ABSTRACT
Gliadin is a major allergen causing allergies occurring also in meat products. Since wheat protein is used as a meat substitute to reduce cost of meat products. Sensitive consumers of these products are really threatened by food allergies in different allergic reaction. The objective of the study was to compare the histochemical, immunochemical (ALERT gliadin screening test) and immunofluorescence methods for the detection of wheat protein in model meat samples and meat products. The limit of detection for the ALERT gliadin screening test was 10 × 104 mg kg−1 of addition, while the histochemical method demonstrated concentration of wheat protein already from 10 × 103 mg kg−1, and the immunofluorescence method from the concentration of 20 mg kg−1. Comparison of the methods using McNemar’s test shows a statistically highly significant difference (p = .01) between the immunofluorescence method and ELISA and a statistically highly significant difference (p = .01) between the immunofluorescence and histochemical methods.
Introduction
Plant proteins are widely used globally for their nutritional value, functional properties, low costs of cultivation, and ease of use in combination with processing technology, which allows utilization of plant proteins in a wide variety of food products (Feiner, Citation2006; Saz & Marina, Citation2007). Wheat protein belongs among cheap forms of proteins and is therefore often used in the manufacture of meat products due to its excellent ability to bind water and to a lesser extent for its ability to emulsify fats. Wheat protein isolate, which contains about 90% protein, has a neutral taste and color; it is an excellent additive to whole muscle products, hamburgers and other meat products. It has very little impact on the natural color and flavor of the meat. Added wheat protein isolate is highly soluble in water and does not increase viscosity in the salt solution, thus it is very easy to apply. Wheat protein is able to support binding between salts and phosphates with proteins of meat in a meat product. Moreover, unlike hydrocolloids or starches, it does not form rubbery texture as gelling characteristics of wheat protein isolate are very similar to those of meat protein. Wheat protein isolate is generally applied in amounts from 1.3% to 4.0%. In the case of whole muscle products, the addition is lower, while in the case of cooked sausages and hamburgers, the addition is higher (Feiner, Citation2006). On the other hand, wheat protein causes allergy in some consumers (Saeed, Bachir, Chen, & Hu, Citation2016). Food allergy is defined as an adverse immune response to food proteins (Sicherer & Sampson, Citation2000). Any protein from foodstuffs can cause an allergic reaction. Wheat, however, belongs among the six foods marked by Codex Alimentarius (Codex, Citation2008), which account for approximately 90% of all food allergies in children and in recent years it has been increasingly recognized as a cause of food anaphylaxis (Hischenhuber, Crevel, & Jarry, Citation2006). Clinical manifestations of allergies to wheat are similar to other food allergies. Symptoms include skin lesions, gastrointestinal tract and respiratory tract disorders (Sicherer & Sampson, Citation2000), with certain differences between adult population and children. The main symptoms in children are atopic dermatitis (Čelakovská, Ettlerová, Ettler, Vaněčková, & Bukač, Citation2015), either alone or in connection with respiratory symptoms and gastrointestinal problems (Moneret-Vautrin, Kanny, Guerin, Flabbee, & Lemerdy, Citation2000). Among adult population, various clinical symptoms, including anaphylaxis, angioedema and eosinophilic esophagitis, were identified (Pasha et al., Citation2013; Scibilia et al., Citation2006). The only possible protection against adverse immune reactions for those suffering from food allergies is strictly excluding the allergen from their diet. Although the number of studies dealing with processes resulting in reduction or loss of allergenicity is increasing, yet these practices are not common. Producers of meat products sometimes fail to comply with legal requirements to mark the addition of wheat protein, which is an allergen, on the package. This obligation is based on the EU regulation (Citation2011); however, using plant proteins lowers the price of products, and this fact could lead manufacturers to use plant proteins without including this ingredient in the product label due to the competitive environment. If an allergic person consumes such unmarked product, they may suffer from an allergic reaction which can in some cases lead to death (Sampson, Citation2004). Many food producers in precaution also warn allergic consumer on the product package that the product may contain an allergen. Analyses of commercially available products show that the presence or absence of such preventive warning hardly corresponds to the actual presence of the allergen in the product (Pele, Brohee, Anklam, & Van Hengel, Citation2007; Spanjersberg, Knulst, Kruizinga, Van Duin, & Houben, Citation2007; Yman, Eriksson, Everitt, Yman, & Karlsson, Citation1994), which could potentially lead to dangerous situations (Sheth et al., Citation2012). As reported by Hischenhuber et al. (Citation2006), determination of wheat protein presence in foodstuffs is not easy because gluten contains several proteins whose composition may vary depending on the climate, cereal variety and geographical location. Moreover, additives used in the food industry may have a different protein composition than the original cereals. Therefore, high sensitivity and specificity of the applied methods are very important for the purposes of allergens detection in order to be effective even in complex food matrices (Petrášová, Pospiech, Řezáčová Lukášková, & Tremlová, Citation2014). In order to prevent misleading consumers and also to protect allergic consumers, analytical methods applicable to all types of foodstuffs have been developed. Most of these methods are based on electrophoretic or chromatographic techniques (Debnath, Martin, & Gowda, Citation2009; Jira & Schwägele, Citation2015; Moen et al., Citation2005; Wieser, Antes, & Seilmeier, Citation1998).
The aim of the study was to develop a new immunofluorescence method including its validation and comparing it with the histochemical method and ELISA with focus on gliadin detection.
Material and methods
Material
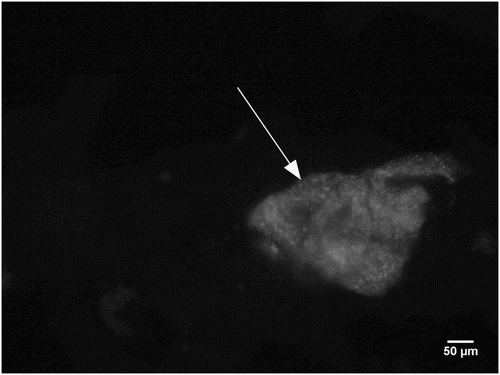
In wheat protein determination, 16 cooked meat products, primarily pâtés, purchased in the market network in the Czech Republic, were processed. In 12 of these products, manufacturers stated on its package that the product contains wheat protein. The remaining four used products had no such information. Besides these commercial products, model samples with additions of wheat protein in the specified quantities were produced: 20 mg kg−1, 100 mg kg−1, 10 × 102 mg kg−1, 10 × 103 mg kg−1 and 10 × 104 mg kg−1. Model samples were prepared from ground chilled pork and beef in the ratio of 1:1. 0.5% of phosphate and 1.5% of salt was added to this meat to improve its water holding capacity. Grinding and stirring was performed using Vorwerk Thermomix 31 appliance (Vorwerk, Wuppertal, GER), speed level 8, stirring for a period of five minutes. Wheat protein was added into the products in the form of edible vital wheat protein PN 56 6310 (Amylon a.s., Havlíčkův Brod, CZE). These model samples were cooked in water bath at 70°C (internal core temperature of the meat samples) for 10 minutes. Four blocks of 1 cm3 were taken from each sample and frozen. Using cryostat HM 550 (Microm, Walldorf, Germany), these blocks were sliced into sections 4 µm thick. These sections were transferred to Thermo Superfrost plus slides (Thermo scientific, Darmstadt, Germany). Three sections with trimming of 50 μm were cut from each block. Prior to use, the sections were left for 30 minutes at room temperature.
Immunofluorescence detection
The immunofluorescence detection method was performed according to the following procedure. Sections were washed: (1) 100% acetone for 5 min; (2) drying for 30 min at room temperature; and (3) washed twice in PBS – Phosphate Buffered Saline, 80 g/l NaCl, 2 g/l KCl, 2 g/l KH2PO4, 23.4 g/l Na2HPO4·2 H2O, 0.16 g/l NaOH adjusted to pH 7.4 for 5 min; (4) antigen retrieval in Citrate buffer, 21 g/l Acidum citricum, 9 g/l NaOH for 5 min at 650 W in a microwave and cooling 20 min at room temperature. The sections were then incubated successively: (5) for 30 min at 25°C with 5% (v/v) Goat normal serum in PBS (Vector Laboratories, Burlingame, GB); (6) incubation with anti-gliadin antibodies isolated from a rabbit (Sigma-Aldrich Company, St. Louis, USA) for 1 h at room temperature diluted (1:1000) with antibody diluent (DakoCytomation ref. S0809, Glostrup, GER); and (7) washed in PBS twice for 5 min; (8) incubation with 25 μl per section of secondary biotinylated anti-rabbit antibody (Vector Laboratories, PK 6101, Burlingtone, USA) for 30 min at 25°C; and (9) than washed in PBS twice for 5 min; (10) incubation with Texas Red Streptavidin SA-5006 (Vector Laboratories, Burlingame, GB) for 15 min at 25°C diluted 1:250 with PBS; and (11) washed in PBS twice for 5 min. The sections were mounted with Vectashield mounting medium for fluorescence (Vector Laboratories, Burlingtone, USA) and covered by micro coverslip (Menzel-Gläser, Braunschweig, GER). These micro coverslips were laid onto each section. The chemicals and other used solutions were obtained from RNDr. Jan Kulich s. r. o. (Prague, CZE) and were used in p.a. quality.
Three controls were performed to ascertain whether the primary and secondary antibodies are specific for target antigens. Only antibody diluent was used in step (6) to perform negative control. A model sample without wheat protein was used for a negative tissue control. A sample with 10 × 103 mg kg−1 of wheat protein was used for positive tissue control.
ELISA detection
ELISA test to detect wheat protein was performed using the ALERT Gliadin Screening Test (Neogen, Michigan, USA) which is a screening test for examination of gluten-free products for the presence of gliadins and prolamins of wheat, barley and rye, and as indicated by the manufacturer, it demonstrates presence of 10 mg kg−1 already. The test is based on the principle of sandwich-like ELISA method where gliadin is first extracted to 40% dilution of ethanol. The acquired extract is diluted by PBS and applied to test wells with conjugated antibodies against gliadin. After incubation and washing off redundant gluten, enzymatically marked antibody is bound to conjugated gliadin if present. Blue color detected in the last stage is considered to be positive, while light pink color is negative. Color is compared to negative and positive control. The test was performed in compliance with the manufacturer’s instructions.
Histochemical examination
The procedure was performed according to Lukášková et al. (Citation2011).
Evaluation of results
For each sample within the immunofluorescence detection, 12 sections were examined in anonymity at a magnification of 40× and 100×. Examination of the samples was performed at Leica DM 3000 fluorescence microscope (Leica, Wetzlar, GER) with digitization using Leica DFC 295 camera (Leica, Wetzlar, GER) and software support of Leica LAS AF (Leica, Wetzlar, GER). The acquired images were saved in the lossless TIFF format.
Validation criteria of the immunofluorescence method to determine wheat protein included specificity, sensitivity and limit of detection (LOD), as recommended by Magnusson and Örnemark (Citation2014).
Furthermore, the wheat protein detection results were compared to the results from ELISA and histochemical examination. The results were evaluated qualitatively with the symbols of (+) for positive, (–) for negative and (±) for doubtful results. If doubtful, in statistical processing by means of McNemar’s test, such a result was evaluated as positive as well as negative.
Statistical evaluation of results
The results were processed by mathematical and statistical methods using the program of Unistat 6.1 (Unistat Ltd. 2012, Brno, CZE). Within the statistical analysis, the absolute numbers of positive and negative samples (or doubtful samples) were determined for each examination method, and their relative abundance was expressed in percent of the entire group of samples examined. Abundances of results obtained by the applied examination methods were compared using McNemar’s test (Hendl, Citation2004) that assesses the demonstrability of differences found between the tested occurrences number by calculating the test criterion χ (chi) square (2 × 2 contingency table).
Results and discussion
Detection of wheat protein was performed in 12 meat products purchased in the market network, where the wheat protein or wheat flour was declared in the label, and 4 products with no flour or wheat protein declaration. In the Czech Republic, use of flour is in compliance with traditional recipes and desired sensory properties of products. With regard to better technological characteristics of wheat protein, however, use of flour is declining and increasingly being replaced by wheat protein. In the analyses, also model samples with additions of wheat protein of 20 mg kg−1, 100 mg kg−1, 10 × 102 mg kg−1, 10 × 103 mg kg−1 and10 × 104 mg kg−1 were therefore used. Results from the immunofluorescence testing of wheat protein were compared with ELISA and histochemical examination. As shown in , LOD for the immunofluorescence method was determined at 20 mg kg−1 of wheat protein addition, for the histochemical examination at 10 × 103 mg kg−1, and for the ALERT gliadin screening test it was at 10 × 104 mg kg−1, even though the manufacturer stated the LOD already at 10 mg kg−1. This deviation can be explained for example by occurrence of doubtful results, when the ELISA method found 9.5% doubtful results (for the histological method, it was 14.28%). Another possibility is the occurrence of false negative results. Scharf, Kasel, Wichmann, and Besler (Citation2013) reported that quantitative results of ELISA kits for determination of gluten showed differences as regards the specificity of antibodies in the test kits. Authors of this study also compared results of the ELISA method from 170 laboratories. Five laboratories showed false negative results which were detected in rather complex food matrices, such as breads and smoked meat products. False negativity was also confirmed by our results (). And another reason may be different extraction of antigen using ELISA kits and different antigen retrieval used to methods based on the immunohistological response (Bednářová, Pospiech, Tremlova, Řezáčová-Lukášková, & Bednář, Citation2015). Food labeled as gluten-free must not exceed 20 mg kg−1 gluten, whereas food containing low levels of gluten has to be lower than 100 mg kg−1 gluten. This recommendation concerning thresholds was taken into European legislation through Commission Regulation (EC) No. 41/2009 of 20 January 2009, concerning the composition and labeling of foodstuffs suitable for people intolerant to gluten (Citation2009). From among the methods validated by our study, the immunofluorescence method only is able to detect this limit in the matrix of meat products and model samples. There are also other sensitive methods that can be used for detection of wheat protein in meat products. LOD in the HPLC-MS/MS method was determined at 3 mg kg−1 (Jira & Schwägele, Citation2015). Another microscopic method, namely the immunohistochemical method, had LOD for the detection of wheat protein at 1000 mg kg−1 (Lukášková et al., Citation2011).
Table 1. Comparison of methods for detection of wheat protein (gluten).
Furthermore, specificity and sensitivity were determined for the immunofluorescence method in all samples according to the protocols by Trullols, Ruisanchez, and Rius (Citation2004). The sensitivity and specificity mean the ability of a method to detect truly positive and negative samples (O’rangers & Condon, Citation2000). Sensitivity was determined at 0.94 and specificity at 1.25. Some parts of meat products can cause false positive reaction as nonspecific reaction between antigen and antibody. False positive reaction can be also caused by enzymatic activity of endogenous biotin in tissue. Biotin contained in meat can be found in concentration range of 27–45 × 109 mg kg−1 (Staggs, Sealey, McCabe, Teague, & Mock, Citation2004). Biotin concentration is decreased by 20% during cooking (Lešková et al., Citation2006). Possibility of false positive reactions mentioned above was disproved by performed controls in immunofluorescence procedure.
Next, correlation of the immunofluorescence test with ELISA was assessed and determined at 52.38% and correlation of the immunofluorescence test with the histochemical method was at 66.67%. To determine correlation or difference between individual methods, the statistical method of McNemar’s test suitable for comparison of qualitative methods was applied (Hendl, Citation2004). Inconsistencies between the immunofluorescent examination, ELISA, and histochemical method are presented in . There was a statistically highly significant difference (p < .01) demonstrated between the immunofluorescence method and ELISA, which means that this method did not achieve similar results compared to the ELISA examination according to the McNemar’s test. The immunofluorescence and histochemical methods were also compared using McNewmar’s test, which again resulted in a statistically highly significant difference (p < .01). The difference between the immunofluorescence and histochemical methods can be explained by a high sensitivity of immunofluorescence compared to light microscopy. Observing cell structures has been traditionally based on light microscopy for centuries. On the other hand, it is limited by its optical resolution. This problem can be solved to some extent by increasing the contrast between the investigated object and its background. This approach using the technique of contrast is better than the absorbance technique in which objects are stained by substances that absorb light, as suggested by Lichtman and Conchello (Citation2005). The difference between the immunofluorescence method and ELISA can be explained by the fact that in case of lower binding of antibodies to the present protein (gluten), identification can by enhanced by knowledge of wheat protein morphology which is based on spongy structure with openings, as described also by Lukášková et al. (Citation2011).
Table 2. Comparison of immunofluorescence, ELISA, and histochemistry, 2x2 contingency table.
Results of examination of meat products purchased in the Czech market point to the frequent use of wheat protein in meat products, which is in line with the statement by Feiner (Citation2006) who confirmed the worldwide use of wheat protein. Our results suggest that the immunofluorescence method can be successfully applied for the detection of wheat protein in meat products. Likewise, Standard for Foods for Special Dietary Use for Persons Intolerant to Gluten (Codex, Citation2008) mentions that immunological methods have their uses for detection of allergens.
Conclusions
To reduce total costs in the meat industry, use of cheaper plant proteins as a meat substitute is considered to be a good strategy. Proteins from various vegetable sources, such as wheat protein, are used as fillers and binders in meat products due to their properties. On the other hand, wheat is ranked among allergens causing even anaphylaxis. Thus, addition of this protein must be under current legislation indicated on the package by the manufacturer. To inspect additions of gliadin, a new immunofluorescence method was developed. The method was validated in a matrix of meat products. Sensitivity of the method was determined at 0.94 and specificity at 1.25. To verify this method, it was compared with a commercial ELISA kit and the histochemical method. Highly significant differences (p < .01) were demonstrated between the immunofluorescence method and ELISA as well as between the immunofluorescence and histochemical methods. Furthermore, LOD was defined for all the methods. LOD for the immunofluorescence was determined at 20 mg kg−1 of added wheat protein, for ELISA it was an addition of 10 × 104 mg kg−1, and for the histochemical method it reached 10 × 103 mg kg−1 (). Since meat products are characterized by a variable matrix with not always described bonds between proteins, masking of the binding epitopes in the protein might occur leading to little or no antigen–antibody reaction. In such cases, the examiner can rely on the morphological structure in microscopic methods. Of these microscopic methods, with regard to the specificity, sensitivity and low LOD, the immunofluorescence method appears to be the most sensitive for the detection of wheat protein in meat products.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Mgr. Michaela Petrášová, Ph.D. was born in Czech Republic in 1985. She received the B.Sc., M.Sc., and Ph.D. degrees at the University of Veterinary and Pharmaceutical Sciences Brno, Czech Republic, in 2008, 2010, and 2015, respectively. Since 2010, she has worked at the University of Veterinary and Pharmaceutical Sciences Brno, where she is currently an Assistant Professor. Main areas of her research are Immunohistochemistry and Immunofluorescence Method for IHC Detection of Allergens (wheat, soy, milk, pea,.. protein). Dr. Michaela Petrášová is also Quality Manager and Laboratory Chief Representative of the Laboratory of Food Analysis no. 1660 accredited by CAI according to CSN EN ISO/IEC 17025:2005. She can be contacted et [email protected].
Matej Pospiech, Ph.D. was born in Slovak Republic in 1980. He received the D.V.M. and Ph.D. degrees at the University of Veterinary and Pharmaceutical Sciences Brno, Czech Republic, in 2005, 2009, respectively. Since 2009 he worked at the University of Veterinary and Pharmaceutical Sciences Brno, where she is currently an Assistant Professor. Main areas of his research are food structural microscopy, immunohistochemistry and food adulteration detection. Dr. Matej Pospiech is also Chief of the Laboratory of Food Analysis no. 1660 accredited by CAI according to CSN EN ISO/IEC 17025:2005. He can be contacted et [email protected].
Bohuslava Tremlová, Ph.D. was born in Czech Republic in 1960. She received the D.V.M., Ph.D. degrees and habilitation at the University of Veterinary and Pharmaceutical Sciences Brno, Czech Republic, in 1984, 1999 and 2003, respectively. Since 1993 he worked at the University of Veterinary and Pharmaceutical Sciences Brno, where she is currently a Doctor of Science. Main areas of her research are food hygiene and technology, food histology and colorimetry of foodstuff. Dr. Bohuslava Tremlová is also Dean of Faculty of Veterinary Hygiene and Ecology at the University of Veterinary and Pharmaceutical Sciences Brno. She can be contacted et [email protected].
Slavomír Marcincák, Ph.D. was born in Slovak Republic in 1975. He received the D.V.M., Ph.D. and habilitation degrees at the University of Veterinary Medicine and Pharmacy in Kosice, Slovak Republic, in 1999, 2004 and 2012, respectively. Since 1999 he worked at the University of Veterinary Medicine and Pharmacy in Košice, where he is currently a Doctor of Science. Main areas of his research are lipids and antioxidant in foodstuff and human nutrition. He can be contacted et [email protected].
Alexandra Tauferová was born in 1984 in Slovak Republic. She recieved the M.Sc. degree at the Slovak University of Agriculture in Nitra, Slovak Republic in 2007. Since the same year she has worked at the University of Veterinary and Pharmaceutical Sciences Brno, Czech Republic as an assistant, where she is currently finishing her Ph.D. studies in the field of hygiene and technology of foodstuffs. In her research she focuses mainly on foodstuffs of plant origin and their sensory and nutritional aspects. She can be contacted at [email protected].
Additional information
Funding
References
- Bednářová, M., Pospiech, M., Tremlova, B., Řezáčová-Lukášková, Z., & Bednář, J. (2015). Antigen retrieval and fixation of sections on slides for immunohistochemical detection of soya protein in meat products. Journal of Food & Nutrition Research, 54(1), 1–8. ISSN 1336-8672
- Codex Alimentarius Commission, Codex Standard 118-1979, 2008, Rome, Italy.
- Debnath, J., Martin, A., & Gowda, L. R. (2009). A polymerase chain reaction directed to detect wheat glutenin: Implications for gluten-free labelling. Food Research International, 42(7), 782–787. doi: 10.1016/j.foodres.2009.02.028
- EC Commission Regulation No. 41/2009, 2009, Brussels, Belgium.
- Čelakovská, J., Ettlerová, K., Ettler, K., Vaněčková, J., & Bukač, J. (2015). Evaluation of food allergy to wheat, cow milk, egg, soy and peanuts in patients suffering from atopic dermatitis. Food and Agricultural Immunology, 26(1), 26–37. doi: 10.1080/09540105.2013.864603
- Feiner, G. (2006). Meat products handbook practical science and technology. Cambridge: Woodhead Publishing. ISBN 9781845690502. 90-91.
- Hendl, J. (2004). Review of statistical methods of data processing (in Czech). (1st ed.). Prague: Portál. ISBN 80-7178-820-1, 583 p.
- Hischenhuber, C., Crevel, R., & Jarry, B. (2006). Review article: Safe amounts of gluten for patients with wheat allergy or coeliac disease. Alimentary Pharmacology and Therapeutics, 23(5), 559–575. doi: 10.1111/j.1365-2036.2006.02768.x
- Jira, W., & Schwägele, F. (2015). HPLC-MS/MS-Detection of caseins and whey proteins in meat products. Procedia Food Science, 5, 129–132. doi: 10.1016/j.profoo.2015.09.037
- Lešková, E., Kubíková, J., Kováčiková, E., Košická, M., Porubská, J., & Holčíková, K. (2006). Vitamin losses: Retention during heat treatment and continual changes expressed by mathematical models. Journal of Food Composition and Analysis, 19(4), 252–276. doi: 10.1016/j.jfca.2005.04.014
- Lichtman, J. W., & Conchello, J. A. (2005). Fluorescence microscopy. Nature Methods, 2(12), 910–919. doi: 10.1038/nmeth817
- Lukášková, Z., Tremlová, B., Pospiech, M., Renčová, E., Randulová, Z., Steinhauser, L., … Bednář, J. (2011). Comparison of immunohistochemical, histochemical and immunochemical methods for detection of wheat protein allergens in meat samples and cooked, dry, raw and fermented sausage samples. Food Additives & Contaminants: Part A, 28(7), 817–825. doi: 10.1080/19440049.2011.572292
- Magnusson, B., & Örnemark, U. (2014). Eurachem guide: The fitness for purpose of analytical methods – A laboratory guide to method validation and related topics (2nd ed.). Eurachem. ISBN 978-91-87461-59-0.
- Moen, L. H., Sletten, G. B., Miller, I., Plassen, C., Gutleb, A. C., & Egaas, E. (2005). Rocket immunoelectrophoresis and ELISA as complementary methods for the detection of casein in foods? Food and Agricultural Immunology, 16(2), 83–90. doi: 10.1080/09540100400029928
- Moneret-Vautrin, D. A., Kanny, G., Guerin, L., Flabbee, J., & Lemerdy, P. (2000). The multifood allergy syndrome. Allerg Immunol (Paris), 32(1), 12–15.
- O’rangers, J. J., & Condon, R. J. (2000). Current issues in regulatory chemistry (p. 207). Gaithersburg, MD: AOAC.
- Pasha, I., Saeed, F., Sultan, M. T., Batool, R., Aziz, M., & Ahmed, W. (2013). Wheat allergy & intolerence; recent updates and perspectives. Critical Reviews in Food Science and Nutrition, 56(1), 13–24. doi: 10.1080/10408398.2012.659818
- Pele, M., Brohee, M., Anklam, E., & Van Hengel, A. J. (2007). Peanut and hazelnut traces in cookies and chocolates: Relationship between analytical results and declaration of food allergens on product labels. Food Additives and Contaminants, 24, 1334–1344. doi: 10.1080/02652030701458113
- Petrášová, M., Pospiech, M., Řezáčová Lukášková, Z., & Tremlová, B. (2014). Comparison of new immunofluorescence method for detection of soy protein in meat products with immunohistochemical, histochemical and ELISA methods. Acta Veterinaria Brno, 83, 65–69. doi: 10.2754/avb201483S10S65
- Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004 Text with EEA relevance. L 304/18, 22/11/2011, s. 18–63.
- Saeed, I., Bachir, D. G., Chen, L., & Hu, Y. G. (2016). The expression of TaRca2-α gene associated with net photosynthesis rate, biomass and grain yield in bread wheat (Triticum aestivum L.) under field conditions. PLoS One, 11(8), e0161308. doi: 10.1371/journal.pone.0161308
- Sampson, H. A. (2004). Update on food allergy. Journal of Allergy and Clinical Immunology, 113(5), 805–819. doi: 10.1016/j.jaci.2004.03.014
- Saz, J. M., & Marina, M. L. (2007). High performance liquid chromatography and capillary electrophoresis in the analysis of soybean proteins and peptides in foodstuffs. Journal of Separation Science, 30, 431–451. doi: 10.1002/jssc.200600247
- Scharf, A., Kasel, U., Wichmann, G., & Besler, M. (2013). Performance of ELISA and PCR methods for the determination of allergens in food: An evaluation of six years of proficiency testing for soy (Glycine max L.) and wheat gluten (Triticum aestivum L.). Journal of Agricultural and Food Chemistry, 61(43), 10261–10272. doi: 10.1021/jf402619d
- Scibilia, J., Pasatorello, E. A., Zisa, G., Ottolenghi, A., Bindslev-Jensen, C., Pravettoni, V., … Ortolani, C. (2006). Wheat allergy: A doubleblind, placebo-controlled study in adults. Journal of Allergy and Clinical Immunology, 117(2), 433–439. doi: 10.1016/j.jaci.2005.10.014
- Sheth, S. S., Waserman, S., Kagan, R., Alizadehfar, R., Primeau, M. N., & Elliot, S. (2012). Role of food labels in accidental exposures in food-allergic individuals in Canada. Annals of Allergy, Asthma & Immunology, 104, 60–65. doi: 10.1016/j.anai.2009.11.008
- Sicherer, S. H., & Sampson, H. A. (2000). Peanut and tree nut allergy. Current Opinion in Pediatrics, 12(6), 567–573. ISBN: 978-3-318-02340-4 doi: 10.1097/00008480-200012000-00010
- Spanjersberg, M. Q., Knulst, M. Q., Kruizinga, A. G., Van Duin, G., & Houben, G. F. (2007). Concentrations of undeclared allergens in food products can reach levels that are relevant for public health. Food Additives & Contaminants: Part A, 27, 169–174. doi: 10.1080/19440040903317513
- Staggs, C. G., Sealey, W. M., McCabe, B. J., Teague, A. M., & Mock, D. M. (2004). Determination of the biotin content of select foods using accurate and sensitive HPLC/avidin binding. Journal of Food Composition and Analysis, 17(6), 767–776. doi: 10.1016/j.jfca.2003.09.015
- Trullols, E., Ruisanchez, I., & Rius, F. X. (2004). Validation of qualitative analytical methods. TrAC Trends in Analytical Chemistry, 23(2), 137–145. doi: 10.1016/S0165-9936(04)00201-8
- Wieser, H., Antes, S., & Seilmeier, W. (1998). Quantitative determination of gluten protein types in wheat flour by reversed-phase high-performance liquid chromatography. Cereal Chemistry Journal, 75(5), 644–650. doi: 10.1094/CCHEM.1998.75.5.644
- Yman, I. M., Eriksson, A., Everitt, G., Yman, L., & Karlsson, T. (1994). Analysis of food proteins for verification of contamination or mislabelling. Food and Agricultural Immunology, 6(2), 167–172. doi: 10.1080/09540109409354827