ABSTRACT
A dual-labelled immunoassay using goldmag nanoparticles (GMNPs) and CdTe quantum dots (QDs) was applied for the quantification of casein in milk. In this method, anti-casein monoclonal antibody bound to GMNPs was used as capture probe, and the anti-casein polyclonal antibody, labelled with CdTe QDs, was employed as detection probe. This dual-labelled immunoassay was optimized and applied in the testing of casein content of three brands of commercial milks. The results showed a good linear tendency between casein concentrations and fluorescent intensities in the range of 4.617–289.9 ng/mL, and the half inhibition concentration (IC50) was 36.59 ng/mL. Besides, the recovery rates of this developed immunoassay were in accordance with those in traditional enzyme-linked immunosorbent assay for rapid detection of casein content in commercial milks. It suggested that this dual-labelled immunoassay was reliable, and could provide important theoretical value and practical significance in the identification and quantification of milk allergens.
Introduction
Milk allergy is one of the most common food allergies in childhood. In recent years, with the improvement of people’s living standards and the widespread use of dairy products, milk allergy has been a worldwide concern (Bennett, Wheatcroft, & Smithers, Citation2001). Casein is a main protein component of bovine milk (about 23 mg/mL), and accounts for approximately 80% of the total milk protein content (Choi, Horne, & Lucey, Citation2011).
Various analytical techniques have been proposed for the detection of potential allergens, like casein, in food products. Protein-based methods usually involved immunochemical detection protocols such as the radio-allergosorbent test (RAST), enzyme allergosorbent test (EAST) (Roberts & Lack, Citation2005), rocket immuno-electrophoresis (RIE) (Moen et al., Citation2005), immunoblotting (Kurien & Scofield, Citation2006) and enzyme-linked immunosorbent assay (ELISA) (Kong, Liu, Song, Kuang, & Xu, Citation2016; Kong, Liu, Xing, Kuang, & Xu, Citation2015; Van Hengel, Citation2007; Wang et al., Citation2015; Xu et al., Citation2016). RIE and immunoblotting rendered only qualitative or semi-quantitative results, while RAST, EAST and ELISA rendered quantitative results. Methods operating on the DNA level were based on an amplification of a specific DNA fragment by the polymerase chain reaction (PCR) (Monaci & Visconti, Citation2010) and real-time PCR (Hird, Lloyd, Goodier, Brown, & Reece, Citation2003), where false-positive results could be obtained. Nowadays, the ELISA technique was the most widely used bio-chemical technique in food analysis due to its precision, simple handling and good potential for standardization (Giovannacci et al., Citation2004). However, classic ELISAs were tedious and time-consuming, requiring of several washes and long reaction times (Khaemba et al., Citation2016; Suryoprabowo, Liu, Peng, Kuang, & Xu, Citation2015). Therefore, it is particularly important to establish an effective assay for casein detection and identification.
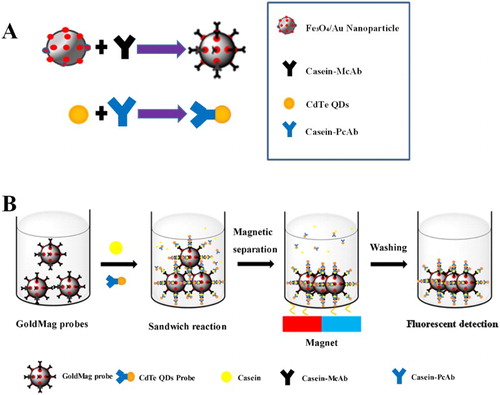
Gold-coated magnetic nanoparticle (GMNP), which possessed optical properties of colloid gold nanoparticles (Daniel & Astruc, Citation2004; Jain, El-Sayed, & El-Sayed, Citation2007; Yang et al., Citation2015) and super paramagnetic features of iron oxide nanobeads (Gupta & Gupta, Citation2005; Ranjbari, Hadjmohammadi, Kiekens, & De Wael, Citation2015; Walz, Citation2002), has broad application prospects in biomedical, environmental monitoring and food safety testing. Moreover, due to gold nanoparticles on the surface of GMNP, the process of coupling antibody on the surface of GMNP was simple and convenient. Meanwhile, CdTe quantum dots (QDs) (semiconductor nanocrystals) exhibit several unique optical properties such as high fluorescence intensity, narrow emission line, wide stimulation spectral line, continuously adjustable emission wavelength along with particle size and resistance to photobleaching (He et al., Citation2007; Wang et al., Citation2016; Zhang, Long, & Zheng, Citation2016), which can be exploited in optoelectronics, biomedicine and food safety detection. In this paper, GMNP bound to the monoclonal antibody (McAb) was used as capture probe, while CdTe QDs labelled with the polyclonal antibody (PcAb) was employed as detection probe. Thus, a dual-labelled immunosensor of “sandwich” assay for the quantification of casein in commercial milks was established and evaluated with main parameters.
Experimental
Materials and reagents
CdTe QDs (Em 611 nm), Fe3O4/Au (180 nm in diameter), McAb and PcAb for casein were prepared and characterized in our laboratory (Wang, Xu, Zhang, & Wei, Citation2013; Wang, Bai, & Wei, Citation2011). Horseradish peroxidase-conjugated PcAb specific for casein were purchased from Shanghai Xinyu Biological Technology Co., Ltd. (Shanghai, China). The β-lactoglobulin, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) were obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). The proteins, such as casein, bovine serum albumin (BSA) and ovalbumin (OVA), were purchased from Solarbio Biological Technology Co., Ltd. (Shanghai, China), while α-lactalbumin and other proteins were purchased from Sigma Chemicals Co (USA). All other reagents were of analytical grade. The Cray/E fluorospectro photometer used was from Varian Co., Ltd (USA).
Development of anti-casein-detection probe labelled with CdTe QDs
The procedure of the detection probe (Trapiella-Alfonso, Costa-Fernandez, Pereiro, & Sanz-Medel, Citation2011) coupled by CdTe QDs and anti-casein PcAb is depicted as follows. Six hundred microlitre of EDC (1 mg/mL) and 800 μL of NHS (1 mg/mL) were added into 3 mL of CdTe QDs solution and incubated at 37°C for 10 min. Then 500 μL PcAb (5 μg/mL) was added and incubated at 37°C for about 90 min. The mixture was centrifuged at 12,000 r/min for 5 min, the precipitation was then dispersed in 6 mL of boric acid buffer (0.2 mol/L, pH7.8).
Development of anti-casein-capture probe labelled with GMNP
GMNP (3 mg) by the solvothermal method (Song et al., Citation2014) was washed three times with 2 mL PBS buffer (0.01 mol/L, pH7.4) and then 500 μL McAb (5 μg/mL) was added to synthesize capture probe (in (A)) by electrostatic adsorption. The mixture was incubated at 37°C for 60 min. GMNP conjugated with McAb was magnetically separated and washed thrice with PBSB buffer (containing 2% BSA) with slight stirring. Finally, this probe was resuspended in PBS buffer and stored at 4°C for further use.
Establishment of the immunoassay based on dual-labelled immunoprobes
The anti-casein McAb labelled with GMNP was employed as capture probe, while the detection probe was developed by conjugation of the anti-casein PcAb and CdTe QDs. The immunosensor of “sandwich” structure (capture probe-casein-detection probe) was established (Li et al., Citation2014) and quantified by fluorescence intensity and concentration of casein.
The procedure of immunoassay based on dual-labelled immunoprobes is briefly shown in (B). The detection probe (1 mL) and casein (500 μL) were firstly added into a tube. Then the capture probe (100 μL) was added and incubated at 37°C for 30 min. The mixture was then washed with borate buffer solution (BBS, 0.05 mol/L, pH 7.5) three times by magnetic separation. The final content was dissolved in 3 mL BBS (0.05 mol/L, pH 7.5) and measured by fluorescence intensity.
Casein standard was dissolved in PBS (0.01 mol/L, pH 7.4) at the following concentrations of 0, 3.125, 6.25, 12.5, 25, 50, 100, 200 and 400 ng/mL. The calibration curve was obtained using the relationship between the values of the fluorescent signal and logarithm of the concentration of casein. The developed immunoassay was compared with the sandwich ELISA method, and the standard curve was established. Data were the means of triplicates. The detection limits (LOD) of the assay were calculated as the mean value of blank samples plus three times standard deviations of the mean.
Assessment of developed dual-labelled immunoassay
Main characters of the developed dual-labelled immunoassay were calculated. Other proteins, including α-lactalbumin, β-lactoglobulin, BSA and OVA, were tested for the selectivity study. Each protein solution (500 μL) at concentrations of 0, 3.125, 6.25, 12.5, 25, 50, 100, 200 and 400 ng/mL was investigated.
The stability of the detection probe was tested by using the same procedures described above with casein standard solution (1 mg/mL) at the 1st, 3rd, 5th, 7th, 9th, 11th, 13th, 15th, 17th, 19th, 21st and, 23rd day.
The accuracy of the established method was assessed by coefficients of variation (CV) (Shiba, Tagaya, Sugiyama, & Hanagata, Citation2015) through calculating intra- and inter-assay. Concentrations of casein standard (10, 40 and 80 ng/mL) were calculated by CV values with intra- and inter-methods and repeated three times.
Quantitative determination of commercial milk samples
To validate the performance of the developed immunoassay, three representative brands of commercial milks were purchased from local supermarket. One millilitre of each sample was centrifuged for 10 min (12,000 r/min, 4°C) to remove fat. Then 10 μL of the milk sample was diluted 100 times with PBS (0.01 mol/L, pH7.4). Casein content was simultaneously measured by ELISA and the developed method, and the results were obtained for conformity determination.
Results and discussion
Characterization of anti-casein-detection probe labelled with CdTe QDs
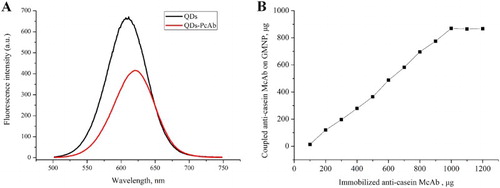
The strategy of the anti-casein-detection probe was established () based on a chemical coupling procedure. Because of the carboxyl group on CdTe QDs surface coated with mercapto propionic acid, anti-casein PcAb could be activated with EDC and NHS, and then was labelled with QDs by covalent binding of carboxyl and amino groups.
CdTe QDs and anti-casein-detection probe were characterized by the Cray/E fluorospectro photometer. The results are shown in (A). Emission wavelength of the pure CdTe QDs was 611 nm, while that of the conjugated probe was 620 nm. This red shift of QDs fluorescent peaks occurred through covalent coupling and was mainly caused by dipole–dipole interaction (Qing-Hui et al., Citation2009).
Characterization of anti-casein-capture probe labelled with GMNP
Because of the different interactions, such as Au and sulfhydryl group, the electrostatic interaction and the hydrophobic interaction, between gold nanoparticles and anti-casein McAb, McAb can be immobilized to the Fe3O4/Au composite. The greater amount of gold nanoparticles that combined onto the as-prepared magnetic composite resulted in the higher coupled capacity of anti-casein McAb on the Fe3O4/Au composite surface.
The coupled capacity of GMNP is shown in (B), which was measured and calculated by determining the absorbance of pre- and post-coupled McAb solution at 280 nm. At the beginning, McAb coupled with GMNP increased with the amount of McAb added in the reaction mixture. However, when the addition of McAb reached 1000 µg, the amount of McAb immobilized reached a plateau. The data also indicated that the maximum McAb immobilized onto Fe3O4/Au composite surface was about 870 µg/mg, which was much higher than a prior study (Cui et al., Citation2006).
Optimization of the immunoassay based on dual-labelled immunoprobes
In order to make this new immunoassay more sensitive, main parameters such as the dosage of probes and the immunoreaction time were optimized based on the dual-labelled immunoprobes.
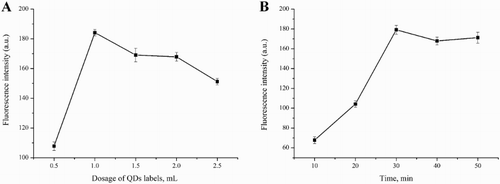
As described, 1.0 mL of capture probe solution and different dosages (0.5, 1.0, 1.5, 2.0, 2.5 mL) of detection probe were used and the results in (A) illustrate the fluorescence intensities of these sandwich compounds. The fluorescent intensity reached a maximum at a dosage of 1.0 mL of QDs detection probe, which was the optimal dosage for this immunoreaction. The effect of reaction time on the fluorescence intensity of fluorescent detection probe was examined and optimized within 10, 20, 30, 40, 50, 60 min in the immunoreaction. The fluorescence intensity of the sandwich compounds reached a maximum in 30 min ((B)), which was then considered to be the optimal time for this immunoreaction.
Development and characterization of the dual-labelled immunoassay
The calibration curve was obtained by investigating the relationships between the fluorescent values and concentrations of casein. Data were the means of triplicates. Detection limit (LOD) of the assay was calculated as the mean value of blanks plus three times standard deviation of the mean (Song et al., Citation2014).
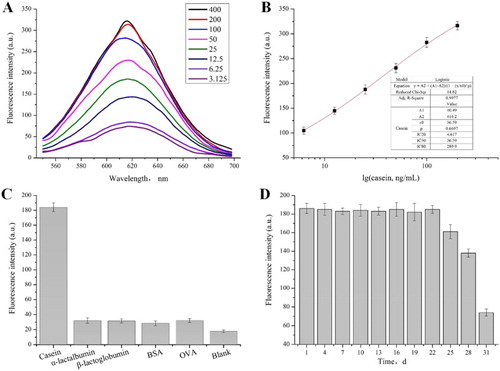
(A) shows fluorescent intensities of the developed sandwich immunoassay at concentrations of 3.125, 6.25, 12.5, 25, 50, 100, 200, 400 ng/mL of standard casein solution. The fluorescence intensity of the detection system increased in accordance with the increasing concentration of casein, and the fluorescence intensity tended to be constant at the concentration of 200 ng/mL.
Based on the fluorescent intensities by different concentrations, standard curve of the developed immunoassay was performed to estimate casein residues. The regression equation shown in (B) indicated that the fluorescent intensity was proportional to the concentration of casein. There was a good linear tendency between casein concentration and the fluorescence intensity in the range of 4.617–289.9 ng/mL, and the half inhibition concentration (IC50) was 36.59 ng/mL.
In comparison, R-Biopharm has quantitatively detected casein in food by using sandwich ELISA with detection limit (LOD) of 0.24 μg/mL, while LOD for whole milk protein in food (casein and beta globulin) is 0.7 μg/mL. Neogen has detected casein in skimmed milk powder in the Veratox detection kit by using sandwich ELISA with the linear range of 2.5–15 μg/mL. The detection linear range and sensitivity of this developed dual-labelled immunoassay were better than those of the available commercial products for casein detection.
Assessment of the dual-labelled immunoassay for casein detection
Casein content was assessed in this dual-labelled immunoassay with main properties, such as specificity, stability and CV. The specificity indicated the ability of the antibody to generate a measurable response only for the target molecule. The specificity of the developed immunoassay was evaluated with α-lactalbumin, β-lactoglobulin, BSA and OVA. Results in (C) show that casein induced an intense fluorescent signal, while other proteins produced negligible intensities which were similar to that of blank at the same concentration. The method showed good specificity and can be applied for the determination of casein.
The stability of this developed assay was tested by using the same procedure described above except using the casein standard solution (1 mg/mL) instead of the sample solution. The fluorescence intensity of the probe did not significantly change in 22 days ((D)), which indicated that the labelled probes could have stable properties for repeated measurements under proper storage.
The CV of inter-assay and intra-assay in were in the range of 5.8–8.5% and 6.6–9.0%, respectively. The results showed that this dual-labelled immunoassay based on immunoprobes of GMNPs and CdTe QDs could be an effective and stable analysis method for casein determination.
Table 1. Coefficients of variation by the developed dual-labelled immunoassay.
Analysis of commercial milk samples
To validate the performance of the dual-labelled immunoassay, commercial milk samples of three different brands were purchased from the local supermarket and casein content of each brand was quantitatively detected. Spike-recovery study is useful to monitor the accuracy of an analytical method or immunoassay. Blank samples were spiked with different amounts of casein and the recoveries were measured. Each sample was evaluated five times in duplicate to verify the repeatability. The concentration of casein was simultaneously measured by the ELISA method and the developed dual-labelled immunoassay, and the results are compared in . The recovery rates were in the range of 93.0–108.0% and 98.0–108.5%, respectively. The results of the two methods were close to each other, which indicated that the developed immunoassay was credible for detection of casein in bovine milk.
Table 2. Casein contents in different commercial milks by dual-labelled immunoassay and traditional ELISA.
Conclusions
In conclusion, we demonstrated a dual-labelled immunoassay for the detection of casein in bovine milk with nanoprobes of GMNPs and CdTe QDs. The linear detection range based on fluorescent intensities was 4.617–289.9 ng/mL with half inhibition concentration (IC50) of 36.59 ng/mL, which could be fulfilled within 30 min without complicated pretreatment procedures. According to the similar results between this developed immunoassay and traditional ELISA, it was demonstrated that this dual-labelled assay would be a reliable tool for rapid detection of casein content in commercial milk products.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Naifeng Xu completed his thesis work related to rapid detection methods of main chemical hazards in aquatic products and received his PhD from School of Food Science and Technology (2015). Dr. Xu is now a teacher-candidate postdoctor at Shanghai Normal University (SHNU) and his primary research interest is in quantitative detection and quality control of allergens in the manufacturing process.
Yanjiao Wang’s work focused on a rapid dual-labelled immunoassay of milk allergens and she received her master’s degree from SHNU in 2015.
Li Pan’s work focused on a GoldMag nanoparticle-based immunoassay for the quantification of milk allergens and she received her master’s degree from SHNU in 2016.
Dr. Yuanfeng Wang obtained her PhD in 2005 from Jiangnan University and has researched for more than 10 years in the field of food safety and extraction, isolation, activity and structure of plant extracts. She is now an associate professor at SHNU and her current research interests focus on innovative “green” process and technology for health-promoting plant ingredients.
Dr. Xinlin Wei received his PhD in 2004 from Jiangnan University and works in the field of food safety and food engineering in plant extract. He became a professor at SHNU in 2007. His current research interests focus on innovative “green” process and technology for health-promoting plant ingredients.
Additional information
Funding
References
- Bennett, L., Wheatcroft, R., & Smithers, G. (2001). A review of allergic and intolerant reactions to dairy protein experienced by non-infant consumers. Australian Journal of Dairy Technology, 56(2), 126.
- Choi, J., Horne, D. S., & Lucey, J. A. (2011). Determination of molecular weight of a purified fraction of colloidal calcium phosphate derived from the casein micelles of bovine milk. Journal of Dairy Science, 94(7), 3250–3261. doi: 10.3168/jds.2010-3762
- Cui, Y. L., Zhang, L. Y., Su, J., Zhang, C. F., Li, Q., Cui, T., … Chen, C. (2006). Synthesis of GoldMag particles with assembled structure and their applications in immunoassay. Science in China Series B-Chemistry, 49(6), 534–540. doi: 10.1007/s11426-006-2005-x
- Daniel, M. C., & Astruc, D. (2004). Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chemical Review, 104(1), 293–346. doi: 10.1021/cr030698+
- Giovannacci, I., Guizard, C., Carlier, M., Duval, V., Martin, J. L., & Demeulemester, C. (2004). Species identification of meat products by ELISA. International Journal of Food Science & Technology, 39(8), 863–867. doi: 10.1111/j.1365-2621.2004.00859.x
- Gupta, A. K., & Gupta, M. (2005). Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials, 26(18), 3995–4021. doi: 10.1016/j.biomaterials.2004.10.012
- He, Y., Sai, L. M., Lu, H. T., Hu, M., Lai, W. Y., Fan, Q. L., … Huang, W. (2007). Microwave-assisted synthesis of water-dispersed CdTe nanocrystals with high luminescent efficiency and narrow size distribution. Chemistry of Materials, 19(3), 359–365. doi: 10.1021/cm061863f
- Hird, H., Lloyd, J., Goodier, R., Brown, J., & Reece, P. (2003). Detection of peanut using real-time polymerase chain reaction. European Food Research and Technology, 217(3), 265–268. doi: 10.1007/s00217-003-0726-z
- Jain, P. K., El-Sayed, I. H., & El-Sayed, M. A. (2007). Au nanoparticles target cancer. Nano Today, 2(1), 18–29. doi: 10.1016/S1748-0132(07)70016-6
- Khaemba, G. W., Tochi, B. N., Mukunzi, D., Joel, I., Guo, L. L., Suryobrobowo, S., … Xu, C. L. (2016). Development of monoclonal antibody and lateral test strip for sensitive detection of clenbuterol and related beta(2)-agonists in urine samples. Food and Agricultural Immunology, 27(1), 111–127. doi: 10.1080/09540105.2015.1079598
- Kong, D. Z., Liu, L. Q., Song, S. S., Kuang, H., & Xu, C. L. (2016). Development of ic-ELISA and lateral-flow immunochromatographic assay strip for the detection of folic acid in energy drinks and milk samples. Food and Agricultural Immunology, 27(6), 841–854. doi: 10.1080/09540105.2016.1183600
- Kong, D. Z., Liu, L. Q., Xing, C. R., Kuang, H., & Xu, C. L. (2015). Sensitive and highly specific detection of Cronobacter sakazakii based on monoclonal sandwich ELISA. Food and Agricultural Immunology, 26(4), 566–576. doi: 10.1080/09540105.2014.998634
- Kurien, B. T., & Scofield, R. H. (2006). Western blotting. Methods, 38(4), 283–293. doi: 10.1016/j.ymeth.2005.11.007
- Li, Y. S., Zhou, Y., Meng, X. Y., Zhang, Y. Y., Liu, J. Q., Zhang, Y., … Liu, Z. S. (2014). Enzyme-antibody dual labeled gold nanoparticles probe for ultrasensitive detection of kappa-casein in bovine milk samples. Biosensors & Bioelectronics, 61, 241–244. doi: 10.1016/j.bios.2014.05.032
- Moen, L., Sletten, G., Miller, I., Plassen, C., Gutleb, A., & Egaas, E. (2005). Rocket immunoelectrophoresis and ELISA as complementary methods for the detection of casein in foods. Food and Agricultural Immunology, 16(2), 83–90. doi: 10.1080/09540100400029928
- Monaci, L., & Visconti, A. (2010). Immunochemical and DNA-based methods in food allergen analysis and quality assurance perspectives. Trends in Food Science & Technology, 21(6), 272–283. doi: 10.1016/j.tifs.2010.02.003
- Qing-Hui, Z., You-Lin, Z., Chuang, D., Kai, S., Ya-Juan, S., Xiao-Min, L., & Xiang-Gu, K. (2009). Investigation on the fluorescent labeling between CdTe/CdS Core/shell quantum dots and protein. Chemical Journal of Chinese Universities-Chinese, 30(6), 1158–1161.
- Ranjbari, E., Hadjmohammadi, M. R., Kiekens, F., & De Wael, K. (2015). Mixed hemi/Ad-micelle sodium dodecyl sulfate-coated magnetic iron oxide nanoparticles for the efficient removal and trace determination of rhodamine-B and rhodamine-6G. Analytical Chemistry, 87(15), 7894–7901. doi: 10.1021/acs.analchem.5b01676
- Roberts, G., & Lack, G. (2005). Diagnosing peanut allergy with skin prick and specific IgE testing. Journal of Allergy and Clinical Immunology, 115(6), 1291–1296. doi: 10.1016/j.jaci.2005.02.038
- Shiba, K., Tagaya, M., Sugiyama, T., & Hanagata, N. (2015). Preparation of luminescent titania/dye hybrid nanoparticles and their dissolution properties for controlling cellular environments. RSC Advances, 5(126), 104343–104353. doi: 10.1039/C5RA23026H
- Song, F., Zhou, Y., Li, Y. S., Meng, X. M., Meng, X. Y., Liu, J. Q., … Zhang, J. H. (2014). A rapid immunomagnetic beads-based immunoassay for the detection of beta-casein in bovine milk. Food Chemistry, 158, 445–448. doi: 10.1016/j.foodchem.2014.02.150
- Suryoprabowo, S., Liu, L. Q., Peng, J., Kuang, H., & Xu, C. L. (2015). Antibody for the development of specific immunoassays to detect nadifloxacin in chicken muscles. Food and Agricultural Immunology, 26(3), 317–324. doi: 10.1080/09540105.2014.914469
- Trapiella-Alfonso, L., Costa-Fernandez, J. M., Pereiro, R., & Sanz-Medel, A. (2011). Development of a quantum dot-based fluorescent immunoassay for progesterone determination in bovine milk. Biosensors & Bioelectronics, 26(12), 4753–4759. doi: 10.1016/j.bios.2011.05.044
- Van Hengel, A. J. (2007). Food allergen detection methods and the challenge to protect food-allergic consumers. Analytical and Bioanalytical Chemistry, 389(1), 111–118. doi: 10.1007/s00216-007-1353-5
- Walz, F. (2002). The Verwey transition – a topical review. Journal of Physics: Condensed Matter, 14(12), R285–R340.
- Wang, J. N., Xu, G. H., Wei, F. D., Yang, J., Zhou, P., & Hu, Q. (2016). A novel Fe3O4/CdTe fluorescence probe for sialic acid detection based on a phenylboronic acid-sialic acid recognition system. RSC Advances, 6(1), 481–488. doi: 10.1039/C5RA17171G
- Wang, W. B., Feng, M., Kong, D. Z., Liu, L. Q., Song, S. S., & Xu, C. L. (2015). Development of an immunochromatographic strip for the rapid detection of Pseudomonas syringae pv. maculicola in broccoli and radish seeds. Food and Agricultural Immunology, 26(5), 738–745. doi: 10.1080/09540105.2015.1023266
- Wang, Y., Bai, Y., & Wei, X. (2011). Conjugation behaviours of CdTe quantum dots and antibody by a novel immunochromatographic method. IET Nanobiotechnology, 5(1), 14–19. doi: 10.1049/iet-nbt.2010.0001
- Wang, Y. F., Xu, F., Zhang, L., & Wei, X. L. (2013). One-pot solvothermal synthesis of Fe3O4-PEI composite and its further modification with Au nanoparticles. Journal of Nanoparticle Research, 15(1), 22.
- Xu, L. G., Song, Y. H., Liu, L. Q., Song, S. S., Zhu, J. P., Kuang, H., & Xu, C. L. (2016). Sandwich ELISA and immunochromatographic strip of Kunitz trypsin inhibitor using sensitive monoclonal antibodies. Food and Agricultural Immunology, 27(6), 772–782. doi: 10.1080/09540105.2016.1160367
- Yang, X. D., Zhang, G. P., Wang, F. Y., Wang, Y. B., Hu, X. F., Li, Q. M., … Zeng, X. Y. (2015). Development of a colloidal gold-based strip test for the detection of chlorothalonil residues in cucumber. Food and Agricultural Immunology, 26(5), 729–737. doi: 10.1080/09540105.2015.1018875
- Zhang, L. Y., Long, Y. J., & Zheng, H. Z. (2016). Rapid size-dependent separation of CdTe quantum dots with prion protein. RSC Advances, 6(42), 35617–35620. doi: 10.1039/C6RA05419F