, T. C., & Tsai, G. J. (2014). Production of Bacillus subtilis-fermented red alga Porphyra dentata suspension with fibrinolytic and immune-enhancing activities bioscience. Biotechnology and Biochemistry, 78, 1074–1081. doi: 10.1080/09168451.2014.915726
- Lin, S., Liu, X., Liu, B., & Yu, Y. (2017). Optimization of pine nut (Pinus koraiensis) meal protein peptides on immunocompetence in innate and adaptive immunity response aspects. Food and Agricultural Immunology, 28, 109–120. doi: 10.1080/09540105.2016.1228835
- Liu, J. Y., Feng, C. P., Li, X., Chang, M. C., Meng, J. L., & Xu, L. J. (2016). Immunomodulatory and antioxidative activity of Cordyceps militaris, polysaccharides in mice. International Journal of Biological Macromolecules, 86, 594–598. doi: 10.1016/j.ijbiomac.2016.02.009
- Liu, X., & Zhao, X. H. (2017). In vitro immune potentials of the soluble extracts of Mucor-fermented Mao-tofu as affected by fermentation times and extracting solvents. Food Science and Biotechnology . (Accepted paper, MS No. E2016-05-027).
- Paredes-López, O., & Harry, G. I. (1988). Food biotechnology review: Traditional solid-state fermentations of plant raw materials-application, nutritional significance, and future prospects. Critical Reviews in Food Science and Nutrition, 27, 159–187. doi: 10.1080/10408398809527483
- Parvez, S., Malik, K., Ah Kang, S., & Kim, H. Y. (2006). Probiotics and their fermented food products are beneficial for health. Journal of Applied Microbiology, 100, 1171–1185. doi: 10.1111/j.1365-2672.2006.02963.x
- Qiao, D., Wei, C., Chen, N., Min, Y., Xu, H., & Chen, R. (2017). Influences of Hyriopsis cumingii polysaccharides on mice immunosignaling molecules and T lymphocyte differentiation. Food and Agricultural Immunology. doi:10.1080/09540105.2017.1306494
- Ren, D., Wang, M., Shen, M., Liu, C., Liu, W., Min, W., & Liu, J. (2016). In vivo assessment of immunomodulatory activity of hydrolysed peptides from Corylus heterophylla fisch. Journal of the Science of Food and Agriculture, 96, 3508–3514. doi: 10.1002/jsfa.7535
- Rhyu, M. R., Kim, E. Y., & Han, J. S. (2002). Antihypertensive effect of the soybean paste fermented with the fungus Monascus. International Journal of Food Science and Technology, 37, 585–588. doi: 10.1046/j.1365-2621.2002.00599.x
- Sanjukta, S., & Rai, A. K. (2016). Production of bioactive peptides during soybean fermentation and their potential health benefits. Trends in Food Science and Technology, 50, 1–10. doi: 10.1016/j.tifs.2016.01.010
- Santiago-López, L., Hernández-Mendoza, A., Vallejo-Cordoba, B., Mata-Haro, V., & González-Córdova, A. F. (2016). Food-derived immunomodulatory peptides. Journal of the Science of Food and Agriculture, 96, 3631–3642. doi: 10.1002/jsfa.7697
- Schmitz, H., & Chevaux, K. (2000). Defining the role of dietary phytochemicals in modulating human immune function. In M. E. Gershwin, J. B. German, & C. L. Keen (Eds.), Nutrition and immunology (pp. 107–119). New York: Humana Press.
- Shen, C., Mao, J., Chen, Y., Meng, X., & Ji, Z. (2014). Extraction optimization of polysaccharides from Chinese rice wine from the Shaoxing region and evaluation of its immunity activities. Journal of the Science of Food and Agriculture, 95, 1991–1996. doi: 10.1002/jsfa.6909
- Shi, M., Yang, Y., Hu, X., & Zhang, Z. (2014). Effect of ultrasonic extraction conditions on antioxidative and immunomodulatory activities of a Ganoderma lucidum polysaccharide originated from fermented soybean curd residue. Food Chemistry, 155, 50–56. doi: 10.1016/j.foodchem.2014.01.037
- Silva, L. H. D., Celeghini, R. M. S., & Chang, Y. K. (2011). Effect of the fermentation of whole soybean flour on the conversion of isoflavones from glycosides to aglycones. Food Chemistry, 128, 640–644. doi: 10.1016/j.foodchem.2011.03.079
- Stevens, T. L., Bossie, A., Sanders, V. M., Fernandezbotran, R., Coffman, R. L., Mosmann, T. R., & Vitetta, E. S. (1988). Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature, 334, 255–258. doi: 10.1038/334255a0
- Unger, W. W. J., van Beelen, A. J., Bruijns, S. C., Joshi, M., Fehres, C. M., van Bloois, L., … van Kooyk, Y. (2012). Glycan-modified liposomes boost CD4+ and CD8+ T-cell responses by targeting DC-SIGN on dendritic cells. Journal Control Release, 160, 88–95. doi: 10.1016/j.jconrel.2012.02.007
- Yanagawa, M., Fukatsu, K., Mitsui, T., Murakoshi, S., Yasuhara, H., & Nishimura, R. (2013). Effects of a new immune-modulating diet enriched with whey-hydrolyzed peptide, fermented milk, and isomaltulose on gut associated lymphoid tissue in mice. e-SPEN Journal, 8, e241–e245. doi: 10.1016/j.clnme.2013.08.003
- Yang, J. H., Mau, J. L., Ko, P. T., & Huang, L. C. (2000). Antioxidant properties of fermented soybean broth. Food Chemistry, 71, 249–254. doi: 10.1016/S0308-8146(00)00165-5
- Yang, Q., Xie, Y., & Depierre, J. W. (2000). Effects of peroxisome proliferators on the thymus and spleen of mice. Clinical and Experimental Immunology, 122, 219–226. doi: 10.1046/j.1365-2249.2000.01367.x
- Yang, R., Zhang, Z., Pei, X., Han, X., Wang, J., Wang, L., … Li, Y. (2009). Immunomodulatory effects of marine oligopeptide preparation from chum salmon (Oncorhynchus keta) in mice. Food Chemistry, 113, 464–470. doi: 10.1016/j.foodchem.2008.07.086
- Yao, M., Kyosuke, Y., Takashi, K., & Munehiko, D. (2008). Effects of hanabiratake (Sparassis crispa) on allergic rhinitis in ova-sensitized mice. Food Science and Technology Research, 14, 589–594. doi: 10.3136/fstr.14.589
- Yasuda, M. (2010). Scientific aspects of the fermented soybean food, tofuyo. Journal of the Japanese Society for Food Science and Technology, 57, 181–190. doi: 10.3136/nskkk.57.181
- Zhao, X. H., & Zheng, X. T. (2009). A primary study on texture modification and proteolysis of mao-tofu during fermentation. African Journal of Biotechnology, 8, 2294–2300.
Appendix 1. Hematological parameters of the mice after an experimental period of 4 weeks.
Appendix 2. Other hematological parameters of the mice after an experimental period of 4 weeks.
Appendix 2. Other hematological parameters of the mice after an experimental period of 4 weeks.
Appendix 3. Mean percentages of T-helper (CD4+) and T-cytotoxic (CD8+) lymphocytes in the collected plasma samples measured by flow cytometry (n = 3). The labeled Q1&4 represent CD8+ and CD4+ cells, respectively. The figures A−I report the assaying results of the plasma samples collected from the mice in Groups I−IX, respectively.