ABSTRACT
To produce specific antibodies against leuco-malachite green (LMG), 15 haptens were synthesized and characterized, and conjugated to carrier protein for immunization. One antigen with excellent reactogenicity and immunogenicity was discovered. Specific monoclonal antibody with high sensitivity for LMG in competitive indirect enzyme-linked immunosorbent assay (ciELISA) was screened and selected. After the screening of a series of heterologous coating antigen and optimization of working conditions, the proposed ciELISA showed the 50% inhibition value (IC50) of 1.16 ng mL−1 and the limit of detection of 0.06 ng mL−1 for LMG. The average recoveries of LMG from spiked fish samples ranged from 78.0% to 101.0%, with coefficients of variation below 15%. Good correlation (R2 = 0.9977) was obtained between the results of ciELISA analysis and those of standard liquid chromatography–tandem mass spectrometry analysis. The proposed ciELISA is ideal for the rapid and sensitive detection of LMG with a low cost and high throughput.
1. Introduction
Malachite green (MG, ) is a kind of industrial dye with suspected carcinogenicity and mutagenicity, which is frequently and illegally applied as therapeutic agents in aquaculture. MG mostly exists as leuco-malachite green (LMG, ), the metabolite form in vivo. Unfortunately, LMG not only tends to accumulate in the tissues for a long period due to its lipophilic nature (Fessard, Godard, Huet, Mourot, & Poul, Citation1999; Plakas, ElSaid, Stehly, Gingerich, & Allen, Citation1996; Plakas, Doerge, & Turnipseed, Citation1999), but also possesses much greater mutagenicity and tumorigenicity than MG (Culp et al., Citation2002; Mittelstaedt et al., Citation2004). Considering that MG and LMG both have various potential health and environmental hazards including carcinogenicity, mutagenicity, teratogenicity, biotoxicity and so on (Culp et al., Citation1999; Culp & Beland, Citation1996; Fernandes, Lalitha, & Rao, Citation1991), they were strictly banned from use in food-borne animals in the USA, Europe (Council Directive 96/23/EC) and China (Ministry of Agriculture Bulletin No. 193). The maximum required performance limit for the sum of MG and LMG was set as 2 μg kg−1 (Commission Decision 2004/25/EC). However, illegal use of MG in aquaculture still exists worldwide because of its good bactericidal activity, cost-effectiveness and ready availability, with the increasing demand in the consumption of aquatic food and the fast growth of the aquaculture industry in the last few years (http://www.vmd.defra.gov.uk/vrc/Reports/annual.htm). Therefore, humans are facing a high risk of exposure to MG and LMG now (Chu, Chimeddulam, Sheen, & Wu, Citation2013). As MG mainly exists as LMG form in vivo, it is necessary to develop accurate and efficient method of directly detecting LMG to efficiently monitor illegal use of MG in aquatic products.
Numerous analytical methods for the determination of LMG residues have been published recently. For instance, liquid chromatography (LC) (Andersen, Turnipseed, & Roybal, Citation2006; Long et al., Citation2009; Xie et al., Citation2013), LC technology with a mass spectrometry (LC–MS; Chen & Miao, Citation2010; Deng et al., Citation2011; Li, Luo, Huang, Zhu, & Wu, Citation2014; López-Gutiérrez, Romero-González, Martinez Vidal, & Frenich, Citation2013; Storey et al., Citation2014; Turnipseed et al., Citation2014; Villar-Pulido, Gilbert-López, García-Reyes, Martos, & Molina-Díaz, Citation2011), LC–MS/MS (Andersen et al., Citation2006; Long et al., Citation2009; Xie et al., Citation2013), surface-enhanced resonance Raman scattering (Zhang, Yu, et al., Citation2015) and micellar electrokinetic capillary chromatography (Jiang, Lv, Cui, & Wang, Citation2012) were used to analyze MG and LMG. However, these instrument-based methods require expensive equipment, sophisticated expert operation, high routine cost, low sample throughput, and time-consuming and complicated sample preparation process (Gui, Jin, Sun, Guo, & Zhu, Citation2009; Wyatt, Garrett, Lee, & Morgan, Citation1999). In the case of rapid screening methods, immunoassay as a specific and sensitive alternative quantitative method has been playing a positive role in the detection of harmful chemical residues in aquatic products, for the purpose of human health and environmental protection (Fuller, Goodwin, & Morris, Citation2006; Li et al., Citation2015; Yang et al., Citation2015). Recently, several immunoassays against LMG and MG were described (Bilandžic, Varenina, Kolanović, Oraić, & Zrnčić, Citation2012; Zhang, Yang, et al., Citation2015). Yang et al. (Citation2007) produced a polyclonal antibody (pAb) against LMG using carboxy-LMG as immunizing hapten. Enzyme-linked immunosorbent assay (ELISA) based on the pAb showed good sensitivity (limit of detection (LOD) of 0.05 μg kg−1, IC50 unavailable) toward LMG. Xing et al. (Citation2009) used the same main structure of hapten but a different spacer (amino group instead of carboxy group) to produce a pAb and developed an ELISA. The assay showed high cross-reactivity (CR) to LMG and MG simultaneously. However, using the two published structure, no specific antibodies against LMG were obtained in our previous work. The reason may be the instability of the proposed hapten structure, or the short space arm between the hapten and carrier protein. Oplatowska, Connolly, Stevenson, Stead, & Elliott (Citation2011) produced a monoclonal antibody (mAb) against MG using carboxy-MG as immunizing hapten, the obtain mAb has no cross-reactivity with LMG. Singh et al. (Citation2011) produced a pAb against LMG using a new hapten by substituting one methyl of LMG with glutaric anhydride. Although the assay showed good sensitivity (IC50 = 2.0 μg kg−1, LOD = 0.1 μg kg−1), the synthesis of hapten is complicated and time consuming.
In this study, with the aim to study the effects of hapten structure on the ability to produce antibody against LMG, a series of haptens with different arm linkers and sites against LMG were designed and synthesized. They were conjugated to carrier protein and used to immunize BALB/c mice. Sera were collected and analyzed for the specific binding to free LMG. Mice secreting sera with good affinity to LMG were further used to produce mAb. Competitive indirect ELISA (ciELISA) was then developed and optimized, and then applied to determine LMG in fortified fish. The results of ciELISA were validated by standard high-performance LC (HPLC)–MS/MS to ensure its accuracy and reproducibility.
2. Material and methods
2.1. Reagents, chemicals, buffers and solutions
MG, LMG, leuco-crystal violet (LCV), crystal violet (CV), leuco-brilliant green (LBG), brilliant green (BG), pararosaniline (PA), methylene blue (MB), 4-hydroxybenzaldehyde, sodium chloroacetate, N,N-diethylaniline, N,N-dimethylaniline, Amberlyst 15 resin were of analytical grade (>90) and obtained from Sigma-Aldrich (Shanghai, China) or Alfa Aesar Company (Augsburg, Germany). Bovine serum albumin (BSA), dicyclohexylcarbodiimide (DCC), N-hydroxysuccinimide (NHS), ovalbumin (OVA), 3,3′,5,5′-tetramethylbenzidine (TMB), complete and incomplete Freund’s adjuvants were received from Sigma-Aldrich (Shanghai, China). Horseradish peroxidase-conjugated goat anti-mouse IgG (GAM-HRP) was supplied by Boster Biotech. Co., Ltd. (Wuhan, China). Polystyrene ELISA plates were obtained from Jiete Biotech., Ltd. (Guangzhou, China). All other chemicals and organic solvents were of analytical grade and were obtained from a local chemical supplier (Yunhui Trade Co., Ltd., Guangzhou, China).
Buffers used were as follows: 50 mmol L−1 carbonate buffer (pH 9.6) was used for coating plates, 10 mmol L−1 PBST solution (10 mmol L−1 phosphate buffer saline (PBS, pH 7.4) containing 0.05% Tween-20, pH 7.4) was used for washing plates, 0.1 mol L−1 sodium citrate and sodium phosphate buffers (pH 5.4) was used for the substrate buffer, and 2 mol L−1 H2SO4 was used as the stopping reagent. TMB solution was prepared with 10 mL substrate buffer, 150 μL of 15 mg mL−1 TMB in N,N-dimethylformamide (DMF) and 2.5 μL of 6% (w/v) H2O2. Ammonium acetate buffer solution (0.1 M, pH 4.5) was prepared by dissolving 4.95 g of ammonium acetate in 900 mL of distilled water, adjusting to pH 4.5 with acetic acid and diluting to 1 L with distilled water.
Cell culture reagents and apparatus: RPMI-1640 media without sodium pyruvate; penicillin–streptomycin; heat inactivated fetal bovine serum (HIFBS); hypoxanthine, aminopterin, thymidine supplement (HAT) and hypoxanthine, thymidine supplement (HT) were purchased from Invitrogen Ltd. (Paisley, UK). Doma Drive hybridoma growth supplement, polyethylene glycol fusion medium 4000 (PEG-4000) and IsoQuick™ Kit for Mouse Monoclonal Isotyping were purchased from Sigma-Aldrich (A Johnson Matthey Company). Myeloma cells SP2/0 were obtained from the Guangdong Provincial Key Laboratory of Food Quality and Safety. The BALB/c mice were purchased from the Guangdong Medical Laboratory Animal Center (Guangzhou, China). All animal studies were performed in accordance with institutional guidelines.
2.2. Instruments
ELISA plates were washed with a Multiskan MK2 microplate washer (Thermo Scientific, USA). ELISA values were read with a Multiskan MK3 microplate reader (Thermo Scientific, USA). Ultraviolet (UV) spectrometry was recorded on a UV-3010 spectrophotometer (Hitachi, Japan). Atmospheric pressure chemical ionization mass spectrometry (APCI-MS) and electrospray ionization mass spectrometry (ESI-MS) analyses were performed with an LCQEDCA series (Thermo Finnigan, USA). LC–MS/MS analysis was carried out by using the API 3000TM Triple Quadrupole system (Perkin-Elmer, USA). Nuclear magnetic resonance (NMR) spectra were obtained with both the DRX-400 and DRX-600 NMR spectrometers (Bruker, Germany-Switzerland).
2.3. Hapten synthesis and characterization
The chemical structure of 15 haptens (H1–H15) are shown in , and the synthetic routes of each hapten referred to the methods described by Cho et al. and Smith et al. with modifications (Burgstahler & Worden, Citation1966; Cho et al., Citation2003; Smith & Opie, Citation1948). MS and NMR spectra were obtained to characterize the synthesized haptens. The synthesis methods and characterization data are shown in Supporting Information Section 1.
Table 1. The chemical structure of LMG and its haptens (H1–H15).
2.4. Preparation of hapten–protein conjugates
The haptens were covalently attached to BSA as immunogens and coupled with OVA as coating antigens according to the active ester method (Langone & Vanvunakis, Citation1982 ) or the diazotization method (Guy, Anne, & Guy, Citation1992). The concentration of hapten–protein conjugates was identified by the 2,2′-bicinchoninic acid (BCA) method (Brenner & Harris, Citation1995). The resulting solution was then dialyzed against 0.01 mol L−1 PBS at 4°C for 24 h with a three-time refreshing of PBS and stored frozen at −20°C. UV–vis spectra data were used to confirm that the hapten was linked successfully to BSA or OVA (Abad, Moreno, & Montoya, Citation1998). The ratios of haptens to carrier proteins were determined using the 2,4,6-trinitrobenzene sulfonic acid (TNBS) method (Habeeb, Citation1966) and UV–vis spectra (Fedenko, Citation1989). The characterization of hapten–protein conjugates is shown in Supporting Information Section 2.
2.5. Production of mAb
Three BALB/c mice per immunogen were subcutaneously immunized with 200 μg of immunogen dissolved in 100 μL of 0.9% sodium chloride solution and emulsified with 100 μL of Freund’s complete adjuvant at 3-week intervals. Booster injections were given at 3-week intervals with the same immunogen suspended in 0.1 mL of Freund’s incomplete adjuvant. Blood samples were collected and screened for the determination of antibody affinities against MG and LMG using ciELISA. The mice showing good responses were selected and immunized with the final dose of the immunogen (100 μg) 4 days before the fusion date. The cells obtained from the spleen of the immunized mouse were fused with myeloma SP2/0 cells using polyethylene glycol 2000 in a modified method described for the first time by Köhler and Milstein (Citation1975). Ten days after the fusion, the hybridomas supernatants were screened using an indirect ELISA format against LMG-ovalbumin conjugate and free ovalbumin proteins. The positive hybridomas were selected and retested in an indirect competitive ELISA with LMG as competitors. The positive hybridomas that secreted antibodies specific to LMG were subsequently subcloned by limited dilution technique, further expanded and subcloned twice (using dilution method) to ensure the monoclonality of a cell line.
2.6. Characterization of mAb
A large amount of mAb was produced in vivo (Beier, Ripley, Young, & Kasier, Citation2001). Ascites fluid was produced in female BALB/c mice and purified from late stationary phase culture supernatants by ammonium sulfate precipitation and protein-G affinity chromatography. The affinity and specificity of the produced antibodies were again confirmed by competitive ELISA. The relative affinity of the monoclonal antibodies for LMG was measured by determining the 50% inhibition of control values (IC50). The isotype of the purified mAb was identified by a commercially available Immunopure Monoclonal Antibody Isotyping Kit I (Sigma). The purified antibodies were stored at −70°C.
2.7. General procedure of ELISA
The ELISA procedure refers to that described by Shen et al. (Citation2012). The result was expressed as percentage inhibition (B/B0), where B is the absorbance of the well containing analyte and B0 is the absorbance of the well without analyte. The inhibition curve was plotted as B/B0 versus the logarithm of LMG concentration. Competitive curves were obtained by plotting the relative absorbance of B/B0 against the logarithm of the analyte concentration. Sigmoid competitive curves were fitted to a four-parameter logistic equation and the IC50 value was calculated (Nix & Wild, Citation2006). The LOD of the assay was defined as the concentration of analyte that provided a 10% reduction of the Amax (IC10). The dynamic range of the assay was established between the values of IC80 and IC20, and they were considered as the upper and lower limits of quantification (LOQ), respectively.
2.8. Assay optimization and characterization
In this study, the effect of the concentration of coating antigen, the concentration of mAb, the concentration of GAM-HRP and the concentration of organic solvent on ELISA performance were investigated and optimized. Meanwhile, the homologous coating antigen with different carrier proteins and the heterologous coating antigen with different haptens were used to establish the ciELISA curve by optimizing the assay conditions. The Amax/IC50 ratio (Amax, the absorbance value at zero concentration of analyte) was a convenient estimation of the influence of some factors on the ELISA sensitivity, with the higher ratio indicating higher sensitivity (Brun, Garces-Garcia, Puchades, & Maquieira, Citation2005; Mercader & Montoya, Citation1997).
2.9. Antibody specificity
CR was tested to determine the specificity of the mAb. Some commercial triphenylmethane dyes, original materials and intermediate of LMG were selected and evaluated. Standard solutions of testing compounds were analyzed by the ELISA procedures. CR was expressed as the ratio between IC50 values acquired from LMG and those from competitors.
2.10. Preparation of spiked samples
Negative fish samples were purchased from a local super market. Head, tail, backbone and skin were removed and the fish meat was homogenized and aliquots were stored at −20°C; 1 g of meat was weighed in a polypropylene tube and 4 mL acetonitrile was added, the sample was vortex mixed for 5 min, and the supernatant was collected after centrifugation (2000 r min−1, 5 min). The above steps were repeated twice and the collected supernatants were combined and evaporated to dryness under vacuum, the residue was dissolved with 250 μL 20:80 (v/v) PBST buffer-methanol, and diluted 4-fold with PBST for analysis.
The spiked samples were prepared by adding different concentrations of LMG standard solution (in acetonitrile) to final concentrations of 0, 1, 3 and 8 ng g−1. The sample pretreatment procedure was the same as described above. The determination of spiked samples was performed by interpolating the mean absorbance values in the standard curve running on the same plate.
2.11. Method validation
The ELISA results were verified using the LC–MS/MS method, which was completed by the Guangzhou Quality Supervision and Testing Institute of Guangdong Province. A Zorbax SB-C18 (4.6 mm × 150 mm, 5 μm particle size) column was used. Mobile phase A was acetonitrile, and mobile phase B consisted of 5 mol L−1 ammonium acetate. The separation used the following gradient profile: 0 min, 70% A, 30% B; 3−14 min, 95% A, 5% B. The flow rate of the mobile phase was 0.5 mL min−1, and the sample injection amount was 20 μL. Analytes were determined by ESI-MS/MS in positive mode. The parameters were as follows: gas temperature, 350°C; nebulizer gas, 70 psi; and capillary voltage, 5.5 kV. High-purity nitrogen (>99.99%) served as the nebulizer and collision gas.
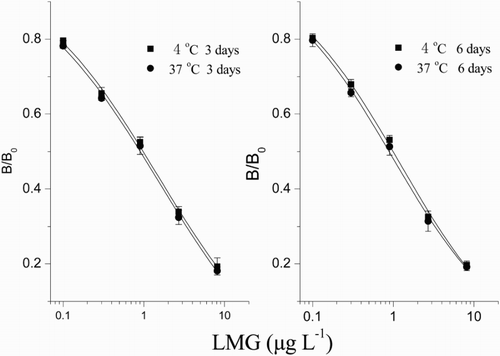
The stability of developed ciELISA was evaluated according to Zhang, Jin, Sun, Xu, & Shan (Citation2012), the experimental procedure with slight changes as follows: the reagent and micro titer plates were placed at 4°C or 37°C for 3 or 6 days, respectively (one day at 37°C is equivalent to 2 months at 37°C according to the Chinese requirements for biological products). Then the calibration curves of ciELISA were plotted as B/B0 versus the logarithm of LMG concentration using different reagents and micro titer plates stored at 4°C or 37°C for 3 or 6 days, respectively. The stability of the developed ciELISA was evaluated by calculating the absorbance and B/B0 values of each concentration of LMG.
3. Results and discussion
3.1. Synthesis and characterization of haptens
Hapten design is a critical factor for the successful preparation of highly specific antibodies against low molecular weight analytes. Commonly, in a hapten molecule, it could play a key and positive role in smoothly eliciting desired antibodies that the linker arm between analyte molecule and carrier protein should possess the rational conjugation site to target molecule, suitable length and terminal active group (Lei et al., Citation2010; Shen et al., Citation2012). In most studies, LMG was derivatized with carboxybenzaldehyle (4-CBA) or aminobenzaldehyde (4-ABA) to form immunizing haptens (Oplatowska et al.,Citation2011; Xing et al., Citation2009; Yang et al., Citation2007). Unfortunately, the repeatability of this strategy was unstable. Four attempts using this strategy to produce an anti-LMG antibody have failed in our previous tests. To further study the effects of hapten structure on LMG antibody generation, we designed and successfully synthesized a series of haptens with different spaced arms, chemical sites, functional groups, based on the chemical structure of LMG, . The synthesized haptens were characterized by ESI (or APCI)-MS and NMR, and the data indicated that the target haptens were synthesized successfully (Section 1 in Supporting Information).
3.2. Synthesis and characterization of artificial antigens
In this study, haptens H1–15 were conjugated to BSA and OVA as artificial antigen. The UV–vis spectra demonstrated qualitative differences between the carrier protein and the corresponding conjugates, suggesting successful hapten conjugation to the carrier protein (Section 2 in Supporting Information). The hapten coupling ratios with carrier proteins BSA were from 15:1 to 35:1, while that for OVA were from 11:1 to 19:1 (Section 2 in Supporting Information) by TNBS (for H1, 2, 5–11, H13–15) or UV–vis spectra (for H3 and H4). The ratios of hapten to carrier proteins that conjugated using diazobenzidine method could not be calculated by TNBS because the TNBS was applied to determinate the free amino groups in proteins. Therefore, for hapten H3 and H4 protein conjugates, we used UV–vis spectra to determine the conjugate ration. The hapten H12 coupling ratios with carrier proteins could not be obtained since the absorbance of antigen at 262 and 420 nm wavelength overlapped with the maximum absorbance wavelength of methods.
3.3. Titer and inhibition screen of sera
Each immunogen was used to immunize three BALB/c mice with the same method. The titer was determined by indirect ELISA, with titer being defined as the times of the serum dilution that results in an absorbance value about 1.0 OD units. The results of the serum titer and inhibition are shown in . The results showed that obvious high titers were obtained from the mice immunized by the haptens H5, H6 and H11. The IC50 of LMG for above positive antiserum was 3.37, 3.25 and 6.23 µg mL−1, respectively. Other sera from the mice immunized by the rest of haptens (H1–H4, H7–H10 and H12–H15) had no specific binding for LMG although the titer of serum was higher than negative serum (obtained before the mice immunized firstly). The reason for the inefficiency of haptens (H1–H4, H7–H10 and H12–H15) could be the length of the space arm. As shown in , the space arm of haptens H1–H4 might be too short to expose the characteristic structure. By contrast, the space arm of H7–H10 conjugates might be too long and easily results the space folding of hapten. Also, the amide bond of hapten may have great impact on the characteristic structure of hapten or it may be metabolized in vivo. The H5 and H6 were synthesized by extending the carbon chain space arm. The antisera obtained from the mice that were immunized by the corresponding artificial antigen were specific to LMG and have no CR with MG. H11 was synthesized using a different method. The length of the carbon chain of H11 was the same as haptens H7–H10, except that the hapten H11 used ether bond as derivative method of spacer arm. The obtained antiserum from corresponding immunized mice showed specific affinity to LMG, but the titer and inhibition were not as good as antisera from haptens H5 and H6. Hapten H12 was synthesized by introducing the spacer arm in the central carbon atoms of triphenylmethane. The results showed that the antiserum from corresponding immunized mice had no specific response to LMG, which may be due to the lively and easy ionization of central carbon atom. Based on the three aromatic ring of LMG, in order to explore the impact of the four methyl functional group on the specific recognition between LMG and the antiserum, the hapten H13 was synthesized with the ethyl replacing methyl, but the antisera from corresponding immunized mice were specific to LBG and had no specific response to LMG. The haptens H14 and H15 were synthesized by using the secondary structure of triphenylmethane. The corresponding antisera from these two immunizing haptens had also no specific response to LMG.
Table 2. The results of antisera from mice immunized by different antigen.
The data in indicate that the functional groups, length of spacer arm, derived method and site were the critical factors for choosing the hapten of LMG. Meanwhile, the chemical structure of LMG was an integral structure in immunological reaction. For these reasons, sodium chloroacetate was selected as the derivation reactant using the etherification reaction at the meta position of aromatic ring of LMG. The resultant derivation product maintained a fully exposed hapten on the surface of the carrier protein, and possessed the antigenic epitopes most likely to mimic that of LMG. This approach worked efficiently by exposing a specific region of the hapten to the animals’ immune systems.
3.4. Production of mAb
BALB/c mice immunized with H6-BSA were used for mAb production. One hybridoma (DC4-A6) secreting mAb stably with high affinity to LMG was obtained. The mAb showed no CR to OVA (or BSA). The mAb was obtained from DC4-A6 by ascites production, and purified using a protein-G column. The mAb isotype was determined as IgG1.
3.5. Effect of heterologous coating on ciELISA
In order to explore the relationship between the specific monoclonal antibodies and coating haptens, the ciELISA were established based on heterologous coating antigen. illustrates the IC50, LOD (IC10) and LOQ (IC20–IC80) of mAb against each of the 15 coating antigens. Comparing the homologous coating antigen, the heterologous coating antigens based on the haptens (H1–H11) that have different functional groups, different chemical sites, different length spacer arms failed to improve the sensitivity of ciELISA. Although the hapten of heterologous coating antigens in this study was different from that of immunogen, the main characteristic structure of all haptens was the parent structure of LMG. As a result, the affinity between coating antigen and antibody was still higher, and therefore leading to no significant improvement on sensitivity. Fortunately, the heterologous coating antigens prepared using the haptens that have the critical secondary structure of LMG showed some interesting results in the development of ciELISA for LMG. The results indicate that although hapten H12 retained the characteristic structure of LMG, the central carbon atom of triphenylmethane was so active that the feature group was hard to expose while conjugated to carrier protein. On the other hand, the results of UV–vis spectra of the artificial antigen H12-OVA show that the antigen had the maximum absorbance at 618 nm, which was the characteristic wavelength of MG. This result could be corroborated by the specificity of mAb. However, the mAb did not recognize H13-OVA, probably because the methyl was changed to the ethyl of aromatic ring. The increase in steric hindrance resulting in the cavity of an Abs has no affinity for the antigen. The obtained mAb showed higher sensitivity in heterologous ELISA format based on the H14-OVA as coating antigen. It can be explained by the weaker recognition of heterologous coating antigens to antibody, which led to better binding of antibody to analyte. Although the mAb could recognize the dimethylaniline of H15-OVA, its affinity was too weak to reflect the titer, which resulted in the poor sensitivity of mAb. Obviously, the mAb that showed the highest specificity and sensitivity for LMG in the heterologous ELISA format should be chosen for further study to develop a specific immunoassay for LMG.
Table 3. The data of ciELISA in different coating antigens (n = 3).
3.6. Optimization of ciELISA conditions
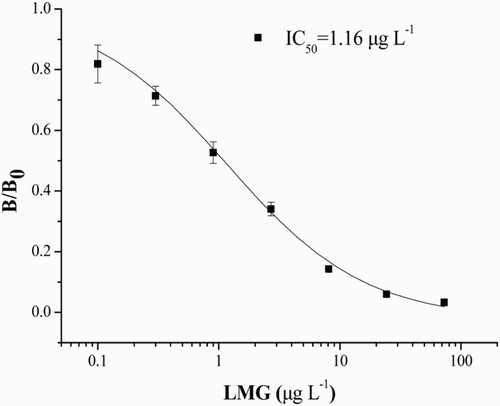
Based on the above results, a heterologous ciELISA using mAb DC4-A6 and coating antigen H14-OVA was developed. The effect of ELISA conditions such as organic solvents, antibodies concentration on ELISA performance was studied. The optimal ELISA conditions were as follows: the concentration of H14-OVA was 0.25 mg L−1, the concentration of mAb was 0.153 mg L−1, the concentration of HRP labeled GAM was 1.67 × 10−4 mg mL−1, the phosphate buffer (pH = 7.4, 50 µL L−1 Tween-20, 10% methanol) (See Section 3 in Supporting Information). Under the optimal ELISA conditions, the calibration curve for the LMG was established and is shown in . The IC50 value for LMG was 1.16 ng mL−1, and the LOD was 0.06 ng mL−1. The linear range ranged from 0.1 ng mL−1 to 8.1 ng mL−1 (y = −0.2737x + 0.4285 R2 = 0.9981). The sensitivity was slightly improved with published literatures (Oplatowska et al., Citation2011; Singh et al., Citation2011; Xing et al., Citation2009).
3.6. Specificity of mAb
To investigate the specificity of ciELISA method and find which structural features of the LMG are important to antibody recognition, the CR against a range of compounds structurally related to LMG was tested using the established ciELISA. The results in show that the mAb generated in this study had excellent specificity to the LMG and only a slight CR was observed toward the triphenylmethane dyes and other similar structure compounds.
Table 4. The CR of mAb based on ciELISA for LMG and analogs.
3.7. Method validation
The accuracy and precision of the established ciELISA were evaluated by determination of spiked samples with LMG and positive samples. lists the recoveries and variation efficiencies of the ciELISA and HPLC–MS/MS. The average recovery of LMG was 91.8% (ranging from 78.0% to 101.0%) by ciELISA, while that was 94.3% (ranging from 82.0% to 105.0%) by HPLC–MS/MS method. In contract, the ciELISA used less pretreatment procedures than HPLC–MS/MS, which is potential for screening of a lot of samples. Coincidentally, the positive sample (perch) was found in the agricultural markets, and was applied to verify the accuracy of the developed ciELISA. No false positive and negative results were obtained in the screening test. The squared coefficient of correlation (R2) was 0.9977 between the results of ciELISA and HPLC–MS/MS using spiked fish samples, which indicated good reliability and accuracy of the proposed ciELISA.
Table 5. The recoveries of fish sample fortified with LMG by ciELISA and HPLC–MS/MS (N = 3).
The stability of developed ciELISA was the basic property, which was also the important indicator to ensure the accuracy of test results. The results of stability test showed that the absorbance value and the sensitivity were not decreased by storing the microtiter plates and the reagents for 3 or 6 days at either 4°C or 37°C (), which indicated that the developed ciELISA could meet the practical requirements of the commercial products.
4. Conclusions
In this work, the impact of hapten structure on the production of specific antibody against LMG was studied by design and synthesis of a series of haptens with different arm linkers and site substitutions. The results indicated that the haptens with a hydroxyacetic acid spacer arm at the para- or ortho-position of benzene ring (R1 or R2 position) of LMG were suitable for producing specific antibody against LMG. mAb was produced and characterized and used to develop a ciELISA. The effects of ELISA conditions and heterologous coating on ELISA performance were carefully studied. The optimized ciELISA showed good sensitivity (IC50 of 1.16 and LOD of 0.06 ng mL−1) and specificity (negligible CR with analogs) for LMG. The results of proposed ciELISA for spiked fishes samples had a good correlation (R2 = 0.9977) with standard HPLC–MS/MS. Due to the good sensitivity, high through-put and low cost, the proposed ciELISA was suitable for LMG screening of large amount samples prior to instrumental analysis.
Supporting_information-Revised_2017.6.7.doc
Download MS Word (2.5 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Yu Wang graduated at 2010 from South China Agricultural University (SCAU) and got his PhD on food science. He is now working at Guangzhou Institute for Food Control.
Jinyi Yang got his PhD on food science at 2008 from SCAU, he is now working on the development of immunoassay kits for food contaminants.
Yudong Shen is a professor at SCAU. He got his PhD on organic chemistry at 2005 from Sun Yat-Sen University. His research interest includes hapten synthesis and antibody production.
Yuanming Sun is a professor at SCAU. He graduated from Southwest University at 1993 and got his PhD on Olericulture. His focused on the development of immunoassays for food contaminants.
Zhili Xiao got her PhD from SCAU on plant protection. She works on immunoassays for pesticides.
Hongtao Lei is a professor at SCAU. He graduated from SCAU at 2005 and got his PhD on food science.
Hong Wang is a professor at SCAU. She got her PhD on molecular biology from South China University of Technology at 2003.
Zhenlin Xu is a professor at SCAU. He graduated from SCAU at 2011 for his PhD on food science. His research interest includes antibody production and development of novel immunoassays.
Additional information
Funding
References
- Abad, A., Moreno, M. J., & Montoya, A. (1998). Hapten synthesis and production of monoclonal antibodies to the N-methylcarbamate pesticide methiocarb. Journal of Agricultural and Food Chemistry, 46(6), 2417–2426. doi: 10.1021/jf980077v
- Andersen, W. C., Turnipseed, S. B., & Roybal, J. E. (2006). Quantitative and confirmatory analyses of malachite green and leucomalachite green residues in fish and shrimp. Journal of Agricultural and Food Chemistry, 54(13), 4517–4523. doi: 10.1021/jf0532258
- Beier, R. C., Ripley, L. H., Young, C. R., & Kasier, C. M. (2001). Production, characterization, and cross-reactivity studies of monoclonal antibodies against the coccidiostat nicarbazin. Journal of Agricultural and Food Chemistry, 49(10), 4542–4552. doi: 10.1021/jf010208j
- Bilandžic, N., Varenina, I., Kolanović, B. S., Oraić, D., & Zrnčić, S. (2012). Malachite green residues in farmed fish in Croatia. Food Control, 26(2), 393–396. doi: 10.1016/j.foodcont.2012.02.001
- Brenner, A. J., & Harris, E. D. (1995). A quantitative test for copper using bicinchoninic acid. Analytical Biochemistry, 226(1), 80–84. doi: 10.1006/abio.1995.1194
- Brun, E. M., Garces-Garcia, M., Puchades, R., & Maquieira, A. N. (2005). Highly sensitive enzyme-linked immunosorbent assay for chlorpyrifos. Application to olive oil analysis. Journal of Agricultural and Food Chemistry, 53(24), 9352–9360. doi: 10.1021/jf0516641
- Burgstahler, A. W., & Worden, L. R. (1966). Coumarone. Organic Syntheses, 43, 28.
- Chen, G. Y., & Miao, S. (2010). HPLC determination and MS confirmation of malachite green, gentian violet, and their leuco metabolite residues in channel catfish muscle. Journal of Agricultural and Food Chemistry, 58(12), 7109–7114. doi: 10.1021/jf9043925
- Cho, B. P., Yang, T. L., Blankenship, L. R., Moody, J. D., Churchwell, M., Beland, F. A., & Culp, S. J. (2003). Synthesis and characterization of N-demethylated metabolites of malachite green and leucomalachite green. Chemical Research in Toxicology, 16(3), 285–294. doi: 10.1021/tx0256679
- Chu, Y. L., Chimeddulam, D., Sheen, L. Y., & Wu, K. Y. (2013). Probabilistic risk assessment of exposure to leucomalachite green residues from fish products. Food and Chemical Toxicology, 62, 770–776. doi: 10.1016/j.fct.2013.10.002
- Culp, S. J., & Beland, F. A. (1996). Malachite green: A toxicological review. International Journal of Toxicology, 15(3), 219–238. doi: 10.3109/10915819609008715
- Culp, S. J., Beland, F. A., Heflich, R. H., Benson, R. W., Blankenship, L. P., Webb, P. J., … Manjanatha, M. G. (2002). Mutagenicity and carcinogenicity in relation to DNA adduct formation in rats fed leucomalachite green. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 506-507, 55–63. doi: 10.1016/S0027-5107(02)00152-5
- Culp, S. J., Blankenship, L. R., Kusewitt, D. F., Doerge, D. R., Mulligan, L. T., & Beland, F. A. (1999). Toxicity and metabolism of malachite green and leucomalachite green during short-term feeding to Fischer 344 rats and B6C3F1 mice. Chemico-Biological Interactions, 122(3), 153–170. doi: 10.1016/S0009-2797(99)00119-2
- Deng, X. J., Yang, H. Q., Li, J. Z., Song, Y., Guo, D. H., Luo, Y., … Bo, T. (2011). Multiclass residues screening of 105 veterinary drugs in meat, milk, and egg using ultra high performance liquid chromatography tandem quadrupole time-of-flight mass spectrometry. Journal of Liquid Chromatography & Related Technologies, 34(19), 2286–2303. doi: 10.1080/10826076.2011.587224
- EC. (2004). Commission Decision 2004/25/EC, as regards the setting of minimum required performance limits (MRPLs) for certain residues in food of animal origin. Official Journal of European Union, L6, 38–39.
- Fedenko, V. S. (1989). Determination of protein in solutions from absorption in the ultraviolet region. Chemistry of Natural Compounds, 25(5), 590–592. doi: 10.1007/BF00598081
- Fernandes, C., Lalitha, V. S., & Rao, K. V. K. (1991). Enhancing effect of malachite green on the development of hepatic pre-neoplastic lesions induced by N-nitrosodiethylamine in rats. Carcinogenesis, 12(5), 839–845. doi: 10.1093/carcin/12.5.839
- Fessard, V., Godard, T., Huet, S., Mourot, A., & Poul, J. M. (1999). Mutagenicity of malachite green and leuco-malachite green in in vitro tests. Journal of Applied Toxicology, 19(6), 421–430. doi:10.1002/(SICI)1099-1263(199911/12)19:6<421::AID-JAT595>3.0.CO;2-6
- Fuller, H. R., Goodwin, P. R., & Morris, G. E. (2006). An enzyme-linked immunosorbent assay (ELISA) for the major crustacean allergen, tropomyosin, in food. Food and Agricultural Immunology, 17(1), 43–52. doi: 10.1080/09540100600572651
- Gui, W. J., Jin, M. J., Sun, L. F., Guo, Y. R., & Zhu, G. N. (2009). Residues determination of carbofuran in vegetables based on sensitive time-resolved fluorescence immunoassay. Food and Agricultural Immunology, 20(1), 49–56. doi: 10.1080/09540100802702221
- Guy, D., Anne, B. D., & Guy, M. R. (1992). Determination of clenbuterol in bovine tissues and urine by enzyme immunoassay. Journal of Agricultural and Food Chemistry, 40(1), 70–75. doi: 10.1021/jf00013a014
- Habeeb, A. F. S. A. (1966). Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Analytical Biochemistry, 14(3), 328–336. doi: 10.1016/0003-2697(66)90275-2
- Jiang, T. F., Lv, Z. H., Cui, X. Y., & Wang, Y. H. (2012). Analysis of malachite green, gentian violet and their leuco metabolites in catfish and carp by micellar electrokinetic capillary chromatography. Journal of Food & Drug Analysis, 20(1), 94–100.
- Köhler, G., & Milstein, C. (1975). Continuous cultures of fused cells secreting antibody of predefined specificity. Nature, 256, 495–497. doi: 10.1038/256495a0
- Langone, J. J., & Vanvunakis, H. (1982). Radio immunoassay of nicotine, cotinine, and gamma-(3-pyridyl)-gamma-oxo-N-methylbutyramide. Methods in Enzymology, 84, 628–640. doi: 10.1016/0076-6879(82)84050-0
- Lei, H. T., Shen, Y. D., Song, L. J., Yang, J. Y., Chevallier, O. P., Haughey, S. A., … Elliott, C. T. (2010). Hapten synthesis and antibody production for the development of a melamine immunoassay. Analytica Chimica Acta, 665(1), 84–90. doi: 10.1016/j.aca.2010.03.007
- Li, Q., Luo, H. T., Huang, X. L., Zhu, Z. X., & Wu, H. Q. (2014). High throughput screening of drug multi residues in fish by quadrupole-time-of-flight mass spectrometry. Chinese Journal of Analytical Chemistry, 42(10), 1478–1485. doi:10.11895/j.issn.0253-3820.140322
- Li, P., Zhang, Y., Lei, H. T., Wang, H., Xu, Z. L., Shen, Y. D., … Yang, J. Y. (2015). Development of chemiluminescent enzyme immunoassay for the determination of aflatoxin M1 in milk products. Food and Agricultural Immunology, 26(2), 157–169. doi:10.1080/09540105.2014.884056
- Long, C. Y., Mai, Z. B., Yang, Y. F., Zhu, B. H., Xu, X. M., Lu, L., & Zou, X. Y. (2009). Determination of multi-residue for malachite green, gentian violet and their metabolites in aquatic products by high-performance liquid chromatography coupled with molecularly imprinted solid-phase extraction. Journal of Chromatography A, 1216(12), 2275–2281. doi: 10.1016/j.chroma.2009.01.047
- López-Gutiérrez, N., Romero-González, R., Martinez Vidal, J. L., & Frenich, A. G. (2013). Analysis of triphenylmethane dyes in seafood products: A review of extraction methods and determination by liquid chromatography coupled to mass spectrometry. Analytical Methods, 5(14), 3434–3449. doi: 10.1039/c3ay40485d
- Mercader, J. V., & Montoya, A. (1997). A monoclonal antibody-based ELISA for the analysis of azinphos-methyl infruit juices. Analytica Chimica Acta, 347(1-2), 95–101. doi: 10.1016/S0003-2670(97)00015-9
- Mittelstaedt, R. A., Mei, N., Webb, P. J., Shaddock, J. G., Dobrovolsky, V. N., McGarrity, L. J., … Heflich, R. H. (2004). Genotoxicity of malachite green and leucomalachite green in female Big Blue B6C3F1 mice. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 561(1-2), 127–138. doi: 10.1016/j.mrgentox.2004.04.003
- Nix, B., & Wild, D. (2006). Calibration curve fitting the immunoassay handbook (pp. 233–245). New York, NY: Nature Publishing Group.
- Oplatowska, M., Connolly, L., Stevenson, P., Stead, S., & Elliott, C. T. (2011). Development and validation of a fast monoclonal based disequilibrium enzyme-linked immunosorbent assay for the detection of triphenylmethane dyes and their metabolites in fish. Analytica Chimica Acta, 698(1-2), 51–60. doi: 10.1016/j.aca.2011.04.047
- Plakas, S. M., Doerge, D. R., & Turnipseed, S. B. (1999). Disposition and metabolism of malachite green and other therapeutic dyes in fish. In M. G. Beconi-Barker, W. H. Gingerich, & D. J. Smith (Eds.), Xenobiotics in fish (pp. 149–166). New York, NY: Plenum Press. doi: 10.1007/978-1-4615-4703-7_11
- Plakas, S. M., ElSaid, K. R., Stehly, G. R., Gingerich, W. H., & Allen, J. H. (1996). Uptake, tissue distribution, and metabolism of malachite green in the channel catfish (ictalurus punctatus). Canadian Journal of Fisheries and Aquatic Sciences, 53(6), 1427–1433. doi: 10.1139/f96-061
- Shen, Y. D., Xu, Z. L., Zhang, S. W., Wang, H., Yang, J. Y., Lei, H. T., … Sun, Y. M. (2012). Development of a monoclonal antibody-based competitive indirect enzyme-linked immunosorbent assay for furaltadone metabolite AMOZ in fish and shrimp samples. Journal of Agricultural and Food Chemistry, 60(44), 10991–10997. doi: 10.1021/jf302913h
- Singh, G., Koerner, T., Gelinas, J. M., Abbott, M., Brady, B., Huet, A. C., … Godefroy, S. B. (2011). Design and characterization of a direct ELISA for the detection and quantification of leucomalachite green. Journal Food Additives & Contaminants: Part A, 28(6), 731–739. doi: 10.1080/19440049.2011.567360
- Smith, L. J., & Opie, J. W. (1948). Synthesis of ο-aminobenzaldehyde. Organic Syntheses, 28, 11. doi: 10.15227/orgsyn.028.0011
- Storey, J. M., Clark, S. B., Johnson, A. S., Andersen, W. C., Turnipseed, S. B., Lohne, J. J., … Madson, M. R. (2014). Analysis of sulfonamides, trimethoprim, fluoroquinolones, quinolones, triphenylmethane dyes and methyltestosterone in fish and shrimp using liquid chromatography-mass spectrometry. Journal of Chromatography B, 972, 38–47. doi: 10.1016/j.jchromb.2014.09.009
- Turnipseed, S. B., Lohne, J. J., Storey, J. M., Andersen, W. C., Young, S. L., Carr, J. R., & Madson, M. R. (2014). Challenges in implementing a screening method for veterinary drugs in milk using liquid chromatography quadrupole time-of-flight mass spectrometry. Journal of Agricultural and Food Chemistry, 62(17), 3660–3674. doi: 10.1021/jf405321w
- Villar-Pulido, M., Gilbert-López, B., García-Reyes, J. F., Martos, N. R., & Molina-Díaz, A. (2011). Multiclass detection and quantitation of antibiotics and veterinary drugs in shrimps by fast liquid chromatography time-of-flight mass spectrometry. Talanta, 85(3), 1419–1427. doi: 10.1016/j.talanta.2011.06.036
- VRC UK. (2001–2010). Veterinary residues committee’s annual report on surveillance for veterinary residues in food in UK for 2001 to 2010. Retrieved from http://www.vmd.defra.gov.uk/vrc/Reports/annual.htm
- Wyatt, G. M., Garrett, S. D., Lee, H. A., & Morgan, M. R. A. (1999). Alteration of the binding characteristics of a recombinant scFv anti-parathion antibody-1. Mutagenesis targeted at the VH CDR3 domain. Food and Agricultural Immunology, 11(3), 207–218. doi: 10.1080/09540109999735
- Xie, J., Peng, T., Chen, D. D., Zhang, Q. J., Wang, G. M., Wang, X., … Deng, J. (2013). Determination of malachite green, crystal violet and their leuco-metabolites in fish by HPLC-VIS detection after immunoaffinity column clean-up. Journal of Chromatography B, 913-914, 123–128. doi: 10.1016/j.jchromb.2012.12.002
- Xing, W. W., He, L., Yang, H., Sun, C. J., Li, D. W., Yang, X. M., … Deng, A. P. (2009). Development of a sensitive and group-specific polyclonal antibody-based enzyme-linked immunosorbent assay (ELISA) for detection of malachite green and leucomalachite green in water and fish samples. Journal of the Science of Food Agriculture, 89(13), 2165–2173. doi: 10.1002/jsfa.3695
- Yang, M. C., Fang, J. M., Kuo, T. F., Wang, D. M., Huang, Y. L., Liu, L. Y., … Chang, T. H. (2007). Production of antibodies for selective detection of malachite green and the related triphenylmethane dyes in fish and fishpond water. Journal of Agricultural and Food Chemistry, 55(22), 8851–8856. doi: 10.1021/jf071195y
- Yang, J. Y., Zhang, Y., Wang, H., Xu, Z. L., Eremin, S. A., Shen, Y. D., … Sun, Y. M. (2015). Development of fluorescence polarisation immunoassay for carbofuran in food and environmental water samples. Food and Agricultural Immunology, 26(3), 340–355. doi: 10.1080/09540105.2014.914890
- Zhang, Y. Z., Jin, P., Sun, X. L., Xu, D., & Shan, J. D. (2012). Establishment of the direct competition ELISA method for deoxynivaleno. Journal of Food Science Biotechnology, 31(1), 29–32.
- Zhang, Y., Yang, J. Y., Lei, H. T., Wang, H., Xu, Z. L., Shen, Y. D., … Sun, Y. M. (2015). Development of chemiluminescent enzyme immunoassay for the determination of malachite green in seafood. Food and Agricultural Immunology, 26(2), 204–217. doi: 10.1080/09540105.2014.884056
- Zhang, Y. Y., Yu, W. S., Pei, L., Lai, K. Q., Rasco, B. A., & Huang, Y. Q. (2015). Rapid analysis of malachite green and leucomalachite green in fish muscles with surface-enhanced resonance Raman scattering. Food Chemistry, 169, 80–84. doi: 10.1016/j.foodchem.2014.07.129