ABSTRACT
This study aimed to explore the effects of neoagarotetraose (NAT) on gut microbiota composition and inflammation after antibiotic treatment. Our results showed that NAT significantly increased the length of colon (P < .01) and decreased the ileocecal valve index (P < .05), and reduced IFN-γ content (P < .01) and downregulated the IFN-γ/IL-4 ratio (P < .01), suggesting that NAT mediated the balance of the Th1/Th2 ratio to maintain the immune equilibrium of the intestinal mucosa. Our data showed that NAT prohibited intestinal inflammation by increasing antimicrobial peptides and gut integrity. Furthermore, NAT administration significantly increased the fecal concentration of total short-chain fatty acids. In addition, NAT had the ability to modulate gut microbiota composition and diversity. In our study, significantly increased Bifidobacterium, Lactobacillus and Prevotella were observed in the NAT group (P < .01). In summary, all of these results demonstrated that NAT was a potential prebiotic for modulating intestinal microbiota and promoting host health.
1. Introduction
There is increasing evidence that a balance in gut microbiota is essential for human health. Alterations in the gut bacterial population have been associated with a number of diseases. Previous studies have shown that the incidence of colorectal cancer and inflammatory bowel diseases (IBDs), such as ulcerative colitis and Crohn’s disease, has increased due to the overuse of antibiotics (Wlodarska & Finlay, Citation2010). Antibiotics can substantially alter the gut microbiota, with effects that can be long-term and may result in infection with antibiotic-resistant bacteria (Dupont & Dupont, Citation2011). Prebiotics are considered to have the potential to reduce disturbances in gut microbiota and gut inflammation induced by antibiotics. Commonly, prebiotics are polysaccharides and oligosaccharides derived from plants or animals. Marine resources, with broad prospects for the development of marine prebiotics, have become a popular research topic.
Seaweed is the most abundant source of polysaccharides, including alginates, agar and agarose, as well as carrageenans. Agarases have been widely applied in agar-derived oligosaccharide production. Compared with the traditional acid degradation method, the enzyme degradation method has a number of remarkable advantages (Fu & Kim, Citation2010). Our group had successfully cloned and expressed the AgWH50A gene, which was a novel β-agarase obtained from a marine microbe (Liu, Mao, Du, Mu, & Wei, Citation2014). We had prepared neoagarotetraose (NAT) by the enzymatic hydrolysis of agar. To date, neoagaro-oligosaccharides have been found to exhibit various biological and physiological functions, such as skin moisturizing, a whitening effect on melanoma cells and anti-inflammatory properties (Hsu et al., Citation2015). NAT was a novel marine oligosaccharide whose biological activities had not yet been reported.
Accumulating evidence has suggested that carbohydrates derived from the diet are not digestible by digestive enzymes in the small intestine. The carbohydrates reach the colon, where they are fermented to short-chain fatty acid (SCFA) and gases by colonic bacteria. Trillions of microbes are crucial for the degradation and absorption of nutrients, playing roles in carbohydrate hydrolysis and monosaccharide uptake from the gut (Michele & Antonio, Citation2010). Considering these facts, we suspected that the function of NAT might affect the gut microbiota. However, the effect of NAT on gut microbiota has not been reported until now. Therefore, this study aimed to explore the effects of NAT on gut microbiota composition in a mouse model induced by antibiotic treatment.
Using quantitative real-time PCR (RT-PCR) and denaturing gradient gel electrophoresis (DGGE), this study revealed the modulation of gut microbiota by NAT for the first time. In addition, we demonstrated that NAT could reshape the microbiota composition, improve gut colonization of anti-inflammatory bacteria and helped to alleviate systemic inflammation caused by antibiotics.
2. Materials and methods
2.1. Materials
NAT was prepared as previously reported (Liu et al., Citation2014). Fructo-oligosaccharide (FOS) was obtained from Solarbio Co, Ltd (Beijing, China). Ceftriaxone sodium was purchased from Shandong Qi-Lu Pharmaceutical Co, Ltd (Jinan, China). All of the reagents used in the animal experiment were of analytical purity.
2.2. Animals and treatment
Male Balb/c mice (18–20 g, four weeks old) were obtained from the Vital River Laboratory Animal Technology Company (Beijing, China). During the experimental period, mice were housed in a room maintained under a 12 h light/dark cycle at 24°C. Mice had free access to fresh water. Mice were fed ad libitum a Maintenance Purified Diet (AIN-93M; also known as #5801-M) throughout the experiment period. After a 7-day acclimation period, mice were randomly assigned to 3 groups with 8 mice each: Untreated group (Control), antibiotic treatment group (AB), FOS and NAT intervention group. FOS was used as a positive control, as a number of studies have revealed the prebiotic effects of this oligosaccharide. During the first seven days, the control group was given an oral administration of normal saline once a day, while the AB, FOS, NAT groups of mice were gavaged with the broad-spectrum antibiotic-ceftriaxone sodium (125 mg/mL, 0.4 mL/day/mice) (Wang, Huang, Tang, & Wei, Citation2006). Over the next 14 days, the control and AB mice were given normal saline, while the FOS, NAT group of mice were gavaged with FOS or NAT, respectively, with a dosage of 150 mg/kg. Animal treatments lasted for 21 days, during which the body weight and food intake of each animal were measured once a day. Fresh stool samples were collected on day 0, 7, 14 and 21 by using a metabolic cage and immediately stored at −80°C for subsequent analysis. At the end of the trial, after 12 h of food deprivation, all animals were sacrificed by cervical dislocation. The animals’ organs were weighed and frozen in liquid nitrogen immediately after sacrifice. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of Ocean University of China (Approved protocol ID SCKK2012-0001).
2.3. Small intestine RNA isolation and cDNA preparation
Total RNA was extracted from isolated small intestine samples using Trizol reagent (Thermo Fisher Scientific Inc., Waltham, MA, USA), as reported in a previous study (Zuo et al., Citation2015). In brief, each sample was dissolved in 1 mL Trizol reagent by homogenization in a homogenizer based on the manufacturer’s instructions. Protein and Trizol were removed with the addition of 0.2 mL chloroform. After isopropanol precipitation, centrifugation at 12,000 × g for 10 min and washing with 75% ethanol, total RNA was extracted and dissolved in diethylpyrocarbonate (DEPC)-treated water. The amount and purity of RNA was quantified spectrophotometrically by a Nanodrop 2000c (Thermo Fisher Scientific Inc.). RNA integrity was checked with agarose gel electrophoresis. RNA (2μg) was converted to cDNA using M-MLV reverse transcriptase and random primers (Sangon Biotech, (Shanghai) Co., Ltd., Shanghai, China). cDNA samples were stored at −80°C until subsequent amplification for analysis.
2.4. Bacteria and genes quantification using RT-PCR
RT-PCR was performed in an iCycler iQ5 system (Bio-Rad Laboratories Inc., Richmond, CA, USA). A reaction volume of 25 μL was used for the quantitative RT-PCR assay that consisted of 12.5 μL Maxima SYBR Green qPCR Master mix (Hoffman-La Roche, Ltd., Basel, Switzerland), 10 μM primers (0.75 μL each of forward and reverse primer), 8.5 μL nuclease-free water and 2.5 μL template. The thermal conditions consisted of an initial denaturation at 95°C for 10 min followed by 45 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 20 s and extension at 72°C for 30 s. Data normalization was accomplished using the endogenous reference β-actin. Gene expression levels were analyzed by relative quantification using a standard curve method. The sequences of the primers used in this study are described in Table 1.
2.5. SCFA measurements
Fecal SCFA content was determined by gas chromatography. Chromatographic analysis was carried out using Agilent 7890A system with a flame ionization detector. One microliter of the upper ether layer was injected into the chromatogram for analysis. The retention times and peak heights of the acids in the standard mix were used as references for the sample unknowns. These acids were identified by their specific retention times and the concentrations determined and expressed as mM concentrations per Kg wet feces (Lawrence et al., Citation2014).
2.6. Fecal bacterial collection and bacterial genomic DNA extraction
Fecal samples were collected from each mouse. Bacterial genomic DNA was extracted from the fecal samples with the magnetic bead DNA extraction kits (Sangon, Shanghai, China) according to the manufacturer’s instruction.
2.7. DGGE analysis of microbiota composition
The variable V3 region of 16S rDNA was amplified using a universal bacterial primer set (Table 1). A GC clamp was attached to the 5’ end of the 338f primer for analysis of the PCR products by DGGE. Primers were produced by Sangon (Shanghai, China). The PCR amplification was carried out in a MJ Mini Personal Thermal Cycler (BIO-RAD, America). Each reaction was performed in a total volume of 50 μL containing 50 ng of DNA template, 5 μL of 10 × PCR buffer, 3.2 μL of dNTP mix (10 mM), 3 μL of MgCl2 (25 mM), 1 μL of each primer (20 mM) and 0.4 μL of Taq DNA polymerase (5 U/μL) (Takara, Japan). The thermal cycler profile consisted of an initial denaturation step of 5 min at 94°C followed by 30 cycles at 94°C for 1 min, 55°C for 45 s, 72°C for 1 min and a final extension at 72°C for 10 min. Purification of the PCR products was performed with a DNA Gel Extraction Kit (Omega, USA). Prior to DGGE analysis, the PCR products were examined by electrophoresis in a 2% agarose gel and visualized under UV light with a Gel-Doc 2000 (Bio-Rad, CA, USA).
DGGE analysis of the PCR amplifications was performed based on a protocol described by Muyzer, Waal, and Uitterlinden (Citation1993). Ten microliters of the PCR products from the amplified DNA of each sample were applied to a 7% (w/v) polyacrylamide gel. Electrophoresis was carried out using a denaturing gradient ranging from 35% to 55% to separate the amplicons. The denaturant contained 7M urea and 40% deionized formamide. Electrophoresis was performed for 5 h at 150 V in 1xTAE buffer at a constant temperature of 60°C. After electrophoresis, the gels were stained using 20 µL of SYBR Green in 200 mL of 1 × TAE buffer for 30 min in the dark. The gel images were converted into digital data using Quantity One software (Li, Song, Liu, Ai, & Yu, Citation2015).
2.8. Statistical analysis
All of the values in the tables and figures are expressed as the mean ± S.E.M. Statistical comparisons of the results were performed using Tukey’s post hoc test (ANOVA) analysis of variance by SPSS 18.0. P < .05 was considered statistically significant.
3. Results
3.1. Dietary NAT increased metabolism homeostasis and organism immunity
We first confirmed the beneficial effects of NAT and FOS on body weight gain and food intake in mice. Compared with the control group, gavage with ceftriaxone sodium induced significant decreases in the body weight and food intake (P < .01), while administration of NAT increased the body weight by 7.77% (P < .05); this same tendency was also observed in the FOS group ((A)). Meanwhile, the AB group mice showed a small degree of recovery, whereas a 26.77% increase in food intake was observed in the NAT group (P < .01) ((B)). Moreover, fecal water content was downregulated in NAT compared with their content in control mice ((C)). Accordingly, NAT increased weight gain and food intake, decreased fecal water contents in antibiotic-treated mice, which effectively improved metabolism homeostasis.
Figure 1. NAT showed remarkable effects on metabolism homeostasis and organism immunity (A) body weight, (B) food intake changes, (C) fecal water content (%), (D) thymus index and (E) Spleen index. Data are presented as mean ± SEM. #P < .05, ##P < .01 indicates significant difference from the control group; *P < .05, **P < .01 indicates significant difference from the AB group.
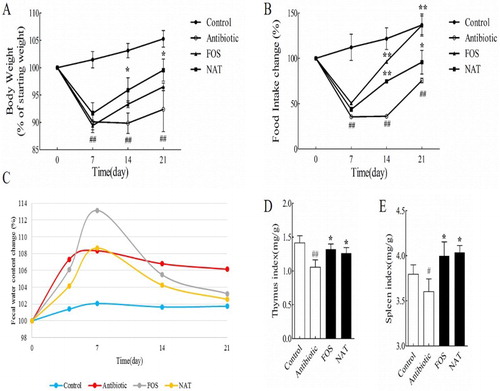
The thymus and spleen index reflect the host immune function on the whole. It showed that antibiotic treatment seriously damaged the thymus (P < .01) and spleen (P < .05) in AB group mice ((D,E)), compared to that of control group mice. Nevertheless, the thymus/bodyweight index of the NAT and FOS group increased significantly compared with the AB group (P < .05), with an 18.87% increase. Similarly, the spleen/bodyweight index of the NAT and FOS group mice got improved significantly (P < .05), with a 12.22% increase compared to that of AB group mice. The results implicates that dietary NAT has an enhancement effect on the immune system.
3.2. NAT significantly reduced gut inflammation caused by antibiotics
To investigate the impact of NAT on gut inflammation, we measured the length of the colon and the ileocecal valve, which had been regarded as key biomarkers of gut inflammation. Our results showed that NAT significantly increased the length of the colon (P < .01) and decreased the ileocecal valve index (P < .05) ((A,B)). To further confirm the anti-inflammation effects of NAT, we next examined the relative mRNA expression of pro-inflammatory cytokines: tumor necrosis factor alpha (TNF-α) and interleukin-1-beta (IL-1β) in intestinal tissue ((C,D)). Notably, the expression of TNF-α was significantly decreased by NAT (P < .05). Also, it is important to note that NAT decreased the mRNA expression of IL-1β by 7.50%. And IL-1β mRNA highly correlated with functional IL-1β expression, as long as inflammasome. In addition, using interleukin-10 (IL-10) as an indicator of anti-inflammation, we also demonstrated that antibiotic treatment increased inflammation, which was effectively reduced by NAT (P < .05) ((E)).
Figure 2. NAT significantly decreased the intestinal inflammation induced by overuse of antibiotics. (A) The length of the colon and (B) the ileocecal valve index. The effects of NAT on the mRNA expression of (C) TNF-α, (D) IL-1β and (E) IL-10, (F) IFN-γ, (G) IL-4 concentration and (H) the ratio of IFN-γ to IL-4 in the intestinal tissue. Data are presented as mean ± SEM. #P < .01, #P < .05 indicates significant difference from the control group; *P < .05, **P < .01 indicates significant difference from the AB group. β-Actin is the loading control.
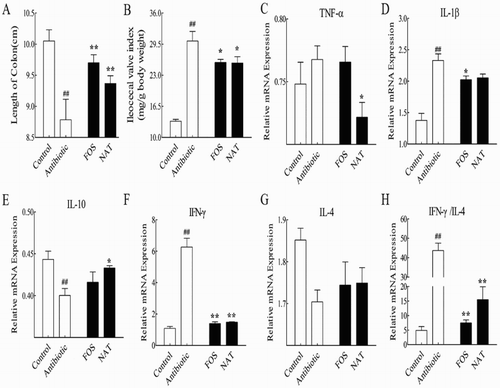
Furthermore, we detected the expression of the representative cytokines secreted by the Th1 and Th2 cells, IFN-γ and IL-4, respectively. However, the IFN-γ/IL-4 ratio is more meaningful than the respective cytokine concentration, since the ratio represents the Th1/Th2 immune balance (Zuo et al., Citation2015). The results demonstrate that NAT treatment notably reduced the IFN-γ content ((F)) (P < .01) and downregulated the IFN-γ/IL-4 ratio ((H)) (P < .01) in mice, compared with that of the AB mice. Taken together, our data suggested that NAT mediated the balance of the Th1/Th2 ratio to maintain the immune equilibrium of the intestinal mucosa.
3.3. NAT relieved inflammation by enhancing the expression of antimicrobial peptides (AMPs) and intestinal integrity
To know if the effect of NAT on prohibiting intestinal inflammation was related to AMPs, the mRNA expression of sPLA2 and Ang4, which are specific AMPs markers of goblet cells and paneth cells in the small intestine, were analyzed between the groups. Compared with the control group, the mRNA expression of sPLA2 and Ang4 had a downward trend in the AB group ((A,B)) (P < .01). The mRNA expression of Ang4 was increased in the NAT group (P < .05), with a 10.00% increase. These results showed a positive link between the NAT intervention and mRNA expression levels of AMPs, which prohibited pathogenic invasion and intestinal inflammation.
Figure 3. The relative mRNA expression levels of the AMPs and tight junction proteins in the small intestine. The relative mRNA expression of (A) sPLA2, and (B) Ang4 in the small intestine. The relative mRNA expression of (C) E-cadherin, (D) Occludin, (E) ZO-1 and (F) Muc2 were detected by qRT-PCR. Data are presented as mean ± SEM. ##P < .01, #P < .05 indicates significant difference from the control group; *P < .05, **P < .01 indicates significant difference from the AB group. β-Actin is the loading control.
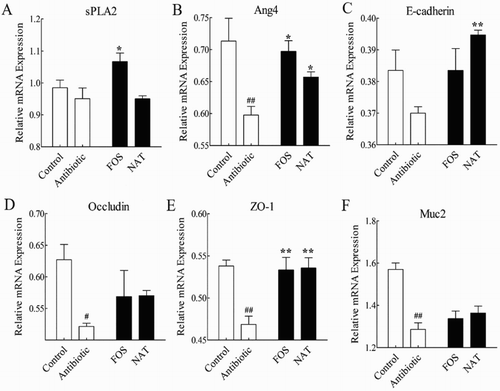
Our results showed that antibiotic feeding reduced expression of the tight junction components, E-cadherin, Occludin and zonula occludens-1 (ZO-1); these effects were reversed by NAT supplementation ((C,E)). It is important to note that NAT increased the expression of E-cadherin, Occludin and ZO-1 by 5.41%, 9.62% and 14.89%, respectively. NAT mice also showed a 5.43% increase in the relative expression of mucin2 ((F)), which provided an insoluble mucous barrier that serves to protect the intestinal epithelium. These findings all suggested that NAT may improve intestinal barrier integrity in antibiotic-treated mice.
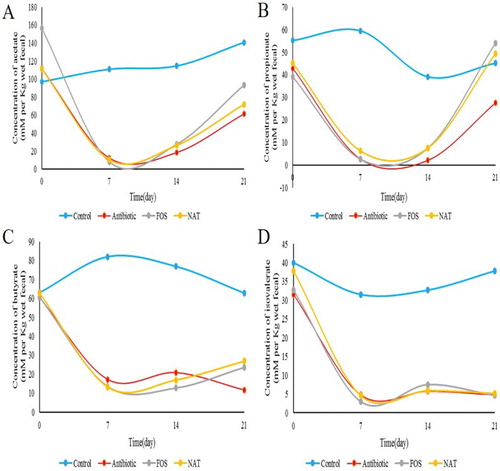
3.4. NAT protected intestinal integrity by increasing microbiota metabolic products-SCFAs
We determined the fecal concentration of SCFAs, including acetic acid, propionic acid, butyric acid and pentanoate acid by gas chromatography (). Acetic acid concentration was increased from 61.43 to 72.10 mM, a 17.37% increase; propionic acid concentration was increased from 27.53 to749.42 mM, a 79.51% increase; butyrate concentration was increased from 11.72 to 26.88 mM, a 129.35% increase; the total SCFAs’ concentration was increased from 105.44 to 153.57 mM, a 45.65% increase. The results indicated that NAT administration significantly altered microbial metabolic activity and increased the fecal concentration of SCFAs.
3.5. Overall structural changes of the gut microbiota in response to NAT treatment
To investigate whether this micro-inflammatory environment could be due to microbiota alterations, colonic microbiota composition was compared according to the 16S rRNA V3 region PCR-DGGE patterns. Unweighted UniFrac principal component analysis (PCA) revealed useful information about the phylogenetic relationship and composition of colonic bacterial microbiota in the different groups. Our results showed that the gut microbiota structure changed significantly in response to antibiotic treatment and NAT administration. Samples from the same group of mice clustered together and separately from samples of the other groups of mice in the plot, with principal component scores that accounted for 47.6% (PC1) and 33.7% (PC2) of the total variance ((A)).
Figure 5. NAT restored antibiotic-induced dysbiosis of gut microbiota. (A) Principal component analysis (PCA) was performed using the Canoco for Windows 4.5 based on DGGE profiles from feces samples. (B) UPGMA cluster analysis of the DGGE profiles. (C) Relative abundances of the Firmicutes and (D) Bacteroidetes phyla in antibiotic-treated mice. (E) The corresponding ratio of the relative abundances of Firmicutes to Bacteroidetes at the phylum level. Data are presented as mean ± SEM. ##P < .01, #P < .05 indicates significant difference from the control group; *P < .05, **P < .01 indicates significant difference from the AB group.
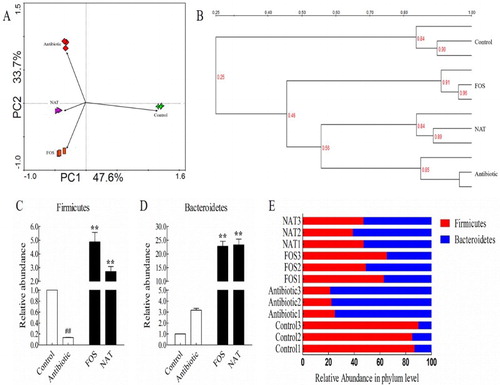
UPGMA cluster analysis based on the DGGE profiles showed that the bacterial communities were clustered into four groups. The AB group had the lowest similarity (only 0.25) to the control group. Alternatively, the similarity coefficients of NAT and FOS with AB were 0.56 and 0.45, respectively ((B)). This revealed that the bacterial community structures were significantly affected by the oligosaccharides’ treatment.
To identify the key phyla of the gut microbiota responding to NAT treatment, we analyzed the relative abundance of Firmicutes ((C)) and Bacteroidetes ((D)). There was a 19.43-fold increase in Firmicutes and 7.34-fold increase in Bacteroidetes induced by NAT. Ceftriaxone sodium cleared away nearly all of the Firmicutes, as Bacteroidetes dominates the gut microbiota. Administration of NAT favored the Firmicutes while hindering the Bacteroidetes ((E)). The percentage of Firmicutes was increased from 22.97% in AB mice to 44.47% in NAT mice, with a 93.60% increase. This indicated that NAT treatment could reshape the microbiota composition under conditions of dysbiosis caused by antibiotics.
3.6. NAT affected microbial diversity and increased the colonization of probiotics in the mice treated with antibiotics
To test whether the observed changes in microbial structure were accompanied by community abundance shifts in the gut microbiome, we examined the total bacteria loaded by RT-PCR. Our results demonstrated that there were significant differences in the total bacterial population among the groups of mice (P < .01) ((A)). A significant increase in total bacterial population was observed in the FOS and NAT group (P < .01). The Shannon diversity index revealed that NAT significantly increased the bacterial diversity of the gut microbiota (P < .05) ((B)). This was confirmed by two other diversity indices: the richness index (P < .01) ((C)) and the evenness index ((D)). However, no significant difference was observed in the evenness index among the mice in AB and NAT treatment groups.
Figure 6. NAT increased microbial diversity and the relative abundance of probiotics in the mice gut microbiome. (A) Total bacteria load, (B) Shannon index and (C) Richness values, (D) Evenness values. The relative abundance of (E) Bifidobacterium, (F) Lactobacillus, (G) Clostridium and (H) Prevotella. Data are presented as mean ± SEM. ##P < .01, #P < .05 indicates significant difference from the control group; *P < .05, **P < .01 indicates significant difference from the AB group.
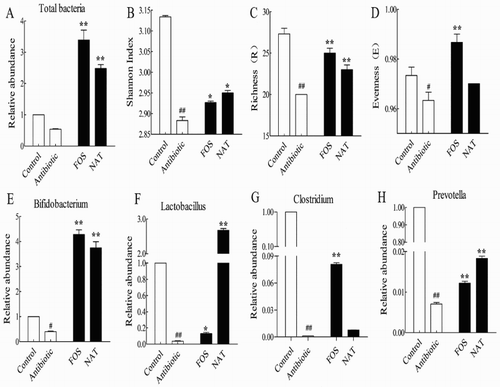
The probiotics and intestinal mucosal immunity are strongly linked (Yurong, Ruiping, ShiMin, & Yibao, Citation2005). To test whether the remarked improvement in intestinal mucosal immunity could be due to the widely expanded colonization of probiotics, we examined the relative abundances of Bifidobacterium, Lactobacillus, Clostridium and Prevotella. In our study, significantly increased Bifidobacterium ((E)) and Lactobacillus ((F)) populations were observed in the NAT group compared with those in the AB group, with a 9.37-fold and 72.16-fold increase, respectively. It is worth mentioning that a significantly increased Clostridium population was also observed in the NAT group ((G)), with a 7.00-fold increase. NAT treatment also increased the abundance of the genus Prevotella ((H)), with a 2.57-fold increase. Taken together, our data suggested that NAT could be a functional food ingredient targeting the microbial ecology.
4. Discussion
With recent advances in our understanding of the intestinal microbiota and its role in regulating immunity, people are becoming increasingly aware of the effects of antibiotics on the microbial ecosystem and how they affect human health (Willing, Russell, & Finlay, Citation2011). Numerous studies have investigated the effect of antibiotic treatment on the composition stability (Ng et al., Citation2013) and function of the intestinal microbiota (Willing et al., Citation2011). Generally, treatment with antibiotics reduces the diversity of the microbiota, thereby influencing its functional capacity and the mutualistic relationship with the host (Modi, Collins, & Relman, Citation2014). Hence, it is essential to find effective food-derived functional components to manipulate the gut microbiota and balance the gut microflora homeostasis (Saeed et al., Citation2016). As a test system, we chose the acute administration of a broad-spectrum antibiotic by gavage, while allowing animal access to untreated drinking water, which more closely mimics antibiotic administration in humans (Ladirat et al., Citation2014).
It has been shown that antibiotics increase the intestinal mucus permeability and bacterial penetration into the epithelial tissue (Pirker et al., Citation2013). Antibiotics damage the delicate crosstalk between the microbiota and the mucosal immune system, thereby leading to alteration of immune homeostasis, which ultimately increases the susceptibility to pathogens (Stecher, Citation2013). Therefore, we aimed to investigate whether NAT has effects on gut inflammation. Thymus and spleen are important immune organs in the host. The main function of the thymus is to produce T lymphocytes and secrete thymosin. And spleen is rich in lymphocytes and macrophages (Wijburg, Heemskerk, Boog, & Van Rooijen, Citation1997). The thymus index and spleen index of the NAT group increased significantly compared with the AB group. The results indicate that dietary NAT has an enhancement effect on the immune system. Notably, in (E), spleen/body weight index is higher in the FOS and NAT group compared with the antibiotic group. One possible reason is splenomegaly, wherein splenomegaly is always related with hyper-inflammation and accumulation of immune cells in the spleen. And such results conflict with , which shows NAT can inhibit inflammatory cytokines’ production and increase anti-inflammatory cytokines such as IL-10. Another reason is that the spleen weight relatively increases with nutrition improvement. Specific reasons leading to an increase in the spleen index need further study.
Our data also showed that NAT could significantly decrease the ileocecal valve index and increase the length of the colon. To further confirm the anti-inflammation effects of NAT, we next examined the relative expression of pro-inflammatory cytokines: TNF-α, IL-1β and pro-inflammatory cytokine: IL-10 in intestinal tissue. Notably, we demonstrated that antibiotic treatment increased inflammation, which was effectively reduced by NAT. In addition, our data also suggested that NAT mediated the balance of the Th1/Th2 ratio to maintain the immune equilibrium of the intestinal mucosa. These results indicated that NAT had the abilities to balance mucosal innate immune cells regulating both gut homeostasis and intestinal inflammation.
Given that intestinal dysbiosis in antibiotic-treated mice may affect gut permeability and subsequently lead to pathogenic invasion into the circulation, we examined whether NAT prohibited intestinal inflammation by increasing gut integrity. Furthermore, we speculated that the expression of AMPs in Paneth cells and Goblet cells may be responsible for the inhibition of intestinal inflammation. Indeed, mRNA levels of various AMPs including sPLA2 and Ang4 in the small intestine of the NAT group were higher than those in the AB group. More profound changes were seen in the FOS-feeding mice. This was further confirmed by increasing gut integrity in the NAT-treated mice. Our findings demonstrate that NAT feeding promotes inflammatory responses in the gut by increasing intestinal barrier integrity and expression of AMPs.
Next, we further explored possible causes of inhibition of intestinal permeability after NAT administration. There have been mixed reports regarding the roles of SCFA in the regulation of intestinal barrier integrity. Accumulated literature supported SCFAs as anti-carcinogenic, anti-inflammatory and barrier protective in the distal gut (Yewei et al., Citation2014). Moreover, butyrate administration can increase IL-1β and IL-6 expression in the gut via the parenteral route. SCFAs have been reported to have anti-inflammatory properties with an attenuation of production of inflammatory cytokines TNF-α, IL-6 and IFN-γ (Allison et al., Citation2016). The results indicated that NAT administration significantly altered microbial metabolic activity and increased the fecal concentration of SCFAs. However, our results suggested that NAT feeding in mice did not appear to significantly affect the expression of SCFA receptors.
To investigate whether this micro-inflammatory environment could be due to microbiota alterations, colonic microbiota composition was compared according to the 16S rRNA V3 region PCR-DGGE patterns. FOS was used as a positive control, as numerous studies have revealed its prebiotic effects. NAT-treated mice had a significantly different microbiota composition compared with the control group mice and the antibiotic-treated mice. Among those bacteria with the most striking difference, we found that NAT remarkably increased the proportion of Firmicutes and decreased the proportion of Bacteroidetes compared with the antibiotic treatment. The balance between the Bacteroidetes and Firmicutes phyla in the gut bacterial community influences host health (Thompson, Oliveira, Djukovic, Ubeda, & Xavier, Citation2015). Shifts in the composition of the microbiota, particularly the Bacteroidetes and the Firmicutes, are associated with the pathogenesis of obesity, diabetes (Qin et al., Citation2012), chronic IBDs (Frank et al., Citation2007) and gastrointestinal cancer, as well as autism and stress (Galley et al., Citation2014). Thompson et al. discovered that despite the streptomycin-induced Bacteroidetes dominance of the microbiota, an increase in the interspecies quorum sensing signal, autoinducer-2 (AI-2), counteracts the antibiotic-induced dysbiosis and favors Firmicutes. AI-2 produced by E. coli favors the Firmicutes while hindering the Bacteroidetes (Thompson et al., Citation2015). Analogously, ceftriaxone sodium nearly clears the Firmicutes, as Bacteroidetes dominates the gut microbiota. Administration of NAT favors the Firmicutes while hindering the Bacteroidetes.
To test whether the observed changes in microbial structure were accompanied by community diversity shifts in the gut microbiome, we examined the total bacteria load and microbial diversity. Microbial diversity is important in all ecosystems to promote stability and performance. Microbiota diversity may become a new biomarker or indicator of health (Hold, Citation2014). Besides, increased bacterial abundance has also been associated with improved health status and alterations in immune system, making multiple connections between host and microbiota (Bermon, Petriz, Prestes, Castell, & Franco, Citation2015). A significantly increased total bacterial population was observed in the FOS and NAT group. The Shannon diversity index revealed that NAT significantly increased the bacterial diversity of the gut microbiota. Furthermore, we demonstrated that NAT administration effectively improved gut colonization of probiotic bacteria following antibiotic treatment. Our study showed that NAT intervention remarkably increased the proportion of the Bifidobacterium, Lactobacillus, Clostridium and Prevotella populations, which enabled the experimental animals a faster recovery from intestinal disorders. Probiotics are increasingly used in food and pharmaceutical applications to modulate intestinal microflora and related dysfunctions in the human gastrointestinal tract. Lactobacillus acidophilus and Bifidobacterium spp. have been reported to be beneficial probiotics that provide excellent therapeutic benefits (Kailasapathy & Chin, Citation2000). Bifidobacteria and Lactobacilli are also able to stimulate the development of the immune system (Hananeh, Citation2015), with certain species of commensal microbiota being required for immune regulation in the gut (Mastromarino, Vitali, & Mosca, Citation2013; Vieira, Teixeira, & Martins, Citation2013). Several studies have demonstrated that Clostridium in the gut can reduce human immune pathological damage by activating Treg cells and by secreting anti-inflammatory cytokines (IL-10). In humans, the abundance of Prevotella was associated with higher carbohydrate or fiber-rich diets (Li et al., Citation2016).
In the future, we plan to further investigate the relationship of the anti-inflammatory effect and gut barrier-protecting function of NAT. Metagenomic studies and molecular dissection of the host–microbiome crosstalk are needed to further elucidate the mechanisms related to NAT.
In summary, our findings suggest that the marked modulation of gut microbiota by NAT had an effect on prohibiting pathogenic invasion and intestinal inflammation. This study also suggests that the nutritional modulation of gut microbiota may be an effective approach to improve human health. Our results may have important implications for NAT as a functional food component, as well as the potential therapeutic utility for manipulating the gut microbiota and alleviating gut inflammation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributor
Na Zhang is a M.A. in Food Nutrition. Presently, she has graduated from the College of Food Science and Engineering of Ocean University of China. Her research interest includes Food Nutrition and Gut Microbiota.
Enling Hou is a M.A. in Food Biotechnology and marine engineering. Presently, she has graduated from the College of Food Science and Engineering of Ocean University of China. Her research interest includes Food Biotechnology.
Jia Song is a M.A. in Food Nutrition. Presently, she is working as a researcher at the College of Food Science and Engineering of Ocean University of China. Her research interest includes Food Nutrition and Gut Microbiota.
Jing Li is a M.A. in Food Nutrition. Presently, she is working as a researcher at the College of Food Science and Engineering of Ocean University of China. Her research interest includes Food Nutrition and Gut Microbiota.
Qingjuan Tang is a Ph.D., Professor in Food Nutrition. Presently, she is working as a teacher and researcher at the College of Food Science and Engineering of Ocean University of China. Her research interest includes Food Nutrition and Gut Microbiota.
Xiangzhao Mao is a Ph.D., Professor in Food Biotechnology and marine engineering. Presently, he is working as a teacher and researcher at the College of Food Science and Engineering of Ocean University of China. His research interest includes Food Biotechnology.
Additional information
Funding
References
- Allison, A., Jérémy, D., Jonathan, T., Margarita, M., Sébastien, M., Pierre, S., … Nicolas, B. (2016). Western diet induces a shift in microbiota composition enhancing susceptibility to adherent-invasive E-coli infection and intestinal inflammation. Scientific Reports, 6, 139–140.
- Bermon, S., Petriz, B., Prestes, J., Castell, L., & Franco, O. (2015). The microbiota: An exercise immunology perspective. Exercise Immunology Review, 21, 70–79.
- Dupont, A. W., & Dupont, H. L. (2011). The intestinal microbiota and chronic disorders of the gut. Nature Reviews Gastroenterology & Hepatology, 8, 523–531. doi: 10.1038/nrgastro.2011.133
- Frank, D. N., St. Amand, A. L., Feldman, R. A., Boedeker, E. C., Harpaz, N., & Pace, N. R. (2007). Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proceedings of the National Academy of Sciences, 104, 13780–13785. doi: 10.1073/pnas.0706625104
- Fu, X. T., & Kim, S. M. (2010). Agarase: Review of major sources, categories, purification method, enzyme characteristics and applications. Marine Drugs, 8, 200–218. doi: 10.3390/md8010200
- Galley, J. D., Nelson, M. C., Yu, Z., Dowd, S. E., Walter, J., Kumar, P. S., … Bailey, M. T. (2014). Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiology, 14, 189. doi: 10.1186/1471-2180-14-189
- Hananeh, W. M. (2015). Small intestine mucosal immune system response to high-fat-high-cholesterol dietary supplementation in male rats. Food & Agricultural Immunology, 26(2), 293–304. doi: 10.1080/09540105.2014.914467
- Hold, G. L. (2014). The gut microbiota, dietary extremes and exercise. Gut, 63, 508–526.
- Hsu, P.-H., Wei, C.-H., Lu, W.-J., Shen, F., Pan, C.-L., & Lin, H.-T. V. (2015). Extracellular production of a novel endo-β-agarase AgaA from pseudomonas vesicularis MA103 that cleaves agarose into neoagarotetraose and neoagarohexaose. International Journal of Molecular Sciences, 16, 5590–5603. doi: 10.3390/ijms16035590
- Kailasapathy, K., & Chin, J. (2000). Survival and therapeutic potential of probiotic organisms with reference to lactobacillus acidophilus and bifidobacterium spp. Immunology and Cell Biology, 78, 80–88. doi: 10.1046/j.1440-1711.2000.00886.x
- Ladirat, S. E., Schuren, F. H. J., Schoterman, M. H. C., Nauta, A., Gruppen, H., & Schols, H. A. (2014). Impact of galacto-oligosaccharides on the gut microbiota composition and metabolic activity upon antibiotic treatment during in vitro fermentation. Fems Microbiology Ecology, 87, 41–51. doi: 10.1111/1574-6941.12187
- Lawrence,, A. D., Corinne,, F. M., Rachel,, N. C., David,, B. G., Julie,, E. B., Benjamin,, E. W., … Michael, A,F. (2014). Discovery and saturation analysis of cancer genes across 21 tumour types. Nature, 505, 495–501. doi: 10.1038/nature12912
- Li, R. W., Li, W., Sun, J., Yu, P., Baldwin, R. L., & Urban, J. F. (2016). The effect of helminth infection on the microbial composition and structure of the caprine abomasal microbiome. Scientific Reports, 6, 20606. doi: 10.1038/srep20606
- Li, H., Song, C., Liu, D., Ai, Q., & Yu, J. (2015). Molecular analysis of biofilms on the surface of neonatal endotracheal tubes based on 16S rRNA PCR-DGGE and species-specific PCR. International Journal of Clinical and Experimental Medicine, 8, 11075–11084.
- Liu, N., Mao, X., Du, Z., Mu, B., & Wei, D. (2014). Cloning and characterisation of a novel neoagarotetraose-forming-β-agarase, agwh50a from agarivorans gilvus, wh0801. Carbohydrate Research, 388C, 147–151. doi: 10.1016/j.carres.2014.02.019
- Mastromarino, P., Vitali, B., & Mosca, L. (2013). Bacterial vaginosis: A review on clinical trials with probiotics. New Microbiol, 36, 229–238.
- Michele, P., & Antonio, M. (2010). Intestinal ecology in the metabolic syndrome. Cell Metabolism, 11, 345–346. doi: 10.1016/j.cmet.2010.04.012
- Modi, S. R., Collins, J. J., & Relman, D. A. (2014). Antibiotics and the gut microbiota. Journal of Clinical Investigation, 124, 4212–4218. doi: 10.1172/JCI72333
- Muyzer, G., Waal, E. C. D., & Uitterlinden, A. G. (1993). Profiling of complex microbial populations by denaturing gradient Gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Applied and Environmental Microbiology, 59, 695–700.
- Ng, K. M., Ferreyra, J. A., Higginbottom, S. K., Lynch, J. B., Kashyap, P. C., Gopinath, S., … Sonnenburg, J. L. (2013). Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature, 502, 96–99. doi: 10.1038/nature12503
- Pirker, A., Stockenhuber, A., Remely, M., Harrant, A., Hippe, B., & Kamhuber, C. (2013). Effects of antibiotic therapy on the gastrointestinal microbiota and the influence of lactobacillus casei. Food & Agricultural Immunology, 24(3), 315–330. doi: 10.1080/09540105.2012.689816
- Qin, J., Li, Y., Cai, Z., Li, S., Zhu, J., Zhang, F., … Shen, D. (2012). A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature, 490, 55–60. doi: 10.1038/nature11450
- Saeed, F., Nadeem, M., Ahmed, R. S., Nadeem, M. T., Arshad, M. S., & Ullah, A. (2016). Studying the impact of nutritional immunology underlying the modulation of immune responses by nutritional compounds – a review. Food & Agricultural Immunology, 27(2), 205–229. doi: 10.1080/09540105.2015.1079600
- Stecher, B. (2013). Finding a sugary foothold: How antibiotics pave the way for enteric pathogens. Cell Host Microbe, 14, 225–227. doi: 10.1016/j.chom.2013.08.008
- Thompson, J. A., Oliveira, R. A., Djukovic, A., Ubeda, C., & Xavier, K. B. (2015). Manipulation of the quorum sensing signal AI-2 affects the antibiotic-treated Gut microbiota. Cell Reports, 10, 1861–1871. doi: 10.1016/j.celrep.2015.02.049
- Vieira, A. T., Teixeira, M. M., & Martins, F. S. (2013). The role of probiotics and prebiotics in inducing gut immunity. Front Immunology, 4, 445. doi: 10.3389/fimmu.2013.00445
- Wang, R. J., Huang, X. F., Tang, H., & Wei, H. (2006). Animal model of intestinal microflora dysbiosis induced by antibiotics. Chinese Journal of Comparative Medicine, 16, 145–149.
- Wijburg, O. L., Heemskerk, M. H., Boog, C. J., & Van Rooijen, N. (1997). Role of spleen macrophages in innate and acquired immune responses against mouse hepatitis virus strain A59. Immunology, 92, 252–258. doi: 10.1046/j.1365-2567.1997.00340.x
- Willing, B. P., Russell, S. L., & Finlay, B. B. (2011). Shifting the balance: Antibiotic effects on host? Microbiota mutualism. Nature Reviews Microbiology, 9, 233–243. doi: 10.1038/nrmicro2536
- Wlodarska, M., & Finlay, B. (2010). Host immune response to antibiotic perturbation of the microbiota. Mucosal Immunology, 3, 100–103. doi: 10.1038/mi.2009.135
- Yewei, J., Shengyi, S., Goodrich, J. K., Kim, H., Poole, A. C., Duhamel, G. E., … Ling, Q. (2014). Diet-induced alterations in gut microflora contribute to lethal pulmonary damage in tlr2/tlr4-deficient mice. Cell Reports, 8, 137–149. doi: 10.1016/j.celrep.2014.05.040
- Yurong, Y., Ruiping, S., ShiMin, Z., & Yibao, J. (2005). Effect of probiotics on intestinal mucosal immunity and ultrastructure of cecal tonsils of chickens. Archives of Animal Nutrition, 59, 237–246. doi: 10.1080/17450390500216928
- Zuo, T., Li, X., Chang, Y., Duan, G., Yu, L., Zheng, R., … Tang, Q. (2015). Dietary fucoidan of acaudina molpadioides and its enzymatically degraded fragments could prevent intestinal mucositis induced by chemotherapy in mice. Food & Function, 6, 415–422. doi: 10.1039/C4FO00567H