ABSTRACT
Ling-Zhi-8 (LZ-8), a 13-kDa protein from mycelia of Ganoderma lucidum, exerts various beneficial functions in mammalian cells. Using murine BV-2 microglial cells, the objective of this study was to determine how LZ-8 modulates the production of pro-inflammatory mediators. Incubation of cells with LZ-8 (up to 10 µg/ml) had no negative effect on cell proliferation. LZ-8 significantly reduced the lipopolysaccharide-stimulated production of nitric oxide, prostaglandin E2 and interleukin-6 up to 31%, 39% and 58%, respectively (p < .05). LZ-8 down-regulated expression of inducible nitric oxide synthase and cyclooxygenase-2 by 69% and 37%, and also suppressed translocation of nuclear factor-kappa B (NF-κB) into the nucleus and reduced gene expression of phosphorylated NF-κB inhibitor and toll-like receptor-4 (TLR-4). In conclusion, LZ-8 reduced TLR-4-mediated NF-κB translocation into nucleus resulting in the suppression of pro-inflammatory mediator expression and production in microglial cells.
Introduction
Ling-Zhi (Ganoderma lucidum, also called reishi mushroom or ganoderma) is a traditional basidiomycetous fungus that has been used as an herbal medicine or medical remedy in Asian countries for centuries. Numerous beneficial effects exerted by Ling-Zhi, such as improvement of immune function and relief of symptoms caused by various chronic diseases might be attributable to a wide variety of other biologically active substances extracted from ganoderma fruiting bodies, mycelia and spores (Wang et al., Citation1997; Wasser, Citation2005). One of these bioactive components is Ling-Zhi-8 (LZ-8), which is a low molecular-weight protein (approximate 13 kDa) (Kino et al., Citation1989). This fungal protein retains its bioactive properties when exposed to heat, freezing and acidic and dehydrating environments (Huang, Yang, Chen, & Chuang, Citation2014; Tong, Chien, Chang, Tsai, & Sheu, Citation2008). Therefore, LZ-8 has long been considered a potentially useful additive in the food-processing and nutraceutical industries.
Recent studies have demonstrated that LZ-8 modulates immune responses by activating cell proliferation and/or cytokine production in murine macrophages and T-cells (Hsu et al., Citation2013; Kino et al., Citation1990; Yeh, Chen, Yang, Chuang, & Sheu, Citation2010), and decreasing the incidence of type-1 diabetes in non-obese diabetic mice (Kino et al., Citation1990). LZ-8 also induces autophagic cell death in human gastric carcinoma SGC-7901 cells, and inhibits A549 human lung cancer cell growth (Liang et al., Citation2012; Wu et al., Citation2011). Furthermore, LZ-8 has been shown to have hepatoprotective properties. For example, oral administration of LZ-8 to rats not only protects them against tetrachloride carbon (CCl4)-stimulated hepatic injury (Yang et al., Citation2014), but also accelerates wound healing in rat liver tissues after monopolar electrosurgery (Lin et al., Citation2014). Taken together, these findings suggest that LZ-8 could be developed as a potent agent in numerous clinical situations, including immunomodulation and anti-carcinogenesis.
However, to date, few studies have been conducted to determine whether LZ-8 can protect the central nervous system (CNS) against exogenous inflammatory stimuli via the modulation of immune responses in microglia, the resident macrophages of the brain and spinal cord. To this end, using the model of lipopolysaccharide (LPS)-stimulated murine microglial BV-2 cells, we firstly determined if incubating cells with LZ-8 would lower the production of pro-inflammatory nitric oxide (NO), prostaglandin E2 (PGE2) and interleukin-6 (IL-6), as well as protein levels of inducible nitric oxide synthase (iNOS) and type-2 cyclooxygenase (COX-2). It is well-known that toll-like receptor-4 (TLR-4)-mediated nuclear factor-kappa B (NF-κB) triggered by LPS are responsible for initiating inflammatory responses by over-expressing numerous pro-inflammatory mediators as above described (Ahn et al., Citation2016; Kim et al., Citation2017; Lu, Yeh, & Ohashi, Citation2008; Taechowisan, Wanbanjob, Tuntiwachwuttikul, & Liu, Citation2010). Furthermore, the inflammation-related signaling NF-κB cascade was assessed to explore the underlying mechanisms, including gene expression of NF-κB inhibitor (IκBα) and translocation of NF-κB into nucleus. Thus, the aim of the present study was to advance understandings regarding the anti-inflammatory properties of LZ-8 in the CNS.
Materials and methods
Chemicals
High purity LZ-8 in phosphate-buffered saline (PBS) (10 mg/mL) was obtained from Yeastern Biotech (Taipei, Taiwan). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), lactate dehydrogenase (LDH) activity kit, LPS (from Escherichia coli O26:B6), n-(1-naphthyl) ethylenediamine, sodium nitrite, sulfanilamide and trypan blue were provided by Sigma Chemical Co. (St. Louis, MO, USA). A PGE2 enzyme immunoassay assay kit was purchased from Cayman Chemicals (Ann Arbor, MI, USA). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), PBS and enzyme-linked immunosorbent assay (ELISA) kits for detecting mouse IL-6 were supplied by Invitrogen (Carlsbad, CA, USA).
Cell culture and growth conditions
The murine microglial BV-2 cell line was a kind gift of Dr Rolis C.-W. Hou. BV-2 cells were grown in DMEM with 10% (v/v) heat-inactivated FBS, and maintained in a 5% CO2 fully humidified atmosphere at 37°C. The Trypan blue solution was used to count cell numbers in all experiments. The methods of MTT assay and LDH activity assay were applied in this study to monitor cell proliferation and cytotoxicity as described elsewhere (Huang, Tsai, Huang, Chen, & Chuang, Citation2014).
Determination of pro-inflammatory mediator production
Two independent studies were carried out to determine if LZ-8 might influence the production of pro-inflammatory NO, IL-6 or PGE2. In the first study, microglial cells (1.5 × 105 cells/mL) were seeded onto a 24-well culture plate and allowed to adhere for 4 h. After the culture medium was replaced by serum-supplemented DMEM with different concentrations (1, 5 or 10 μg/mL) of LZ-8 for 24 h, the cells were then washed with sterile PBS, and stimulated with LPS (0.1 μg/mL) for 16 h. In the second study, attached microglial cells were incubated with LZ-8 (1, 5 or 10 μg/mL) and LPS (0.1 μg/mL) together for 16 h. Following these two types of LPS stimulation, cell-free supernatants were collected to measure nitrite concentration using the Griess reaction as a surrogate of NO production (Green et al., Citation1982). The concentrations of secreted IL-6 and PGE2 were assessed using commercial ELISA kits.
Detection of iNOS, COX-2 and IκB expression by western blot analysis
BV-2 cells (1.5 × 105 cells/mL) were cultured in the presence of LZ-8 (5 or 10 μg/mL) for 24 h, followed by LPS stimulation (0.1 μg/mL) for 16 h to assess iNOS and COX-2 expression, or for 30 min to test for IκB phosphorylation. After harvest, total cellular protein was determined using the BCA Protein Assay Reagent Kit (Pierce, Rockford, IL, USA). Heat-denatured protein samples (10 μg) were separated on 10% (w/v) sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and transferred onto immobile nitrocellulose polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). After non-specific blocking and rinsing with 3% (w/v) non-fat-dried milk in TBS-T (0.1% Tween 20 (v/v) in Tris-buffered saline) and TBS-T, membranes were incubated with rabbit anti-mouse primary antibody (1:1000 dilution of iNOS or COX-2; BD Biosciences, Franklin Lakes, NJ, USA), or anti-mouse IκB or phospho-IκB antibody (1:1000; Cell Signaling Technology, Danvers, MA, USA). Membranes were then reacted with a goat anti-rabbit secondary antibody conjugated with horseradish peroxidase (1:5,000; Sigma, St. Louis, MO, USA). The interested immuno-complexes were visualized using enhanced chemiluminescence (Merck, Darmstadt, Germany).
Determination of nuclear translocation of NF-κB p65
To examine whether nuclear transcription factor NF-κB activation was affected by LZ-8, BV-2 cells (1.5 × 106 cells/mL) were incubated with LZ-8 (5 μg/mL) for 24 h, and then stimulated with LPS (0.1 μg/mL) for 30 min. The cytosolic NF-κB p65 translocation into the nucleus was detected using an NF-κB/p65 ActivELISA™ kit (Imgenex, San Diego, CA, USA) (Huang, Huang, Li, & Chuang, Citation2011).
Flow cytometry
To determine whether BV-2 cells were stimulated by the supplementation of LZ-8, the expression of ionized calcium-binding adaptor molecule 1 (Iba-1) and TLR-4 was evaluated by flow cytometry. Briefly, cells were collected and mixed well with FACS buffer (PBS containing 0.5% (w/v) bovine serum albumin and 0.09% (w/v) sodium azide), and then fixed with fixation buffer (BioLegend, SanDiego, CA, USA) for 20 min at room temperature. Next, cells were rinsed twice with permeabilization buffer (BioLegend) and stained with fluorescein isothiocyanate-conjugated anti-mouse Iba-1 (Abcam, Cambridge, MA, USA) or allophycocyanin (APC)-conjugated anti-mouse TLR-4 (Abcam, Cambridge, MA, USA) in permeabilization buffer for 20 min. After rinsing three times with FACS buffer, cells were suspended in 0.5 ml FACS buffer and then analyzed using a FACSCanto II instrument (BD Biosciences, San Jose, CA, USA).
Analysis of TLR-4 mRNA level by real-time reverse transcription polymerase chain reaction
Total RNA of BV-2 cells was isolated using the TRIzol reagent (Invitrogen; Carlsbad, CA, USA), and cDNA was generated from 2 μg of total RNA using an oligo (dT) primer and 1 μL of reverse transcriptase (Promega, Madison, WI, USA). To amplify cDNA, primer sets for TLR-4 (forward primer, 5′-CTGGTGGCTGTGGAGACA-3′; reverse primer, 5′-ATGTACTAGGTTCGTCAGATTGGA-3′) and glyceraldehydes 3-phosphate dehydrogenase (GAPDH) (forward primer, 5′-TCCAAGGAGTAAGAAACC-3′; reverse primer, 5′-GAAATTGTGAGGGAGATG-3′) were used for polymerase chain reaction (PCR). Real-time PCR amplification was performed using an iCycler iQ Real-Time detection system (Bio-Rad, Hercules, CA, USA) using iQ™ SYBR Green Supermix (Bio-Rad). Thermal cycling conditions were applied for all assays as described elsewhere (Huang, Tsai, et al., Citation2014). The melting curve of each tube confirmed the presence of a single peak. The relative amounts of the PCR products were analyzed using iQ™5 optical system software (ver. 2.1). The mRNA level of each sample that was analyzed for TLR-4 gene was normalized to that of the GAPDH mRNA.
Lipid extraction and fatty acid analysis
After harvest, the cell pellets were washed with PBS, and total cellular lipids were extracted using the modified Folch’s method described elsewhere (Folch, Lees, & Sloane-Stanley, Citation1957). The extracted lipids were re-constituted with 0.25 mL of chloroform, and the total phospholipids were separated by thin layer chromatography using a developing solvent consisting of hexane/diethyl ether/acetic acid (80:20:1, v/v/v). FAME derived from the total phospholipids were analyzed using an Agilent 6890 gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) equipped with an autosampler system, a flame ionization detector and a fused-silica capillary column (Omegawax; 30 m × 0.32 mm, i.d., film thickness 0.25 μm, Supelco, Bellefonte, PA, USA) (Huang et al., Citation2011). To quantify fatty acids, a known amount of triheptadecanoin was added to each sample as internal standard.
Statistical analyses
Data were analyzed by analysis of variance and Tukey’s range test using SPSS software (SPSS for windows 17.0; SPSS Inc. Chicago, IL, USA) to determine differences between means. Mean differences were considered significant at the p ≤ .05 levels.
Results
Effect of LZ-8 on cell proliferation
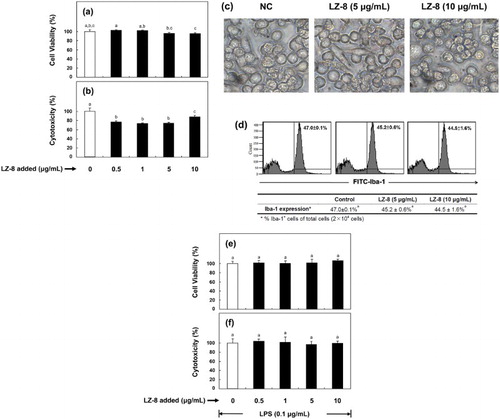
In this study, there was no inhibitory effect on cell proliferation when BV-2 cells were incubated with LZ-8 concentrations below 10 μg/mL ((a)). With regard to toxicity, we found that LZ-8 supplementation ( ≥0.5 μg/ml) significantly reduced lactate dehydrogenase release by up to 26% as compared to the control ((b)). Results from (c) demonstrated that no obvious morphological difference between control and LZ-8-treated cells was observed. Similarly, using flow cytometric analysis, we determined that there was no significant difference in the expression levels of Iba-1, an indicator of activated microglia, among the three test samples ((d)). Similarly, no negative effect of LZ-8 on cell proliferation and cytotoxicity when BV-2 microglia incubated with LZ-8 for 24 h, followed by 16-h LPS stimulation ((e,f)).
Figure 1. Effect of LZ-8 on the cell viability (a), cytotoxicity (b), cellular morphology (c) and expression of ionized calcium-binding adapter molecule-1 (Iba-1) (d) in regular murine microglial BV-2 cells; and on the cell viability (e), cytotoxicity (f) in LPS-stimulated BV-2 cells. To examine cellular proliferation and cytotoxicity, cells were incubated in medium supplemented with different concentrations of LZ-8 (0, 0.5, 1, 5 or 10 μg/mL) for 24 h (regular), followed by LPS stimulation (0.1 µg/mL) for 16 h (LPS-stimulated). The MTT and LDH activity assays were used to evaluate cell viability and cytotoxicity of both experimental groups (regular and LPS-stimulated) of cells, respectively. To examine cellular morphology, BV-2 cells were incubated in medium supplemented with none, 5 or 10 μg/mL of LZ-8 for 24 h, and photographed under an inverted microscope (× 400). To examine the expression of Iba-1 in BV-2 cells, control and LZ-8-treated cells were (2 × 104 cells) determined by flow cytometry with fluorescein isothiocyanate-conjugated anti-mouse Iba-1. The bars or numbers indicate the mean ± SD of three independent experiments. The values with different letters are significantly different at p < .05.
Effect of LZ-8 on pro-inflammatory mediator production
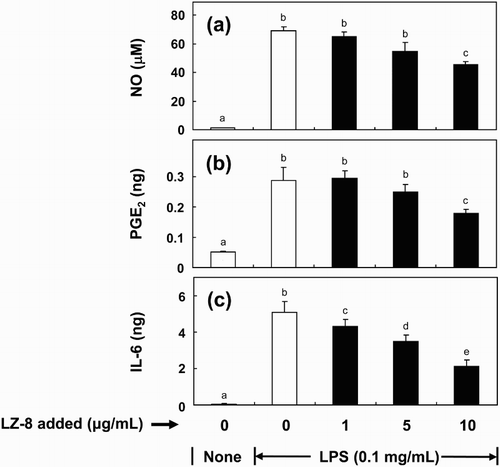
To determine whether LZ-8 might affect inflammatory responses by modulating production of NO, PGE2 and IL-6, BV-2 cells were incubated with increasing concentrations (1–10 μg/mL) of LZ-8 for 24 h, followed by LPS stimulation (0.1 μg/mL) for 16 h. LPS markedly stimulated the synthesis of four pro-inflammatory mediators, as compared to the untreated control (). LZ-8 significantly suppressed the production of NO, PGE2 and IL-6 up to 31%, 39% and 58%, respectively ().
Figure 2. Effects of pre-treatment of LZ-8 on the production of nitric oxide (NO) (a), prostaglandin E2 (PGE2) (b) and interleukin-6 (IL-6) (c) by LPS-stimulated BV-2 microglial cells. Cells were pre-treated with LZ-8 (1, 5 or 10 μg/mL) for 24 h, and then stimulated with 0.1 µg/mL of LPS for 16 h. The bars indicate the mean ± SD of three independent experiments. The values with different letters are significantly different at p < .05.
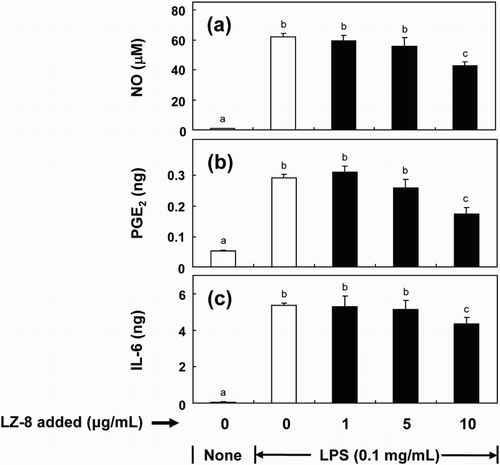
In addition to pre-treat cells with LZ-8 before LPS stimulation, we determined if LZ-8 exerted a similar anti-inflammatory effect when incubated BV-2 cells with LZ-8 and LPS together for 16 h. shows that LZ-8 significantly reduced LPS-stimulated NO, PGE2 and IL-6 production when the concentration was at 10 μg/mL.
Figure 3. Effects of co-culture of LZ-8 on the production of nitric oxide (NO) (a), prostaglandin E2 (PGE2) (b) and interleukin-6 (IL-6) (c) by LPS-stimulated BV-2 microglial cells. Cells were incubated with LZ-8 (1, 5 or 10 μg/mL) and LPS (µg/mL) for 16 h. The bars indicate the mean ± SD of three independent experiments. The values with different letters are significantly different at p < .05.
Effect of LZ-8 on pro-inflammatory iNOS and COX-2
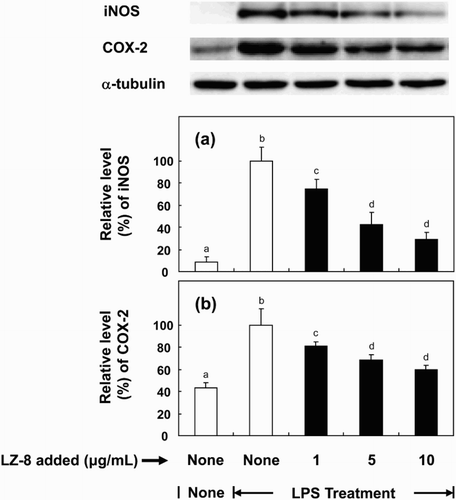
To investigate mechanisms underlying the modulatory effect of LZ-8 on the production of NO and PGE2 by microglia, we examined the effect of LZ-8 on the expression of iNOS and COX-2 in BV-2 cells. shows that iNOS and COX-2 were over-expressed in response to LPS stimulation. However, over-expression of both enzymes was significantly suppressed up to 69% and 37% when the culture medium was supplemented with LZ-8.
Figure 4. Effects of LZ-8 on the expression of iNOS and COX-2 in LPS-stimulated BV-2 microglial cells. Microglial cells were incubated with only medium, or medium containing LZ-8 (1, 5 or 10 μg/mL) for 24 h, followed by LPS treatment (0.1 µg/mL) for 16 h. iNOS and COX-2 expression was determined by Western blot analysis. α-Tubulin was used as a loading control. Each value represents the mean ± SD of three independent experiments. Values with different letters are significantly different from each other at p < .05.
Effect of LZ-8 on NF-κB activation and IκBα phosphorylation
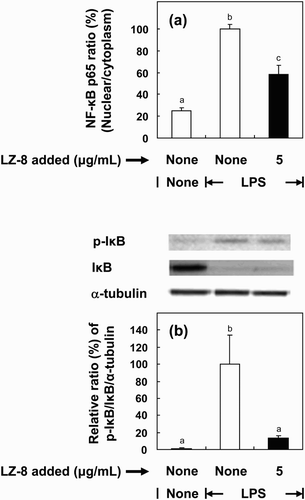
We also determined whether NF-κB activation might play a role in the modulation of pro-inflammatory mediators. The results in (a) show that the level of translocated NF-κB was increased in response to LPS stimulation as compared to control. Incubation of BV-2 cells with LZ-8 suppressed NF-κB p65 activation by 43% (p < .001). Furthermore, IκBα, an inhibitor of NF-κB, was phosphorylated when the culture medium was added with LPS ((b)). However, when LZ-8-preincubated cells were induced by LPS, expression of phosphorylated IκBα was significantly reduced by 85% relative to the LPS-stimulated control.
Figure 5. Effects of LZ-8 on the activation of NF-κB and phosphorylation of IκB in BV-2 microglial cells. Cells were incubated with normal medium, or medium containing LZ-8 (5 μg/mL) for 24 h, followed by LPS treatment (0.1 μg/mL) for 30 min. The degree of augmentation of NF-κB p65 translocation into the nucleus from the cytosol was determined using an NF-κB/p65 ActivELISA™ kit. IκB and phosphor-IκB expression was determined by Western blot analysis. α-Tubulin was used as a loading control. Each value represents the mean ± SD of three independent experiments. Values with different letters are significantly different from each other at p < .05.
Effect of LZ-8 on TLR-4 expression
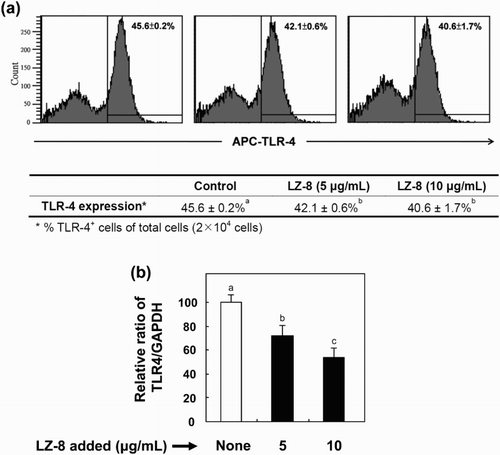
We inquired whether LZ-8 affects TLR-4 expression at translational and transcriptional levels by incubating BV-2 cells with LZ-8 (0, 5 or 10 μg/mL) for 24 h. As determined by flow cytometry, supplementation of LZ-8 significantly lowered TLR-4 expression from 45.6% to 40.6% ((a)). Treatment of LZ-8 significantly reduced the level of TLR-4 mRNA by up to 45% relative to the untreated control ((b)).
Figure 6. Effects of LZ-8 on the expression of TLR-4 protein (a) and mRNA (b) levels in BV-2 microglial cells. To examine the expression of TLR-4 in BV-2 cells, control and LZ-8-treated cells were (2 × 104 cells) determined by flow cytometry with fluorescein allophycocyanin (APC)-conjugated anti-mouse TLR-4 and real-time reverse transcription polymerase chain reaction. The numbers indicate the mean ± SD of three independent experiments. The values with different letters are significantly different at p < .05.
Effect of LZ-8 on phospholipid fatty acid compositions in BV-2 cells
The LPS-stimulated PGE2 production was reduced by LZ-8 supplementation, and the reduction could be caused by lowering the availability of arachidonic acid (AA) substrate, the precursor of PGE2, in cellular phospholipids. To assess the effect of LZ-8 on cellular phospholipid AA content, BV-2 cells were incubated in culture medium supplemented with LZ-8 (5 μg/mL) for 24 h, followed by LPS (0.1 μg/mL) for 16 h; or in culture medium supplemented with LZ-8 and LPS for 16 h. Analysis of cellular fatty acid content by GC showed that the levels of AA significantly increased from 6.0% to 9.0% (by 49%) of total phospholipid fatty acids when cells were stimulated by LPS (). The proportions of AA were also raised 47% or 35% as compared to the untreated control, respectively, when the LZ-8 supplementation was applied before LPS addition or contemporaneously with LPS. However, among three experimental groups (LPS control, pre-treated and co-culture groups), there is no significant difference in the AA proportion was found.
Table 1. Effects of LPS or/and LZ-8 on the percentage of cellular phospholipid arachidonic acid (AA).
Discussion
The most important finding of the present study was that LZ-8 reduced TLR-4-mediated NF-κB translocation into the nucleus resulting in the suppression of pro-inflammatory mediator expression and production in microglial cells.
(a,e) demonstrate that incubation of BV-2 cells with LZ-8 did not affect cell viability, but significantly reduced the cytotoxicity up to 26% in comparison with the untreated control ((b,f)). These results are in accord with our previous observation that supplementation of the culture medium with LZ-8 decreased release of LDH by murine macrophages, indicating that LZ-8 might protect cultured cells from various stimuli or toxic substances in the culture environment (Huang, Yang, et al., Citation2014). Furthermore, LZ-8 was previously reported to promote pseudopodia elaboration by macrophages and aggregation of cells at relatively high concentrations of LZ-8 (Huang, Yang, et al., Citation2014). However, no obvious changes in the morphology of LZ-8 treated BV-2 cells were observed under light microscopy ((c)), and the marker of activated microglia, Iba-1, was not evident, indicating that BV-2 cells were not activated by LZ-8 ((d)) (Ito, Tanaka, Suzuki, Dembo, & Fukuuchi, Citation2001). Furthermore, while LZ-8 activates murine macrophages and spleen cells by enhancing transcriptional expression or production of TNF-α (Chang, Chien, Tong, & Sheu, Citation2007; Li, Wen, Liu, Zhou, & Chen, Citation2012; Yang et al., Citation2014; Yeh, Yeh, Hsu, Luo, & Lin, Citation2008), no such stimulatory effect on TNF-α production in BV-2 microglia was detected (data not shown). It is well established that microglia serve as the resident macrophages in the brain and spinal cord, but, based on results of the present study, murine BV-2 microglia and RAW264.7 macrophages do not appear to have the same cellular responses to LZ-8. The present study demonstrated that LZ-8 suppressed pro-inflammatory NO and PGE2 production ( and ), and down-regulated iNOS and COX-2 over-expression in BV-2 microglial cells (). LZ-8 also inhibited the cytokine IL-6 synthesis ((c) and (c)). These findings confirm the anti-inflammatory properties of LZ-8 previously reported using rats and murine RAW264.7 macrophages, respectively (Huang, Yang, et al., Citation2014; Yang et al., Citation2014). To investigate the mechanisms underlying the anti-inflammatory effect of LZ-8, we firstly examined whether LZ-8 affected the activation of NF-κB, which is responsible for initiating the expression of a wide array of inflammatory genes (Kawai & Akira, Citation2007). Our results suggest that LZ-8 might block the NF-κB signaling cascade by inhibiting the phosphorylation of IκB and translocation of p65 into nucleus (). We also investigated how LZ-8 modulated the LPS-stimulated NF-κB pathway. An expanding body of evidence strongly suggests that LPS interacts with TLR-4, resulting in activation of numerous cell signaling cascades, including NF-κB (Kawai & Akira, Citation2007; Lin et al., Citation2009; Palsson-McDermott & O'Neill, Citation2004). Results of flow cytometric and PCR analyses demonstrated that LZ-8 significantly inhibit expression of TLR-4 (), indicating that LZ-8 interferes with LPS-activated TLR-4 signal transduction pathways, such as NF-κB. Taken together, these results support the hypothesis that LZ-8 suppressed the production of pro-inflammatory mediators and down-regulated the over-expression of iNOS and COX-2 by interfering with TLR-4-mediated NF-κB signaling activated by LPS.
Recent studies have indicated that LZ-8 activates mammalian dendritic cells by means of an interaction with the TLR-4 which enhances naïve T cell-stimulatory capacity, cytokine secretion or induction of antigen-specific T cell activation (Lin et al., Citation2009, Citation2011). To probe this possible mechanism further, Lin and coworkers neutralized human dendritic cells with antibodies against TLR-4, and found that it inhibited both LZ-8- and LPS-induced IL-12 p40 and IL-10 production (Lin et al., Citation2009). In a separate study, they showed, too, that LZ-8 and LPS stimulated TLR-4- or TLR-4/MD2-transfected human embryonic kidney 293 (HEK293) cells to induce IL-8 production (Lin et al., Citation2009), thereby pointing to a critical role for TLR-4 in cell signaling upon incubation of cells with LZ-8. Since LZ-8 appears to act as a TLR-4 ligand, the reduced translational level of TLR-4 by LZ-8 in the cytometric analysis ((a)) could be due in part to hindrance of TLR-4 recognition by antibody by the LZ-8-TLR-4 interaction. However, the results in (b) show that LZ-8 significantly reduced the level of TLR-4 mRNA, suggesting the suppression of TLR-4-mediated inflammatory responses by LZ-8 might be mainly attributed to the down-regulation of TLR-4 expression, but not to the interaction of LZ-8 and TLR-4. Furthermore, this finding might explain why cells incubated with LZ-8 before or during LPS stimulation would differentially modulate production of pro-inflammatory IL-6. For example, BV-2 cells were pre-treated with LZ-8, the suppression of TLR-4 by LZ-8 for 24 h prior to the LPS treatment would reduce LPS-mediated activation, thereby lowering the IL-6 level by more than 50%, while IL-6 production was lowered by only 19% in cells co-cultured with LPS and LZ-8 ((c) and (c)). This result suggested that activation of TLR-4-mediated signaling by LPS might contribute to the attenuation of the anti-inflammatory effects of LZ-8 through the suppression of TLR-4 expression.
In contrast to cytokine production, results of (b) and (b) demonstrate the similar effects of LZ-8 on PGE2 production when cells are exposed to LZ-8 before or simultaneous with LPS stimulation. This could be due to the result of the comparable effects of LZ-8 on COX-2 expression (data not shown) and cellular phospholipid AA content () in the two experimental protocols. Furthermore, the extent of reduction of NO levels in the two LZ-8 protocols was similar ((a) and (a)). These findings suggest that supplementation of LZ-8 at different timing exerts comparable efficacy on the production of PGE2 and NO, but not on that of IL-6. Since pre-treatment of BV-2 cells with LZ-8 is associated with larger effects on pro-inflammatory substances compared with cotemporaneous treatment with LZ-8 and LPS, it seems reasonable to postulate that LZ-8 or some derivative thereof might be useful as a preventive agent rather than a suppressive drug in the future medical or nutraceutical applications.
A limitation of the current study and its implications is that it remains to be shown if LZ-8 crosses the highly selective permeable blood-brain barrier due to its size (13 kDa) and polarity characteristics. Thus, LZ-8 may not protect the brain or CNS against pathogens or neurotoxins directly through its anti-inflammatory properties. However, intraperitoneal administration of LZ-8 prevents systemic anaphylactic reactions in mice, and significantly alleviates mouse footpad edema during the Arthus reaction (Kino et al., Citation1989). Furthermore, oral administration of LZ-8 has been shown to suppress carbon tetrachloride (CCl4)-induced hepatic inflammation and lower serum inflammatory mediator production (Yang et al., Citation2014). These findings suggest that LZ-8 can modulate not only local immune responses but also the systemic immune system. Thus, whether LZ-8 would improve systemic immune functions and exert the similar anti-inflammatory properties in brain is unclear. Future studies of LZ-8 administration with animal brain models to assess these possibilities are required.
In conclusion, we have demonstrated that LZ-8 modulated the LPS-activated immune responses of BV-2 cells by suppressing production of pro-inflammatory mediators, including NO, PGE2, IL-6, as well as expression of iNOS and COX-2, at least in part, by suppressing TLR-4-mediated NF-κB signaling. Furthermore, the incubation of BV-2 microglial cells with LZ-8 prior to the LPS simulation exerted greater anti-inflammatory effects than co-culture of cells with LZ-8 and LPS. Thus, LZ-8 is a potential anti-inflammatory agent for modulating in vitro immune responses involved in neural inflammation.
Acknowledgements
The authors are grateful to Professor Robert H. Glew, PhD, for editing our manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahn, S.-I., Kim, J.-S., Hong, C.-Y., Gu, G.-J., Shin, H.-M., Paek, J. H., … Youn, H.-S. (2016). Eupatorium makinoi suppresses toll-like receptor signaling pathways. Food and Agricultural Immunology, 27, 242–250. doi: 10.1080/09540105.2015.1086315
- Chang, H.-H., Chien, P.-J., Tong, M.-H., & Sheu, F. (2007). Mushroom immunomodulatory proteins possess potential thermal/freezing resistance, acid/alkali tolerance and dehydration stability. Food Chemistry, 105, 597–605. doi: 10.1016/j.foodchem.2007.04.048
- Folch, J., Lees, M., & Sloane-Stanley, G. H. (1957). A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry, 226, 497–509. Retrieved from http://www.jbc.org/content/226/1/497.long
- Green, L. C., Wagner, D. A., Glogowski, J., Skipper, P. L., Wishnok, J. S., & Tannenbaum, S. R. (1982). Analysis of nitrate, nitritie and [15N]nitrate in biological fluids. Analytical Biochemistry, 126, 131–138. doi: 10.1016/0003-2697(82)90118-X
- Hsu, H. Y., Kuan, Y. C., Lin, T. Y., Tsao, S. M., Hsu, J., Ma, L. J., & Sheu F. (2013). Reishi protein LZ-8 induces FOXP3(+) Treg expansion via a CD45-dependent signaling pathway and alleviates acute intestinal inflammation in mice. Evidence-Based Complementary and Alternative Medicine, 2013, 513–542. doi: 10.1155/2013/513542
- Huang, Y.-S., Huang, W.-C., Li, C.-W., & Chuang, L.-T. (2011). Eicosadienoic acid differentially modulates production of pro-inflammatory modulators in murine macrophages. Molecular and Cellular Biochemistry, 358, 85–94. doi: 10.1007/s11010-011-0924-0
- Huang, W.-C., Tsai, P.-J., Huang, Y.-L., Chen, S.-N., & Chuang, L.-T. (2014). PGE2 production suppressed by chemically-synthesized Δ7-eicosatrienoic acid in macrophages through the competitive inhibition of COX-2. Food and Chemical Toxicology, 66, 122–133. doi: 10.1016/j.fct.2014.01.031
- Huang, W.-N., Yang, C.-Y., Chen, D.-C., & Chuang, L.-T. (2014). Correlation of the structure and bioactivity of recombinant fungal immunomodulatory protein, Ling-Zhi-8 (LZ-8) following exposure to denaturing conditions. Journal of Food Biochemistry, 38, 328–336. doi: 10.1111/jfbc.12057
- Ito, D., Tanaka, K., Suzuki, S., Dembo, T., & Fukuuchi, Y. (2001). Enhanced expression of Iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke, 32, 1208–1215. doi: 10.1161/01.STR.32.5.1208
- Kawai, T., & Akira, S. (2007). Signaling to NF-kappaB by toll-like receptors. Trends in Molecular Medicine, 13, 460–469. doi: 10.1016/j.molmed.2007.09.002
- Kim, J.-S., Kim, A.-Y., Shin, H.-M., Ahn, S.-I., Shim, H.-J., Nam, K.-W., … Youn, H.-S. (2017). Aster yomena suppresses LPS-induced cyclooxygenase-2 and inducible nitric oxide synthase expression. Food and Agricultural Immunology, 28, 202–210. doi: 10.1080/09540105.2016.1251395
- Kino, K., Mizumoto, K., Sone, T., Yamaji, T., Watanabe, J., Yamashita, A., … Tsunoo, H. (1990). An immunomodulating protein, Ling Zhi-8 (LZ-8) prevents insulitis in non-obese diabetic mice. Diabetologia, 33, 713–718. doi: 10.1007/BF00400340
- Kino, K., Yamashita, A., Yamaoka, K., Watanabe, J., Tanaka, S., Ko, K., … Tsunoo, H. (1989). Isolation and characterization of a new immunomodulatory protein, Ling Zhi-8 (LZ-8), from Ganoderma lucidum. Journal of Biological Chemistry, 264, 472–478. Retrieved from http://www.jbc.org/content/264/1/472.long
- Liang, C., Li, H., Zhou, H., Zhang, S., Liu, Z., Zhou, Q., & Sun F. (2012). Recombinant LZ-8 from Ganoderma lucidum induces endoplasmic reticulum stress-mediated autophagic cell death in SGC-7901 human gastric cancer cells. Oncology Reports, 27, 1079–1089. doi: 10.3892/or.2011.1593
- Lin, H. J., Chang, Y. S., Lin, L. H., Huang, C. F., Wu, C. Y., & Ou, K. L. (2014). An immunomodulatory protein (Ling Zhi-8) from a Ganoderma lucidum induced acceleration of wound healing in rat liver tissues after monopolar electrosurgery. Evidence-Based Complementary and Alternative Medicine, 2014, 916531. doi: 10.1155/2014/916531
- Lin, Y.-L., Liang, Y.-C., Tseng, Y.-S., Huang, H.-Y., Chou, S.-Y., Hseu, R.-S., … Chiang, B.-L. (2009). An immunomodulatory protein, Ling Zhi-8, induced activation and maturation of human monocyte-derived dendritic cells by the NF-κB and MAPK pathways. Journal of Leukocyte Biology, 86, 877–889. doi: 10.1189/jlb.0708441
- Lin, C.-C., Yu, Y.-L., Shih, C.-C., Liu, K.-J., Ou, K.-L., Hong, L.-Z., … Chu, C.-L. (2011). A novel adjuvant Ling Zhi-8 enhances the efficacy of DNA cancer vaccine by activating dendritic cells. Cancer Immunology, Immunotherapy, 60, 1019–1027. doi: 10.1007/s00262-011-1016-4
- Li, F., Wen, H., Liu, X., Zhou, F., & Chen, G. (2012). Gene cloning and recombinant expression of a novel fungal immunomodulatory protein from Trametes versicolor. Protein Expression and Purification, 82, 339–344. doi: 10.1016/j.pep.2012.01.015
- Lu, Y. C., Yeh, W. C., & Ohashi, P. S. (2008). LPS/TLR4 signal transduction pathway. Cytokine, 42, 145–151. doi: 10.1016/j.cyto.2008.01.006
- Palsson-McDermott, E. M., & O'Neill, L. A. (2004). Signal transduction by the lipopolysaccharide receptor, toll-like receptor-4. Immunology, 113, 153–162. doi: 10.1111/j.1365-2567.2004.01976.x
- Taechowisan, T., Wanbanjob, A., Tuntiwachwuttikul, P. & Liu, J. (2010). Anti-inflammatory effects of lansai C and D cause inhibition of STAT-1 and NF-κB activations in LPS-induced RAW 264.7 cells. Food and Agricultural Immunology, 21, 57–64. doi: 10.1080/09540100903419592
- Tong, M.-H., Chien, P.-J., Chang, H.-H., Tsai, M.-J., & Sheu, F. (2008). High processing tolerances of immunomodulatory proteins in Enoki and Reishi mushrooms. Journal of Agricultural and Food Chemistry, 56, 3160–3166. doi: 10.1021/jf800205g
- Wang, S. Y., Hsu, M. L., Hsu, H. C., Tzeng, C. H., Lee, S. S., Shiao, M. S., & Ho, C. K. (1997). The antitumor effect of Ganoderma lucidum is mediated by cytokines released from activated macrophages and T lymphocytes. International Journal of Cancer, 70, 699–705. doi: 10.1002/(SICI)1097-0215(19970317)70:6<699::AID-IJC12>3.0.CO;2-5
- Wasser, S. P. (2005). This is a chapter. In P. Coates, M. R. Blackman, G. Cragg, M. Levine, J. Moss, & J. White (Eds.), Encyclopedia of dietary supplements: And Reishi or Ling Zhi (Ganoderma lucidum) (pp. 603–622). New York, NY: Marcel Dekker.
- Wu, C. T., Lin, T. Y., Hsu, H. Y., Sheu, F., Ho, C. M., & Chen, E. I. (2011). Ling Zhi-8 mediates p53-dependent growth arrest of lung cancer cells proliferation via the ribosomal protein S7-MDM2-p53 pathway. Carcinogenesis, 32, 1890–1896. doi: 10.1093/carcin/bgr221
- Yang, C.-Y., Chuang, L.-T., Huang, W.-C., Hou, C.-W., Chen, D.-C., Jeng, K.-C.G., & Kao, T.-Y. (2014). Preventive effects of borage oil and Ling-Zhi-8 protein on carbon tetrachloride-induced acute hepatic toxicity in rats. Current Topics in Nutraceutical Research, 12, 91–100. Retrieved from http://ctnr.newcenturyhealthpublishers.com/
- Yeh, C. H., Chen, H. C., Yang, J. J., Chuang, W. I., & Sheu F. (2010). Polysaccharides PS-G and protein LZ-8 from Reishi (Ganoderma lucidum) exhibit diverse functions in regulating murine macrophages and T lymphocytes. Journal of Agricultural and Food Chemistry, 58, 8535–8544. doi: 10.1021/jf100914m
- Yeh, C. M., Yeh, C. K., Hsu, X. Y., Luo, Q. M., & Lin, M. Y. (2008). Extracellular expression of a functional recombinant Ganoderma lucidium immunomodulatory protein by Bacillus subtilis and Lactococcus lactis. Applied and Environmental Microbiology, 74, 1039–1049. doi: 10.1128/AEM.01547-07
