ABSTRACT
Nitrofurans are a class of drugs, which are typically used as antibiotics or antimicrobials illegally in aquiculture. In this paper, indirect competitive enzyme-linked immunosorbent assay and strip sensor were developed for detection of furazolidone metabolite, 3-amino-2-oxazolidinone (AOZ). These two methods could detect AOZ with high sensitivity and specificity. The immunosorbent assay with the half-inhibitory concentration of 0.074 ng/mL has a good recovery from 79.9% to 119.8% for detection of AOZ in pork, chicken, fish, shrimp, pork liver and chicken liver. Based on the developed strip sensor, the detection limit was 0.05 ng/g by the naked eyes and the cut-off value was 0.3 ng/g. The cross-reactivity with other nitrofurans, metabolites and some other compounds was all less than 0.1%. The sample recoveries ranged from 97.3% to 115.5%. Both these methods could be used for on-site detection of furazolidone metabolite at trace level in animal tissues.
Introduction
Nitrofurans are Shiff’s base derivatives of nitrofuraldehyde, which were widely used in medical and veterinary industries for the treatment of ulcers and gastrointestinal infection. They also have a preventive and therapeutic effect against a variety of host parasites and pathogen infection in poultry, pigs, rabbits, fish, etc. However, evidence suggested that furazolidone could result in abnormal progression of DNA in the human hepatocellular carcinoma cell, arresting cell cycle in the S phase (Jin et al., Citation2011). On the other hand, furazolidone will be quickly metabolized to 3-amino-2-oxazolidinone (AOZ) in the body, causing the concentration of the parent compound in plasma to decrease dramatically. After the metabolism of nitrofurans in the body, the metabolites could accumulate in the protein tissue and steadily exist for months (McCracken, Blanchflower, Rowan, McCoy, & Kennedy, Citation1995). Due to its carcinogenicity and mutagenicity, the abuse of furazolidone in food-producing animals will damage the health of animals, accumulate in the tissue and ultimately affect human health through the food chain (Basak, Citation1995; Gajewska, Szczypka, Tudek, & Szymczyk, Citation1990). Based on the obvious negative effect, the use of furazolidone in food-producing animals has been forbidden by the EU since 1995 and China’s Ministry of Agriculture also prohibited the addition of nitrofurans in animal feed and drinking water in 2002. However, in many countries, nitrofurans are still used illegally in livestock and aquaculture production, especially furazolidone.
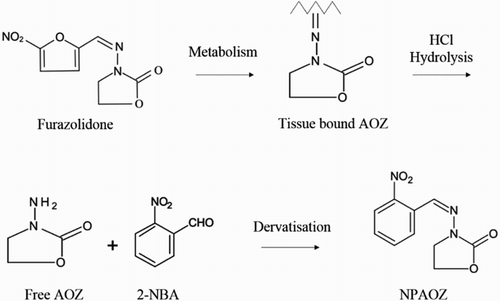
For the purpose of environment protection and food safety, it is very important to monitor the furazolidone residue in the livestock production and aquafarms and the surrounding water environment. However, it was difficult to detect the furazolidone itself as the fast molecular metabolism. The metabolites (AOZ) could be detected after a few hours in the furazolidone-treated body. Through the study of furazolidone marked by 14C, the metabolites of furazolidone are combined with protein and the complete AOZ side chains could be obtained by mildly acid-hydrolyzed protein (Horne, Cadogan, O’Keeffe, & Hoogenboom, Citation1996). AOZ is stable in the tissue. There are researches that indicate that AOZ could be detected in pig muscle six weeks after stopping drug injection (Hoogenboom, Berghmans, Polman, Parker, & Shaw, Citation1992a).
Based on the metabolic and structural characteristics of furazolidone, AOZ in combination with protein or other tissues are detected by most of the current detection methods to quantify furazolidone indirectly. On the other hand, due to low molecule weight (AOZ, molecular weight 102), no absorption in the UV spectrum and quick elution from the chromatographic column, AOZ reacts with 2-nitrobenzaldehyde (2-NBA) to form a stable compound, 3-[[2-nitrophenyl]methylene]amino-2-oxazolidinone (NPAOZ), in most instrument detection methods. At present, high-performance liquid chromatography (HPLC; Hoogenboom, Berghmans, Polman, Parker, & Shaw, Citation1992b; Horne et al., Citation1996; Mccracken & Kennedy, Citation1997; McCracken et al., Citation1995) and liquid chromatography mass spectrometry (LC-MS; Khong et al., Citation2004; Leitner, Zollner, & Lindner, Citation2001) have been widely used for the detection and analysis of furazolidone. However, chromatographic detection methods need expensive instruments which are unsuitable for on-site detection.
Enzyme-linked immunosorbent assays (ELISAs) and strip biosensor are characteristic of high sensitivity, low cost, small sample size and high-throughput screening (Bai et al., Citation2012; Isanga et al., Citation2017; Nath, Arun, & Chanda, Citation2015; Peng et al., Citation2014; Shi, Tian, Wu, Li, & Yu, Citation2015; Singh, Sharma, & Nara, Citation2015; Wang et al., Citation2016; Yu, Su, Zhang, Wei, & Guo, Citation2017; Zhang et al., Citation2017). The ELISAs have been widely used for rapid detection of substances such as low molecular analytes, nucleic acids, proteins, etc. (Chen et al., Citation2017; Choi et al., Citation2016; Pavankumar, Engstrom, Liu, Herthnek, & Nilsson, Citation2016; Xing et al., Citation2013; Xing et al., Citation2015; Xing, Liu, Zhang, Kuang, & Xu, Citation2014; Yin, Liu, Song, Kuang, & Xu, Citation2015; Zhang et al., Citation2013). Many researchers have suggested the immunological detection method for furazolidone metabolite detection. Kevin M. Cooper had established the ELISA method for rapid detection of AOZ in shrimp tissue based on the polyclonal antibody which could be interacted with NPAOZ (Cooper, Caddell, Elliott, & Kennedy, Citation2004; Cooper, Elliott, & Kennedy, Citation2004). These polyclonal antibodies were stimulated by the same immunogen synthesized from 3-{[(3-carboxyphenyl)-methylene]amino-2-oxazolidinone (CPAOZ). Zonghui Yuan established a similar detection method that is based on new polyclonal antibodies against 4-CPAOZ hapten. The sample was treated with an Oasis MAX purification column and then derivatization by a new derivatization reagent of benzaldehyde. High accuracy and precision were achieved for sensitive detection of PAOZ derivative from the AOZ (Chang, Peng, Wu, Wang, & Yuan, Citation2008). M. Vass has prepared a monoclonal antibody against CPAOZ hapten and established the ELISA method to detect AOZ in shrimp, poultry, pork and beef (Vass et al., Citation2005). Tzong-fu Kuo established an immunological detection method based on polyclonal antibody produced from a new immunogen containing hapten 2-NP-HXA-AOZ. The established method could detect 2-NP-AOZ with high specificity, sensitivity and stability (Cheng et al., Citation2009). Through coupling furazolidone to carrier protein directly by diazotization and glutaraldehyde reaction, Jianping Wang produced polyclonal antibody and established an immunological method for rapid detection of four kinds of furan derivatives including furazolidone in feed (Li, Liu, & Wang, Citation2009). Anping Deng has developed an ultrasensitive competitive immunochromatographic assay for direct detection of 3-amino-5-methylmorpholino-2-oxazolidinone (AMOZ) in tissue and urine samples. Surface-enhanced Raman scattering was used as the output signal (Li et al., Citation2015). The limit of detection of the assay for AMOZ was 0.28 pg/mL. However, this highly sensitive discrimination was achieved based on Raman enhancement which could not be realized through naked eye. Lingwen Zeng developed a lateral flow biosensor based on magnetic beads as both the extraction and color-developing media. On the beads, the antibody against nitrofuran derivative was functionalized. This method has avoided tedious extraction procedure and could visually detect four metabolites with a detection limit of 0.1 μg/L (Lu, Liang, Dong, Fang, & Zeng, Citation2016). In this method, ractopamine derivative must be prepared first and the process of synthesis was complex. Chuanlai Xu has reported a new anti-2-NPAOZ antibody 5G12 and established an indirect competitive enzyme-linked immunosorbent assay (IC-ELISA) with a half-inhibitory concentration (IC50) of 0.2 ng/mL (Ding, Liu, Song, Kuang, & Xu, Citation2017). Xie Yong has developed a novel immunochromatography test strip and the visual cut-off level was 10 μg/L (Xie, Zhang, & Le, Citation2017).
In this paper, a high-sensitive indirect competitive ELISA and lateral flow biosensor were developed based on furazolidone monoclonal antibody. The samples including pork, chicken, fish, shrimp, pork liver and chicken liver were detected by the ELISA. The IC50 was 0.074 ng/mL. As to the strip sensor, the standard curve was obtained by detecting series of the trace furazolidone added in fish tissue and the detection results could be easily judged by visual discrimination.
Materials and methods
Materials and instruments
Furazolidone monoclonal antibody and furazolidone bovine serum albumin conjugates were kindly offered by Jiangsu MeiZheng Biological Technology Co. Ltd. Furantoin and its metabolite of 1-aminohydantoin (AHD), furacilin and its metabolite of semicarbazide (SEM), furazolidone and its metabolite of AOZ, furaltadone and its metabolite of AMOZ and 2-NBA were all purchased from Sigma-Aldrich (St Louis, MO, USA). Goat anti-mouse IgG was purchased from Jackson. Other reagents were all analytical reagents.
The CT300 CNC strip cutting machine, HM3030 XYZ three-dimensional dispensing platform and ZQ3500 CNC fast chopping machine were purchased from shanghai Kinbio Tech Co., Ltd and used to manufacture the test strips. Strip scanning reader (HM12003) was purchased from Jiangsu MeiZheng Biological Technology Co. Ltd. Sartorius CN 140 nitrocellulose (NC) membrane, H5015 thick absorbent pad, Ahlstrom 8964 sample pad, PVC backplane were supplied by Shanghai Jieyi Biotechnology Co., Ltd.
Preparation of indirect competitive ELISA
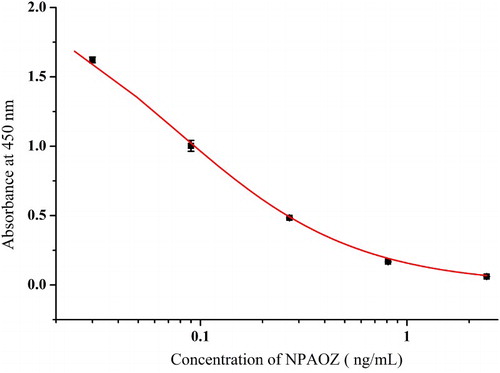
The sensitivity of the monoclonal antibody was determined based on the immunosorbent assay. The optimized coating antigen and antibody concentration were obtained based on the minimum IC50 values and the optical density values of negative wells around 2.0. Under these conditions, the assay was stable and sensitive. Different standard concentrations of NPAOZ (0, 0.03, 0.09, 0.27, 0.81 and 2.43 ng/mL) were used. Different tissue samples including pork, chicken, fish, shrimp, pork liver and chicken liver are measured by ELISA. Original concentration of the tissue samples was detected by LC–MS/MS and no AOZ was detected.
Preparation of gold nanoparticles labeled furazolidone monoclonal antibody
Firstly, the pH of gold nanoparticles (GNPs) solution (20 nm) was adjusted by K2CO3 to 8.2. In this condition, antibody could be attached to the surface of GNPs by the electrostatic adsorption. At the same time, the concentration of furazolidone antibody was diluted to 0.2 mg/mL by 0.002 M borate buffer solution (pH8.2). Then, 60, 80, 100 and 120 µg diluted antibody solutions were added to a 15-mL sterile centrifuge tube containing 10 mL GNPs solution. The solution was shaken up and down slightly and rotated for 30 min on the automatic rotating instrument. To block the GNPs’ surface, 500 µL 10% bovine serum albumin (BSA) solution was added to the nanoparticles solution for 2 h incubation. The conjugate was centrifuged at 8000 rpm for 30 min. Excess antibody and BSA on the supernatant were discharged and the sediments of antibody-conjugated GNPs were washed according to the same process by phosphate-buffered solution (PBS) containing 2% (w/v) BSA, 2% (w/v) sucrose and 0.02% (w/v) sodium azide (protective solution). Finally, the GNPs labeled antibody sediment was redispersed to a concentration of 1 mL and stored under 4°C.
Derivatization process of furazolidone in animal tissue
In , the release of bound AOZ under mildly acidic conditions and derivatization are illustrated. In particular, the skin and fat of fish were removed and the tissue portion was collected and homogenized. Then 1 M hydrochloric acid (5 mL) and 0.05 M 2-NBA (400 μL) were added to a 50 mL centrifuge tube containing 1.0 g homogenized sample with vigorous shaking for 2 min. Then the centrifuge tube was incubated in a water bath at 65°C for 3 h. 0.1 M dipotassium phosphate (5 mL) and 2 M sodium hydroxide (1 mL) were added to the tube. The mixture was oscillated violently for 1 min. The ethyl acetate (8 mL) was added to the solution immediately and shaken for 2 min. The reaction solution was centrifuged for 5 min at 4000 r/min. The supernatant (4 mL) was taken into a 5 mL centrifuge tube and air dried at 60°C. Two milliliters of n-hexane was added to re-dissolve the NPAOZ completely through vigorous oscillation for 1 min before adding 1 mL 0.1 M PBS. The n-hexane and PBS mixture was shaken for 1 min and centrifuged at 4000 r/min for 1 min. The lower solution was collected and measured by ELISA. As to strip detection, the sample treatment process was the same as described above except the sample weight was 4.0 g.
Preparation of the strip sensor
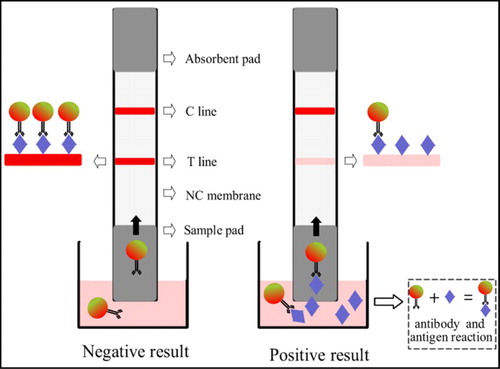
The strip sensor was prepared as per the following process. The concentration of colloidal gold conjugates was estimated by UV scanning and diluted to 1, 2, 4 and 6 nM by protective solution. Fifty microliters of each diluted solution was added to the 96-well microplate for freeze drying. The freeze-dried conjugates were sealed for protection. At the same time, furazolidone–BSA conjugates were diluted by PBS solution to 2.0, 1.5, 1, 0.5 and 0.25 mg/mL. The coating solution was sprayed on the NC membrane as the detection area. The concentration of goat anti-mouse IgG was diluted to 0.2, 0.3, 0.4 and 0.5 mg/mL and sprayed on the NC membrane as the control area. Then the NC membrane was air dried under 37°C. The NC membrane sprayed with antigen and goat anti-mouse antibody, sample pads and absorbent pads were assembled according to and cut into strips of 2 mm width.
Adding 200 µL PBS extraction solution to the well containing freeze-dried gold labeled antibody, the solution was pipetted several times and incubated for 5 min at room temperature. The sample pad of strips was inserted into the solution. The solution was migrated into the NC membrane. The antibody-labeled colloidal gold reacted with the furazolidone–BSA on the detection area and formed T line and goat anti-mouse IgG on the control area and formed a C line. After 10 min, obvious control line and detection line will be observed by the naked eye resulting from nanoparticles’ gathering induced by antibody–antigen interaction. The color density on the T line will decrease as the concentration of AOZ increases in the sample. The detection results could be judged visually depending on the color density of the T line and C line.
Sensitivity determination
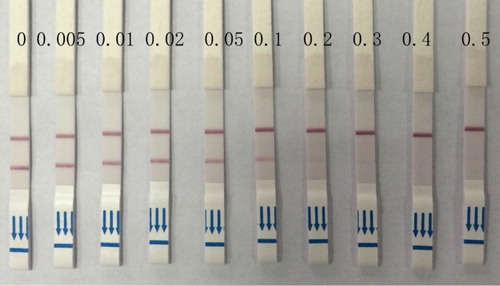
Different concentrations of AOZ (0, 0.005, 0.01, 0.02, 0.05, 0.1, 0.2, 0.3, 0.4 and 0.5 ng/g) were added to the fish tissue and pretreated according to the process described before. The obtained sample solution was detected by the strip sensor. The detection results were judged visually and recorded by the strip reader for quantitative detection.
Specificity determination and sample analysis by strip sensor
Frantoin, furacilin, furazolidone, furaltadone, AHD, SEM, AMOZ and 2-NBA were tested to calculate their cross-reactivity with AOZ. Fish samples were fortified with four concentrations of AOZ (0.02, 0.05, 0.1 and 0.2 ng/g) and treated according to the preparation method. The extracts were detected by the strip and the results were judged by the naked eye and strip reader simultaneously. The result was expressed as the mean of three assay repeats for each fortified concentration.
Results and discussion
ELISA establishment based on antibody
The ELISA was prepared and used for detection of different meat samples. As showed in , the IC50 was 0.074 ng/mL when the absorbance value of the negative well was around 2.2 at 450 nm. The established method was applied for detection of AOZ in different samples including pork, chicken, fish, shrimp, pork liver and chicken liver. The spiked AOZ concentrations were 0.2, 0.5, 1 and 2 ng/g. The spiked samples were detected by ELISA after derivatization. The results are shown in . The ELISA possessed a satisfactory repeatability with recovery ranging from 79.9% to 119.8% and coefficient of variation (CV) ranging from 0.3% to 23%. From the results, when the lowest concentration AOZ was spiked (0.2 ng/g), the results have a higher coefficient of variation. This may be induced by the matrix effects and these effects weakened as the spiked concentration increased. The established method was accurate and could be used as a fast screening tool for different meat samples.
Table 1. Recovery results in spiked meat samples based on ELISA.
Preparation of antibody–GNP conjugates
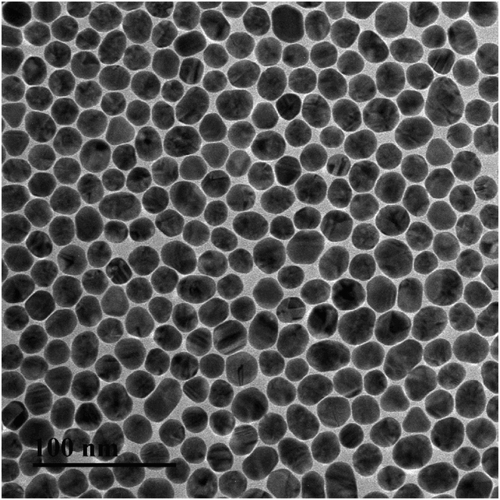
The GNPs were prepared according the Frens method with some modifications (Yan et al., Citation2012). The particle size could be controlled by the amount of citric acid added. Gold nanoparticles with 10 and 15 nm show light red color and the change of color density would not be easily observed by the naked eyes. GNPs with 30 and 40 nm tend to aggregate in the process of antibody–GNP conjugates formation. And the stability of freeze-dried antibody–GNP conjugates was poor. In this experiment, 20 nm GNPs were used and the transmission electron microscopy image is shown in .
Establishment of the furazolidone strip sensor
The results of judgment of the strip sensor have two approaches. The first one was semi-quantitative determination. The detected results were read visually by comparison of color density of T line and C line. The second one was quantitative determination. Colloidal GNPs aggregated in the detection area and control area depended on the chromatography and antibody–antigen reaction. The strip reader will scan and record the color density of the T line and C line and calculate the ratio of T value to C value. Depending on the standard curve input before, the results of the sample could be read automatically.
At the optimal condition, 8 µg antibody was used to label a milliliter of GNPs. The concentration of colloidal gold conjugates was diluted to 4 nM for freeze drying. The furazolidone–BSA conjugates were diluted to 1 mg/mL and sprayed as the T line. The concentration of goat anti-mouse IgG was diluted to 0.4 mg/mL and sprayed on the NC as the C line. In these conditions, obvious red lines could be observed by the naked eyes and the sensitivity of the strip detected by the strip reader was best.
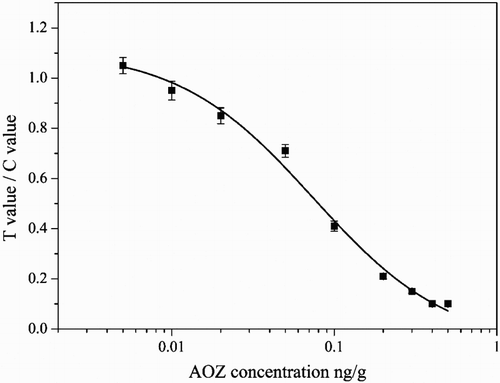
Fish tissue was fortified by different concentrations of AOZ derived according to the mentioned method and detected by the strip sensor. As seen in , the color density of the T line decreased gradually and the color density of C line remained constant. When the AOZ concentration in the sample was more than 0.05 ng/g, the color density of the T line was obviously lighter than that of the control line. When AOZ concentration in the sample was more than 0.3 ng/g, the detection line disappeared. From the detection results, the detection limit was 0.05 ng/g by the naked eye and the cut-off value was 0.3 ng/g. The strip was also analyzed by the strip reader. The ratio of T value to C value decreased from 1.13 to 0.95 when 0.01 ng/g AOZ was found in the fish sample. Here, as seen in , the detection limit was 0.01 ng/g when the ratio of T value to C value was less than 1.
Specificity determination and sample analysis
Different analogues including frantoin, furacilin, furazolidone, furaltadone, AHD, SEM, AMOZ and 2-NBA were detected to evaluate their cross-reactivity with AOZ. When the concentration of these chemicals was 50 ng/g, the detection results were still negative. As shown in , the cross-reactivity was less than 0.1%. This indicated that the monoclonal antibody of furazolidone was highly specific. Fish samples spiked with AOZ at concentrations of 0.02, 0.05, 0.1 and 0.2 ng/g were analyzed. From the intra-assay and inter-assay test results in , the strip sensor possessed a satisfactory repeatability with recovery ranging from 97.3% to 115.5% and CV ranging from 3.7% to 7.3%. As the standard curve in the strip method was obtained based on the spiked fish samples, the repeatability of the strip sensor was better than that of ELISA. The results indicated that the strip sensor is very sensitive and stable and could detect AOZ in animal food samples at trace concentrations.
Table 2. The specificity of the strip sensor.
Table 3. Recovery test of AOZ in fish samples by the strip sensor.
Conclusion
In this study, we have developed ELISA and the strip sensor for detecting AOZ with high sensitivity and specificity. Different meat tissues spiked with AOZ could be detected by immunosorbent assays with good performance. The developed strip sensor could be judged by the naked eyes directly. Combined with strip reader, the detection limit of the strip could reach 0.01 ng/g. These two methods have good recoveries and could be used as a powerful tool for analysis of nitrofuran drugs of aquatic products.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Changrui Xing got his Ph.D in food science in 2016 from Jiangnan University, Wuxi, China and then became a faculty in College of food science and engineering in Nanjing University of Finance and Economics. His research interests are immunochromatographic strip design and application.
Xuexue Jing got her bachelor from Bohai University and then she began to study in Nanjing University of Finance and Economics as a graduate student in food safety. Her research interests are immunoassay applications in food.
Xun Zhang got his Ph.D in food science in 2014 from Jiangnan University, Wuxi, China and then became the director of technology in Jiangsu MeiZheng Biological Technology Co. Ltd (Wuxi, China). His interests are fast detection technology and food safety evaluation.
Jian Yuan is a full professor of College of food science and engineering in Nanjing University of Finance and Economics. His research interests are food quality and safety evaluation.
Additional information
Funding
References
- Bai, Y., Tian, C., Wei, X., Wang, Y., Wang, D., & Shi, X. (2012). A sensitive lateral flow test strip based on silica nanoparticle/CdTe quantum dot composite reporter probes. RSC Advances, 2, 1778–1781. doi: 10.1039/c2ra00976e
- Basak, J. (1995). Inter-strand cross-linking of Vibrio cholerae DNA induced by furazolidone: a quantitative assay by four simple methods. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 327, 5–15. doi: 10.1016/0027-5107(94)00059-E
- Chang, C., Peng, D., Wu, J., Wang, Y., & Yuan, Z. (2008). Development of an indirect competitive ELISA for the detection of furazolidone marker residue in animal edible tissues. Journal of Agricultural and Food Chemistry, 56, 1525–1531. doi: 10.1021/jf0726684
- Cheng, C.-C., Hsieh, K.-H., Lei, Y.-C., Tai, Y.-T., Chang, T.-H., Sheu, S.-Y., … Kuo, T.-F. (2009). Development and residue screening of the furazolidone metabolite, 3-amino-2-oxazolidinone (AOZ), in cultured fish by an enzyme-linked immunosorbent assay. Journal of Agricultural and Food Chemistry, 57, 5687–5692. doi: 10.1021/jf900859r
- Chen, G., Jin, M., Du, P., Zhang, C., Cui, X., Zhang, Y., … Zheng, L. (2017). A review of enhancers for chemiluminescence enzyme immunoassay. Food and Agricultural Immunology, 28, 315–327. doi: 10.1080/09540105.2016.1272550
- Choi, J. R., Liu, Z., Hu, J., Tang, R., Gong, Y., Feng, S., & Xu, F. (2016). Polydimethylsiloxane-paper hybrid lateral flow assay for highly sensitive point-of-care nucleic acid testing. Analytical Chemistry, 88, 6254–6264. doi: 10.1021/acs.analchem.6b00195
- Cooper, K. M., Caddell, A., Elliott, C. T., & Kennedy, D. G. (2004). Production and characterisation of polyclonal antibodies to a derivative of 3-amino-2-oxazolidinone, a metabolite of the nitrofuran furazolidone. Analytica Chimica Acta, 520, 79–86. doi: 10.1016/j.aca.2004.05.074
- Cooper, K. M., Elliott, C. T., & Kennedy, D. G. (2004). Detection of 3-amino-2-oxazolidinone (AOZ), a tissue-bound metabolite of the nitrofuran furazolidone, in prawn tissue by enzyme immunoassay. Food Additives and Contaminants Part A-chemistry Analysis Control Exposure & Risk Assessment, 21, 841–848.
- Ding, X., Liu, L., Song, S., Kuang, H., & Xu, C. (2017). Rapid and ultrasensitive detection of 3-amino-2-oxazolidinone in catfish muscle with indirect competitive enzyme-linked immunosorbent and immunochromatographic assays. Food and Agricultural Immunology, 28, 463–475. doi: 10.1080/09540105.2017.1297778
- Gajewska, J., Szczypka, M., Tudek, B., & Szymczyk, T. (1990). Studies on the effect of ascorbic acid and selenium on the genotoxicity on nitrofurans: nitrofurazone and furazolidone. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 232, 191–197. doi: 10.1016/0027-5107(90)90124-M
- Hoogenboom, L. A. P., Berghmans, M. C. J., Polman, T. H. G., Parker, R. M., & Shaw, I. C. (1992a). Depletion of protein-bound furazolidone metabolites containing the 3-amino-2-oxazolidinone side-chain from liver, kidney and muscle tissues from pigs. Food Additives and Contaminants Part A-chemistry Analysis Control Exposure & Risk Assessment, 9, 623–630.
- Hoogenboom, L. A. P., Berghmans, M. C. J., Polman, T. H. G., Parker, R., & Shaw, I. C. (1992b). Depletion of protein-bound furazolidone metabolites containing the 3-amino-2-oxazolidinone side-chain from liver, kidney and muscle tissues from pigs. Food Additives and Contaminants, 9, 623–630. doi: 10.1080/02652039209374117
- Horne, E., Cadogan, A., O’Keeffe, M., & Hoogenboom, L. A. P. (1996). Analysis of Protein-bound metabolites of Furazolidone and furaltadone in pig liver by high-performance liquid chromatography and liquid chromatography-mass spectrometry. The Analyst, 121, 1463–1468. doi: 10.1039/AN9962101463
- Isanga, J., Mukunzi, D., Chen, Y., Suryoprabowo, S., Liu, L., Kuang, H., & Xu, C. (2017). Development of a monoclonal antibody assay and a lateral flow strip test for the detection of paromomycin residues in food matrices. Food and Agricultural Immunology, 28, 355–373. doi: 10.1080/09540105.2016.1272551
- Jin, X., Tang, S., Chen, Q., Zou, J., Zhang, T., Liu, F., … Xiao, X. (2011). Furazolidone induced oxidative DNA damage via up-regulating ROS that caused cell cycle arrest in human hepatoma G2 cells. Toxicology Letters, 201, 205–212. doi: 10.1016/j.toxlet.2010.12.021
- Khong, S., Gremaud, E., Richoz, J., Delatour, T., Guy, P. A., Stadler, R. H., & Mottier, P. (2004). Analysis of matrix-bound nitrofuran residues in worldwide-originated honeys by isotope dilution high-performance liquid chromatography-tandem mass spectrometry. Journal of Agricultural and Food Chemistry, 52, 5309–5315. doi: 10.1021/jf0401118
- Leitner, A., Zollner, P., & Lindner, W. (2001). Determination of the metabolites of nitrofuran antibiotics in animal tissue by high-performance liquid chromatography-tandem mass spectrometry. Journal of Chromatography A, 939, 49–58. doi: 10.1016/S0021-9673(01)01331-0
- Li, J., Liu, J. X., & Wang, J. P. (2009). Multidetermination of four nitrofurans in animal feeds by a sensitive and simple enzyme-linked immunosorbent assay. Journal of Agricultural and Food Chemistry, 57, 2181–2185. doi: 10.1021/jf8035098
- Li, M., Yang, H., Li, S., Liu, C., Zhao, K., Li, J., … Deng, A. (2015). An ultrasensitive competitive immunochromatographic assay (ICA) based on surface-enhanced Raman scattering (SERS) for direct detection of 3-amino-5-methylmorpholino-2-oxazolidinone (AMOZ) in tissue and urine samples. Sensors and Actuators B: Chemical, 211, 551–558. doi: 10.1016/j.snb.2014.12.135
- Lu, X., Liang, X., Dong, J., Fang, Z., & Zeng, L. (2016). Lateral flow biosensor for multiplex detection of nitrofuran metabolites based on functionalized magnetic beads. Analytical and Bioanalytical Chemistry, 408, 6703–6709. doi: 10.1007/s00216-016-9787-2
- McCracken, R. J., Blanchflower, W. J., Rowan, C., McCoy, M. A., & Kennedy, D. G. (1995). Determination of furazolidone in porcine tissue using thermospray liquid chromatography-mass spectrometry and a study of the pharmacokinetics and stability of its residues. The Analyst, 120, 2347–2351. doi: 10.1039/AN9952002347
- Mccracken, R. J., & Kennedy, D. G. (1997). Determination of the furazolidone metabolite, 3-amino-2-oxazolidinone, in porcine tissues using liquid chromatography-thermospray mass spectrometry and the occurrence of residues in pigs produced in Northern Ireland. Journal of Chromatography B: Biomedical Sciences and Applications, 691, 87–94. doi: 10.1016/S0378-4347(96)00448-3
- Nath, P., Arun, R. K., & Chanda, N. (2015). Smart gold nanosensor for easy sensing of lead and copper ions in solution and using paper strips. RSC Advances, 5, 69024–69031. doi: 10.1039/C5RA14886C
- Pavankumar, A. R., Engstrom, A., Liu, J., Herthnek, D., & Nilsson, M. (2016). Proficient detection of multi-drug-resistant mycobacterium tuberculosis by padlock probes and lateral flow nucleic acid biosensors. Analytical Chemistry, 88, 4277–4284. doi: 10.1021/acs.analchem.5b04312
- Peng, T., Yang, W., Lai, W.-H., Xiong, Y.-H., Wei, H., & Zhang, J. (2014). Improvement of the stability of immunochromatographic assay for the quantitative detection of clenbuterol in swine urine. Analytical Methods, 6, 7394–7398. doi: 10.1039/C4AY01173B
- Shi, Z., Tian, Y., Wu, X., Li, C., & Yu, L. (2015). A one-piece lateral flow impedimetric test strip for label-free clenbuterol detection. Analytical Methods, 7, 4957–4964. doi: 10.1039/C5AY00706B
- Singh, J., Sharma, S., & Nara, S. (2015). Nanogold based lateral flow assay for the detection of Salmonella typhi in environmental water samples. Analytical Methods, 7, 9281–9288. doi: 10.1039/C5AY02271A
- Vass, M., Kotkova, L., Diblikova, I., Nevorankova, Z., Cooper, K. M., Kennedy, D. G., & Franek, M. (2005). Production and characterisation of monoclonal antibodies for the detection of AOZ, a tissue bound metabolite of furazolidone. Vet. Med.-Czech, 50, 300–310.
- Wang, S., Wang, L., Chen, H., Wang, Y., Cai, J., Yang, M., & Liu, F. (2016). Development of an eco-friendly immunochromatographic test strip and its application in detecting Hg2+ without chelators. RSC Advances, 6, 8729–8735. doi: 10.1039/C5RA22752F
- Xie, Y., Zhang, L., & Le, T. (2017). An immunochromatography test strip for rapid, quantitative and sensitive detection of furazolidone metabolite, 3-amino-2-oxazolidinone, in animal tissues. Food and Agricultural Immunology, 28, 403–413. doi: 10.1080/09540105.2017.1293013
- Xing, C., Feng, M., Hao, C., Xu, L., Wang, L., & Xu, C. (2013). Visual sensor for the detection of trace Cu(II) Ions using an immunochromatographic strip. Immunological Investigations, 42, 221–234. doi: 10.3109/08820139.2012.752378
- Xing, C., Liu, L., Song, S., Feng, M., Kuang, H., & Xu, C. (2015). Ultrasensitive immunochromatographic assay for the simultaneous detection of five chemicals in drinking water. Biosensors and Bioelectronics, 66, 445–453. doi: 10.1016/j.bios.2014.12.004
- Xing, C., Liu, L., Zhang, X., Kuang, H., & Xu, C. (2014). Colorimetric detection of mercury based on a strip sensor. Analytical Methods, 6, 6247–6253. doi: 10.1039/C3AY42002G
- Yan, W., Xu, L., Xu, C., Ma, W., Kuang, H., Wang, L., & Kotov, N. A. (2012). Self-assembly of chiral nanoparticle pyramids with strong R/S optical activity. Journal of the American Chemical Society, 134, 15114–15121. doi: 10.1021/ja3066336
- Yin, Y., Liu, L., Song, S., Kuang, H., & Xu, C. (2015). Development of a highly sensitive icELISA to detect semicarbazide based on a monoclonal antibody. Food and Agricultural Immunology, 26, 356–365. doi: 10.1080/09540105.2014.914891
- Yu, J., Su, J., Zhang, J., Wei, X., & Guo, A. (2017). CdTe/CdS quantum dot-labeled fluorescent immunochromatography test strips for rapid detection of Escherichia coli O157:H7. RSC Advances, 7, 17819–17823. doi: 10.1039/C7RA00821J
- Zhang, X., Feng, M., Liu, L., Xing, C., Kuang, H., Peng, C., … Xu, C. (2013). Detection of aflatoxins in tea samples based on a class-specific monoclonal antibody. International Journal of Food Science & Technology, 48, 1269–1274. doi: 10.1111/ijfs.12086
- Zhang, Y., Yang, J., Lu, Y., Ma, D.-Y., Qi, M. G., & Wang, S. (2017). A competitive direct enzyme-linked immunosorbent assay for the rapid detection of deoxynivalenol: development and application in agricultural products and feedstuff. Food and Agricultural Immunology, 28, 516–527. doi: 10.1080/09540105.2017.1306491