 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Fruits of Euterpe spp. are rich in phenolic compounds, mainly anthocyanins, which are endowed with a high antioxidant capacity. The objective of the study was to evaluate the effects of derivatives from Euterpe spp. fruits on oxidative metabolism and inflammatory mediators. The oil (OE), total lyophilized pulp (LEE) and defatted pulp (LEDE) were obtained from the fruits of Euterpe edulis. Thirty-six animals were divided into four experimental groups: G1: Control; G2: OE (4%), G3: LEE (10%), G4: LEDE (10%), each of which received a particular extract in their diet for 50 days. The activities of catalase, glutathione-S-transferase, superoxide dismutase, malondialdehyde produced in liver and expression of pro-inflammatory cytokines tissue were lower in G4 than in the other groups. The study indicates that dietary supplementation with extracts of E. edulis has no deleterious effects and may be beneficial, especially for LEDE extracts containing high concentrations of anthocyanin.
Introduction
The use of fruits and medicinal plants is an ancient practice that has shown worldwide growth (Ferreira & Pinto, Citation2010; Rufino et al., Citation2010). However, to demonstrate the curative or preventive effects of a compound it is necessary determine its potential, biochemical, oxidative and inflammatory aspects. There is increasing interest in naturally occurring antioxidants for use in foods to replace synthetic antioxidants (Kanyaiya, Digambar, Arora, Kapila, & Singh, Citation2014). In this context, although the fruit of the jussara palm tree (Euterpe edulis) is widely distributed in the Brazilian Atlantic Forest, its consumption and processing is not as well explored in the different regions as that of another palm tree fruit known as açaí (Euterpe oleraceae) (Sousa De Brito et al., Citation2007). The final product of jussara fruit processing is a thick purple pulp with considerable nutritional value that can be used in the manufacturing of a variety of beverages, sweets and ice cream (Borges et al., Citation2011). The nutritive potential of this fruit is well justified due to the high content of polyphenols, especially anthocyanins and polyunsaturated fatty acids (Rufino et al., Citation2010). Anthocyanins can stimulate the activity of immunocompetent cells, capture free radicals directly, repair DNA and repair damaged cells (Hartati, Widjanarko, Widyaningsih, & Rifa’i, Citation2017). Studies have shown that anthocyanins have a high antioxidant capacity and activate important pathways in the fight against metabolic reactors, which highlights its importance in the fight against cellular oxidation and consequently in the pathogenesis of cardiovascular diseases, hepatic diseases, diabetes and cancer. Thus, the analysis of foods rich in these compounds has been steadily growing because dietary supplementation with tropical fruit pulp rich in antioxidants has been presented as an effective curative and preventive therapy for the treatment of several conditions associated with pathological conditions (Felzenszwalb, da Costa Marques, Mazzei, & Aiub, Citation2013; Freitas et al., Citation2016; Pereira et al., Citation2014; Prior & Wu, Citation2006). The fruits of jussara contain two main anthocyanins identified as cyanidin 3-glucoside and cyanidin 3-rutinoside (Harborne, Saito, & Detoni, Citation1994), in addition to minor compounds such as cyanidin 3-sambubioside, pelargonidine 3-glycoside, cyanidin 3-rhamnoside and pelargonidine 3 rutinoside identified by LC-DAD-ESI/MS (Rufino et al., Citation2010). The lipid fraction obtained from the pulp of jussara (although little used) is also suitable for consumption due to its high content of polyunsaturated fatty acids, remarkable concentration of oleic acid, palmitic and linoleic acids, similar to E. oleraceae, and lower saturated lipid concentration compared to other oils (Freitas et al., Citation2016). The saturated fatty acids present in this fruit represent 24.32–28.89% of the total lipid content (Neida & Elba, Citation2007; Schauss et al., Citation2006). Considering the increased consumption of E. edulis fruit, it is prudent to carry out a toxicological analysis of the extracts to guarantee their safe consumption. In this context, hepatic toxicity analysis is an important tool because the bioactivation of the metabolites present in food extracts is able to result in interactions with the hepatocytes leading to DNA damage, loss of protein function, lipid peroxidation and consequently to tissue oxidative stress (Wang, Lee, Chang, Liou, & Ho, Citation2001). In addition, liver dysfunction also has the capacity to initiate immune reactions, which contribute to the progression of liver injury through the production of inflammatory cytokines (Lacour, Gautier, Pallardy, & Roberts, Citation2005). According to Schauss et al. (Citation2010), the lyophilized pulp of E. oleraceae does not present mutagenic, clastogenic, cytotoxic or genotoxic effects. However, the biosafety of the pulp of E. edulis requires analysis to determine its effects on biochemical, oxidative and cellular parameters and, consequently, to observe its action on energy metabolism. The implications highlighted in this study on the potential of E. edulis extracts may open new avenues for dietary supplementation and represent a promising path to be explored by the food and pharmaceutical industry. In addition, health professionals may associate conventional treatments for several pathologies with diets rich in antioxidant molecules obtained inexpensively and that are accessible to the general population. Therefore, we evaluated the safety of the administration of OE, lyophilized extract (LEE) and lyophilized and defatted extract (LEDE) of E. edulis supplemented with the standardized diet of male Wistar rats following the guidelines recommended by ANVISA Resolution 16 e 17/99, which establishes basic guidelines for risk assessment and food safety (BRASIL, Citation1999).
Material and methods
Plant material and obtaining OE, LEE and LEDE
The fruits of E. edulis were collected in a remnant area of the Atlantic forest, located in the “Zona da Mata” (Latitude: −20.97337439; Longitude: −42.52883943; Height: 744661) in the State of Minas Gerais, Brazil. The ripe fruits were selected, washed, weighed, disinfected with chlorinated water, cooked in water, pulped (Itametal® Depolpadeira Bonina 0.25DF) and refined. The pulp was passed through a fine mesh screen and then lyophilized (Lyophilizer Liotop LP510, Liobras®), and the LEE extract was obtained. This extract served as raw material for the production of the defatted extract (LEDE) and the oil (OE) of E. edulis. The LEDE was obtained from Soxhet Quimis® (Model Q308-16B) for 12 h, using ethyl ether as the separation solvent. The three extracts were conditioned in nitrogen atmosphere and stored at −20°C.
Identification of anthocyanins by UPLC-DAD-ESI/MS
The chemical composition of LEE and LEDE was analyzed using ACQUITY Ultra Performance LC equipment (Walters, Milford, MA, USA) simultaneously coupled to PDA 2996 photodiode detector (Waters, Milford, MA, USA) and ACQUITY TQ detector (Waters MS Technologies, Manchester, UK), equipped with electrospray Z-spray (ESI) ionization with source operating in positive mode. MassLynx software (version 4.1, Waters, Milford, MA, USA) was used. Sample solutions (3 µL; 0.5 mg mL−1) were injected into a reversed phase column using a Waters Acquity SDS C-18 column (50 mm, 2.1 mm diameter, 4.6 µm particle size). Chromatographic analysis was performed using a mobile phase gradient consisting of (A) water with 0.1% formic acid and (B) acetonitrile with 0.1% formic acid with the initial condition set at 95% A. The mobile phase was then linearly increased to 95% B in 10 min, maintained at this concentration for 1 min, and then returned to initial conditions at 13 min.
Animals and treatment
The method used to evaluate in vivo toxicity was authorized by the Ethics Committee for the Use of Animals (CEUA)/UFV through process no 60/2012. Thirty-two Wistar rats (Rattus norvergicus) at 4 weeks of age were randomly divided into four experimental groups: G1: animals that received a commercial ration (Control), G2: commercial ration + E. edulis oil (OE), G3: commercial ration + freeze-dried extract of E. edulis (LEE 10%) and G4: commercial ration + lyophilized and defatted extract (LEDE 10%). The animals received the extracts mixed with commercial feed for 50 days. All diets and water were provided ad libitum. All procedures took place under controlled conditions of brightness (cycles of 12 h clarity/darkness), temperature (21 ± 2°C) and relative humidity (60%).
Serum biochemistry and organ weight
After 50 days, the animals were anesthetised in a halothane chamber followed by euthanasia by hypovolemia with collection of blood by puncture of the abdominal aorta. Blood was centrifuged at 1750g for 10 min at 4°C for the determination of serum levels: ultra-sensitive C-reactive protein, glucose, total cholesterol (TC), triglycerides, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were processed according to the manufacturer’s instructions. The animals were weighed before euthanization to evaluate possible differences between the groups that received E. edulis extracts and the control group. Immediately after euthanasia, the organs (liver, adipose tissue, kidneys, brain, gonads and heart) were removed and weighed.
Analysis of redox status
Aliquots (100 mg) of frozen liver fragments were homogenized in phosphate-buffered saline, centrifuged at 3500g under refrigeration (5°C), and the supernatant was used for analysis of catalase (CAT), glutathione-S-transferase (GST) and superoxide dismutase (SOD). CAT activity was evaluated by measuring the rate of decomposition of hydrogen peroxide according to the method described by Aebi (Citation1984). SOD activity was evaluated by the xanthine oxidase method based on the production of hydrogen peroxide and the reduction of nitroblue tetrazolium (Sarban, Kocyigit, Yazar, & Isikan, Citation2005). GST activity was followed by spectrophotometry to measure the product formed from the complexation of reduced glutathione with 1-chloro-2,4-dinitrobenzene according to Keen, Habig, and Jakoby (Citation1976). Total protein levels in hepatic tissue were measured by the method of Bradford (Citation1976). The determination of lipid peroxidation in the liver homogenates was performed by the detection of malondialdehyde (MDA) (Buege & Aust, Citation1978).
Inflammatory mediators
Total RNA was extracted from hepatic tissue using the Trizol reagent (Life Technologies). Fractions of 40 mg of hepatic tissue from each animal were macerated in liquid nitrogen. Total RNA was treated with RNAse-free DNAse (Life Technologies) and used for cDNA synthesis, using the M-MLV Reverse transcriptase kit (Invitrogen Brazil Ltd.). Real-time PCR reactions were performed using the 7500 Real Time PCR Systems (Applied Biosystems) apparatus, specific oligonucleotides (), cDNAs from the treatments and SYBR Green PCR Master Mix (Applied Biosystems). The amplification conditions were: 95°C for 10 min, and 40 cycles of 94°C for 15 s and 60°C for 1 min. For the quantification of gene expression, the comparative methods of Ct: and
were used. As endogenous control for the normalization of qRT-PCR data, primers specific for Rattus norvegicus var. albinus β-actin were used ().
Table 1. List of primers that were used in real-time PCR reactions.
Stereometry and hepatic karyometry
The volume density of hepatocytes (Vv [hep],%), interstitium (Vv [int],%), sinusoidal capillaries (Vv [inf],%), and inflammatory cells of lipids (Vv [lipid],%) were estimated using 4-µm thick histological sections stained with hematoxylin and eosin (H&E) (Gonçalves et al., Citation2012). Fifty histological fields of each group (40× objective lens) were sampled randomly, and a total of 3.65 × 104 µm2 liver areas were analyzed. To avoid repeated analysis of the same histological site, the sections were evaluated in semi-serial sections using one in every 20 cuts. For stereology analysis, a 300-point test system was used in a standard test area (At) of 73 × 103 µm2. The volume densities (vv) were estimated by counting points using the following formula: vv = PP[structure]/PT; where PP is the number of points located along the structure in question and PT are the total test points (Gonçalves et al., Citation2012; Novaes et al., Citation2013). The volume of 50 hepatocyte nuclei for each animal was determined according to Novaes et al. (Citation2013). All morphological analyses were performed using the Pro-Plus image analysis software 4.5 (Media Cybernetics, Silver Spring, MD, USA).
Statistical analysis
Data were expressed as the mean ± standard deviation (mean ± SD). The normality of the data distribution was verified by D’Agostino–Pearson’s test. Morphological data were subjected to the Kruskal–Wallis test, and the biochemical and molecular data were analyzed by the unidirectional analysis of variance, followed by Student–Newman–Keuls post hoc test for multiple comparisons. All tests were performed using the GraphPad Prism 5.01 statistical software (GraphPad Software, Inc., CA, USA), and statistical significance was set at p < .05.
Result
Identification of anthocyanins
The UPLC-DAD-ESI/MS chromatogram analysis of the LEE and LEDE extracts showed the presence of two major peaks when analyzed in the absorption region at 254 nm. The UV/vis absorption spectra of these peaks showed two bands with λmax between 240 and 280 nm (band II) and other between 500 and 540 nm (band I), characteristic of anthocyanin compounds. The ESI/MS full scan data showed for the two major anthocyanins molecular ion [M + H]+ at m/z 449 and [M + H]+ ion at m/z 595 MS/MS, both with fragments at [M + H −162]+, can be assigned to cyanidin 3-glucoside and cyanidin 3-rutinoside, respectively. Anthocyanin standards were used to confirm these compounds.
Analysis of organ weights
There was no significant difference between weight gain and absolute body weight (p < .05) ().
Table 2. Weight variation and absolute weight of organs of Wistar rats fed various extracts (OE, LEE and LEDE) of E. edulis.
Serum biochemistry
The values of TC were lower in the groups that received extract of LEDE (G4) incorporated into the diet (p < .05). Analysis of C-reactive protein, glucose, triglycerides, HDL, AST and ALT did not show significant differences between the different groups (p < .05) ().
Table 3. Biochemical parameters of serum (mg/dL) of control animals (G1) and animals receiving three different Euterpe edulis extracts incorporated into the diet (G2, G3 and G4).
Redox status
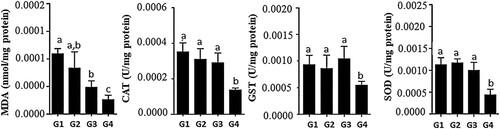
The analysis of the redox potential showed that LEDE (G4) was effective in reducing the production of malondialdehyde (MDA) and consequently decreased the activity of the antioxidant enzymes CAT, GST and SOD when compared to the other treatment groups. LEE (G3) was also effective in reducing MDA levels when compared to G1 and G2 ().
Figure 1. Redox status of the hepatic tissue of rats receiving the Euterpe edulis extract-supplemented diet. MDA: malondialdehyde; CAT: catalase; GST: glutathione-S-transferase; SOD: superoxide dismutase. G1: commercial ration; G2: commercial ration + 4% OE; G3: commercial feed + 10% LEE; G4: commercial ration + 10% LEDE defatted. a, b, c Different letters in the columns denote a statistical difference between groups at p < .05.
Inflammatory mediators
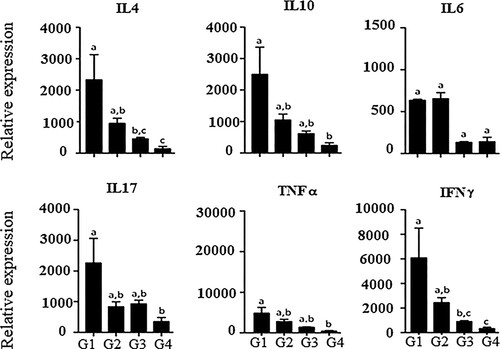
Analyses of the expression of proinflammatory mediators (IL-17, IFN-γ and TNF-α) revealed a decrease of all the mediators in animals that received the LEDE-supplemented diet (G4) when compared to the other treatment groups. The LEDE-supplemented group was also associated with a decrease in expression of the anti-inflammatory mediators (IL-4 and IL-10) compared to that of the other treatment groups (p < .05) ().
Figure 2. Expression of cytokine mRNA in the liver tissue of rats treated with Euterpe edulis in the IL diet, interleukin; TNF, tumor necrosis factor alpha; IFN-γ, or gamma interferon. G1: commercial ration; G2: commercial ration + 4% OE; G3: commercial feed + 10% LEE; G4: commercial ration + 10% LEDE defatted. a, b, c Different letters in the columns denote a statistical difference between groups at p < .05.
Stereological parameters
Stereological analysis of hepatic tissue showed that the fat volume density in hepatocytes was higher in the G2 and G3 groups, which received commercial feed + E. edulis oil and commercial ration + E. edulis extract lyophilized, respectively. The fat deposition of the LEDE group (G4), which received the defatted extract, was similar to that of the control group (p < .05). The other analyzed parameters did not present a significant difference between the groups ( and ).
Figure 3. Effect of the extract of Euterpe edulis on liver tissue of Wistar rats. G1: commercial ration; G2: commercial ration + 4% OE; G3: commercial feed + 10% LEE; G4: commercial ration + 10% LEDE. Color: hematoxylin and eosin. Magnification: 400×.
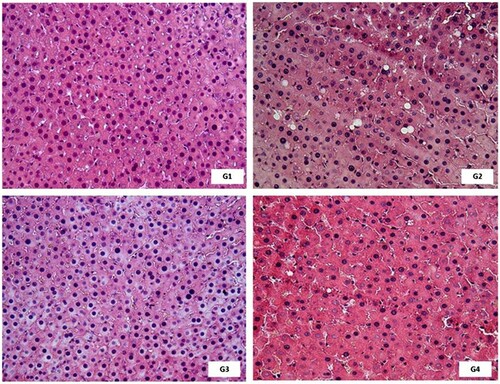
Table 4. Stereological parameters of the liver tissue of rats treated with Euterpe edulis extract in their diets.
Discussion
Our results showed that the defatted extract of E. edulis presents a great potential for dietary supplementation due to its capacity to positively modulate the redox potential and to induce the release of inflammatory mediators. These findings are important because they show that the consumption of E. edulis extract does not induce toxicity to the organism and can be safely consumed. In addition, the beneficial potential of the consumption of this extract on the body is evident from its modulation of important metabolic pathways involved in oxidative stress and tissue inflammation. It is interesting to observe that dietary supplementation with LEDE was able to reduce the serum levels of TC in the blood and in this way, decrease the possibility of endothelial damage and consequently its deposition in cells that normally do not store this molecule. These results corroborate the findings of Basu et al. (Citation2010), which demonstrated that the increase of dietary flavonoid content decreases the markers of atherosclerotic lesions in the metabolic syndrome. Furthermore, it has been observed that anthocyanin-rich foods are capable of promoting an improvement in the lipid profile (Basu et al., Citation2016; Hwang et al., Citation2011).
Analysis of the oxidative status of hepatic tissue showed that there was a decrease in the production of MDA, an important marker of lipid peroxidation and consequently a decrease in the activity of antioxidant defense enzymes (CAT, GST and SOD) in the groups that received LEDE dietary supplementation. Lipid peroxidation is a process generally associated with a significant production of free radicals within the cell, which can culminate in the destruction of membranes, proteins and DNA damage. The antioxidant enzymes act to accelerate the process of passage of electrons and consequently neutralize the action of free radicals. Therefore, a decrease in free radical production may correlate with the reduced expression of antioxidant enzymes (Lei et al., Citation2016). These data are supported by the findings of some studies that have demonstrated that flavonoid-rich extracts have a high antioxidant effect, promoting a reduction of the chain peroxidation process, reducing the formation of lipoperoxyl radicals and consequently causing less damage to cellular structures (Novaes et al., Citation2012; Virgili & Marino, Citation2008; Zhou et al., Citation2009). In addition, anthocyanins have been shown to exert a positive effect on the inhibition of free radical synthesis, thereby contributing to the maintenance of cellular function. Anthocyanin being one of the most popular antioxidants (Afanas’ev, Dcrozhko, Brodskii, Kostyuk, & Potapovitch, Citation1989; Dwijayanti, Widodo, Ibrahim, & Rifa’i, Citation2016; Fukumoto & Mazza, Citation2000; Gonçalves et al., Citation2012).
In addition to the oxidative status, the action of E. edulis on the release of inflammatory mediators was evaluated, because the pathways related to the inflammatory process are closely related to the cellular redox status (Reuter, Gupta, Chaturvedi, & Aggarwal, Citation2010). Our results showed that the consumption of an LEDE-supplemented diet was associated with a reduction in the release of pro-inflammatory mediators, and there was a consequent decrease in the release of anti-inflammatory mediators. These mediators are released in small amounts and in a similar proportion in order to maintain tissue homeostasis (Zhang, Liu, & Tsao, Citation2016).
The release of pro-inflammatory mediators occurs at the beginning of the inflammatory process, and as the inflammation is resolved the production of anti-inflammatory mediators outweighs the production of pro-inflammatory molecules (Tilg, Kaser, & Moschen, Citation2006). Thus, a reduction in pro-inflammatory mediator production generally indicates no inflammation and results in a decrease in anti-inflammatory mediators (Jiang et al., Citation2016). During the common cellular migration of phagocytes a process known as respiratory explosion occurs in which the cells produce free radicals to aid in the destruction of aggressive agents and apoptotic remnants of cells derived from their own tissue (Hussain, Hofseth, & Harris, Citation2003). This may result in the significant association between the production of ROS and the inflammatory process (Pohl et al., Citation2002). In our study, although no inflammation was induced, we observed that the LEDE extract was effective in inhibiting the release of inflammatory mediators. These results suggest that the consumption of this extract as a food supplement should positively regulate the release of these mediators and consequently modulate the inflammatory response. We believe that this potential is due to the high concentration of the anthocyanin flavonoid in the fruits of E. edulis. The modulating effect of anthocyanins on the inflammatory process has been previously demonstrated to occur through the control of cell migration and proliferation, as well as by inhibiting the production of inflammatory mediators and effectively improve liver damage (Youdim, Martin, & Joseph, Citation2000; Zhang, Liu, et al., Citation2016; Zhang, Pan, Jiang, & Mo, Citation2016). These data corroborate the results shown in the histopathological analysis of the liver in which the groups that received fat-rich extract had a high concentration in the amount of fat droplets in the hepatic tissue when compared to the control and the group supplemented with degreased LEDE. The accumulation of liver fat may be indicative of a reversible degenerative process called steatosis, which is closely related to the production of ROS and tissue inflammation (Gonçalves et al., Citation2012; Novaes et al., Citation2012). The more inflammatory mediators are released, the greater the production of ROS within phagocytes, the greater the chance of extravasation of these reactive molecules, which increases the probability of lipid peroxidation and consequently the development of a degenerative process(Gu, Wu, & Li, Citation2013; Hammerich & Tacke, Citation2014; Mourtzikou et al., Citation2014; Zhang, Miao, Han, & Dou, Citation2011). In the present study, the defatted extract showed morphological characteristics similar to those of the control, indicating that the consumption of this fruit is not toxic and can be safely consumed. It is interesting to note that the maintenance of the normal hepatic architecture in the groups treated with LEDE can be justified by the positive regulation in the redox status and in the release of inflammatory mediators.
This study includes novel data on the hepatic toxicology standard of three E. edulis extracts (LEE, LEDE and OE). Dietary supplementation with the extracts revealed no change in weight and absolute body weight as well as biochemical abnormalities in the blood of the animals. The oxidative status and inflammatory mediators generally had a positive modulation in the groups treated with the different extracts. However, the OE and LEE groups showed an increase in the amount of fat droplets when compared to the control and the degreased LEDE group. Based on these findings, we believe that among the extracts tested, the best results were observed in the animals treated with LEDE because there was a significant modulation of the oxidative and inflammatory status in this group compared to that of the other groups. In addition, no morphological changes were observed in the hepatic tissue of these animals. Therefore, our results demonstrate that extract of LEDE is a safe and promising compound with possible antioxidant and anti-inflammatory activities. However, additional studies are required to associate this extract with pathologies associated with an inflammatory process to elucidate the mechanism of action of this extract.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
R. B. Freitas
Rodrigo de Barros Freitas is D.S. in Agricultural Biochemistry. Currently, he works as a professor and researcher at the College Governador Ozanam Coelho in Brazil. The interest of their research includes Immunological processes and oxidative stress.
D. N. Rômulo
Rômulo Dias Novaes is D.S. in Cellular and Structural Biology. Currently, he works as a professor and researcher at the Federal University of Alfenas in Brazil. The interest of their research includes Clinical and Experimental Pathology and Parasitology.
G. M. Bianca
Bianca Gazolla Mendonça is Biochemistry and Medical. He currently resides in a medical clinic. Brazil. The interest of their research includes Oncology and Hematology.
C. S. Eliziária
Eliziária Cardoso dos Santos is D.S. in Cellular and Structural Biology. Currently, he works as a professor and researcher at the Federal University Of The Jequitinhonha And Mucuri Valleys in Brazil. The interest of their research includes morphophysiology with emphasis on the cardiovascular, respiratory, renal and digestive systems.
S. A. Murilo
Murilo Siqueira Alves is D.S. in Agricultural Biochemistry. Currently, he works as a postdoctoral fellow at Instituto Tecnológico Valein, Brazil. The interest of their research includes molecular pathways of response to stresses.
G. F. Luciano
Luciano Gomes Fietto is D.S. in Biochemistry and Molecular Biology. Currently, he works as a professor and researcher at the Federal University of Viçosa in Brazil. The interest of their research includes molecular biotechnology.
M. L. Luciana
Luciana Moreira Lima is D.S. in Pharmaceutical Sciences . Currently, he works as a professor and researcher at the Federal University of Viçosa in Brazil. The interest of their research includes Biochemical and hemostatic tests in diseases of high prevalence, in an attempt to identify possible markers of diagnosis and prognosis that may be useful for clinical use.
P. Maria do Carmo
Maria do Carmo Peluzio is D.S. in Biochemistry and Immunology . Currently, he works as a professor and researcher at the Federal University of Viçosa in Brazil. The interest of their research includes food with claim of functional property in the prevention of chronic non-communicable diseases.
V. G. Reggiani
Reggiani Vilela Gonçalves is D.S. in Cellular and Structural Biology . Currently, he works as a professor and researcher at the Federal University of Viçosa in Brazil. The interest of their research includes biotechnological potential for the development of drugs that promote cutaneous, hepatic and pulmonary repair.
V. L. João Paulo
João Paulo Viana Leite is D.S. Organic Chemistry. Currently, he works as a professor and researcher at the Federal University of Viçosa in Brazil. The interest of their research includes bioprospecting of secondary metabolites and their applications.
References
- Aebi, H. (1984). [13] Catalase in vitro. Methods in Enzymology, 105(C), 121–126. doi: https://doi.org/10.1016/S0076-6879(84)05016-3
- Afanas’ev, I. B., Dcrozhko, A. I., Brodskii, A. V., Kostyuk, V. A., & Potapovitch, A. I. (1989). Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochemical Pharmacology, 38(11), 1763–1769. doi: https://doi.org/10.1016/0006-2952(89)90410-3
- Basu, A., Fu, D. X., Wilkinson, M., Simmons, B., Wu, M., Betts, N. M., … Lyons, T. J. (2010). Strawberries decrease atherosclerotic markers in subjects with metabolic syndrome. Nutrition Research, 30(7), 462–469. doi: https://doi.org/10.1016/j.nutres.2010.06.016
- Basu, A., Morris, S., Nguyen, A., Betts, N. M., Fu, D., & Lyons, T. J. (2016). Effects of dietary strawberry supplementation on antioxidant biomarkers in obese adults with above optimal serum lipids. Journal of Nutrition and Metabolism, 2016. doi: https://doi.org/10.1155/2016/3910630
- Borges, G. D. S. C., Vieira, F. G. K., Copetti, C., Gonzaga, L. V., Zambiazi, R. C., Mancini Filho, J., & Fett, R. (2011). Chemical characterization, bioactive compounds, and antioxidant capacity of jussara (Euterpe edulis) fruit from the Atlantic Forest in southern Brazil. Food Research International, 44(7), 2128–2133. doi: https://doi.org/10.1016/j.foodres.2010.12.006
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. doi: https://doi.org/10.1016/0003-2697(76)90527-3
- BRASIL. (1999). Resolução no 16, de 30 de abril de 1999—Regulamento técnico de procedimentos para registro de Alimentos e ou novos ingredientes. Agência Nacional de Vigilância Sanitária, 6–8. doi: https://doi.org/10.1007/s13398-014-0173-7.2
- Buege, J. A., & Aust, S. D. (1978). Microsomal lipid peroxidation. Methods in Enzymology, 52(C), 302–310. doi: https://doi.org/10.1016/S0076-6879(78)52032-6
- Dwijayanti, D. R., Widodo, Ibrahim, M., & Rifa’i, M. (2016). EMSA eritin polyherbal can suppress NF-κB activation and decrease IL-17 cytokine in an irradiated mice model. Food and Agricultural Immunology, 27(3), 422–433. doi: https://doi.org/10.1080/09540105.2015.1126233
- Felzenszwalb, I., da Costa Marques, M. R., Mazzei, J. L., & Aiub, C. A. F. (2013). Toxicological evaluation of Euterpe edulis: A potential superfruit to be considered. Food and Chemical Toxicology, 58, 536–544. doi: https://doi.org/10.1016/j.fct.2013.05.029
- Ferreira, V. F., & Pinto, A. C. (2010). A fitoterapia no mundo atual. Química Nova, 33(9), 1829. doi: https://doi.org/10.1590/S0100-40422010000900001
- Freitas, R. B., Novaes, R. D., Goncalves, R. V, Mendonca, B. G., Santos, E. C., Ribeiro, A. Q., … Leite, J. P. (2016). Euterpe edulis extract but not oil enhances antioxidant defenses and protects against nonalcoholic fatty liver disease induced by a high-fat diet in rats. Oxidative Medicine & Cellular Longevity, 2016, 8173876. Retrieved from http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=prem&AN=27418954http://sfx.ucl.ac.uk/sfx_local?sid=OVID:medline&id=pmid:27418954&id=doi:10.1155%2F2016%2F8173876&issn=1942-0994&isbn=&volume=2016&issue=&spage=8173876&pages=8173876&date=
- Fukumoto, L. R., & Mazza, G. (2000). Assessing antioxidant and prooxidant activities of phenolic compounds. Journal of Agricultural and Food Chemistry, 48(8), 3597–3604. doi: https://doi.org/10.1021/jf000220w
- Gonçalves, R. V., Novaes, R. D., Leite, J. P. V, Vilela, E. F., Cupertino, M. C., Nunes, L. G., & Matta, S. L. P. (2012). Hepatoprotective effect of Bathysa cuspidata in a murine model of severe toxic liver injury. International Journal of Experimental Pathology, 93(5), 370–376. doi: https://doi.org/10.1111/j.1365-2613.2012.00835.x
- Gu, C., Wu, L., & Li, X. (2013). IL-17 family: Cytokines, receptors and signaling. Cytokine, 64, 477–485. doi: https://doi.org/10.1016/j.cyto.2013.07.022
- Hammerich, L., & Tacke, F. (2014). Interleukins in chronic liver disease: Lessons learned from experimental mouse models. Clinical and Experimental Gastroenterology, 7, 297–306. doi: https://doi.org/10.2147/CEG.S43737
- Harborne, J. B., Saito, N., & Detoni, C. H. (1994). Anthocyanins of cephaelis, cynomorium, euterpe, lavatera and pinanga. Biochemical Systematics and Ecology, 22(8), 835–836. doi: https://doi.org/10.1016/0305-1978(94)90088-4
- Hartati, F. K., Widjanarko, S. B., Widyaningsih, T. D., & Rifa’i, M. (2017). Anti-Inflammatory evaluation of black rice extract inhibits TNF-α, IFN-γ and IL-6 cytokines produced by immunocompetent cells. Food and Agricultural Immunology, 105(June), 1–10. doi: https://doi.org/10.1080/09540105.2017.1332006
- Hussain, S. P., Hofseth, L. J., & Harris, C. C. (2003). Radical causes of cancer. Nature Reviews Cancer, 3(4), 276–285. doi: https://doi.org/10.1038/nrc1046
- Hwang, Y. P., Choi, J. H., Han, E. H., Kim, H. G., Wee, J. H., Jung, K. O., … Jeong, H. G. (2011). Purple sweet potato anthocyanins attenuate hepatic lipid accumulation through activating adenosine monophosphate-activated protein kinase in human HepG2 cells and obese mice. Nutrition Research, 31(12), 896–906. doi: https://doi.org/10.1016/j.nutres.2011.09.026
- Jiang, M., Klein, M., Zanger, U. M., Mohammad, M. K., Cave, M. C., Gaikwad, N. W., … Xie, W. (2016). Inflammatory regulation of steroid sulfatase: A novel mechanism to control estrogen homeostasis and inflammation in chronic liver disease. Journal of Hepatology, 64(1), 44–52. doi: https://doi.org/10.1016/j.jhep.2015.07.022
- Kanyaiya, M., Digambar, S. P., Arora, S., Kapila, S., & Singh, R. R. B. (2014). In vivo, effect of herb (Withania somnifera) on immunomodulatory and antioxidative potential of milk in mice. Food and Agricultural Immunology, 25(3), 443–452. doi: https://doi.org/10.1080/09540105.2013.837032
- Keen, J. H., Habig, W. H., & Jakoby, W. B. (1976). Mechanism for the several activities of the glutathione S transferases. Journal of Biological Chemistry, 251(20), 6183–6188.
- Lacour, S., Gautier, J.-C., Pallardy, M., & Roberts, R. (2005). Cytokines as potential biomarkers of liver toxicity. Cancer Biomarkers: Section A of Disease Markers, 1(1), 29–39. doi: https://doi.org/10.3233/CBM-2005-1105
- Lei, X. G., Zhu, J.-H., Cheng, W.-H., Bao, Y., Ho, Y.-S., Reddi, A. R., … Arnér, E. S. J. (2016). Paradoxical roles of antioxidant enzymes: Basic mechanisms and health implications. Physiological Reviews, 96(1), 307–364. doi: https://doi.org/10.1152/physrev.00010.2014
- Mourtzikou, A., Alepaki, M., Stamouli, M., Pouliakis, A., Skliris, A., & Karakitsos, P. (2014). Evaluation of serum levels of IL-6, TNF-α, IL-10, IL-2 and IL-4 in patients with chronic hepatitis. Inmunologia, 33, 41–50. doi: https://doi.org/10.1016/j.inmuno.2014.01.001
- Neida, S., & Elba, S. (2007). Caracterización del acai o manaca (Euterpe olerdcea Mart.): Un fruto del Amazonas. Archivos Latinoamericanos de Nutricion, 57(1), 94–98.
- Novaes, R. D., Gonçalves, R. V., Cupertino, M. C., Marques, D. C. S., Rosa, D. D., Peluzio, M. do C. G., … Leite, J. P. V. (2012). Bark extract of Bathysa cuspidata attenuates extra-pulmonary acute lung injury induced by paraquat and reduces mortality in rats. International Journal of Experimental Pathology, 93(3), 225–233. doi:https://doi.org/10.1111/j.1365-2613.2012.00808.x
- Novaes, R. D., Penitente, A. R., Gonçalves, R. V., Talvani, A., Peluzio, M. C. G., Neves, C. A., … Maldonado, I. R. S. C. (2013). Trypanosoma cruzi infection induces morphological reorganization of the myocardium parenchyma and stroma, and modifies the mechanical properties of atrial and ventricular cardiomyocytes in rats. Cardiovascular Pathology, 22(4), 270–279. doi: https://doi.org/10.1016/j.carpath.2012.12.001
- Pereira, A. C. D. S., Dionísio, A. P., Wurlitzer, N. J., Alves, R. E., Brito, E. S. De, Silva, A. M. D. O. E., … Mancini Filho, J. (2014). Effect of antioxidant potential of tropical fruit juices on antioxidant enzyme profiles and lipid peroxidation in rats. Food Chemistry, 157, 179–185. doi: https://doi.org/10.1016/j.foodchem.2014.01.090
- Pohl, L. R., Shah, A. G., George, J. W., Amouzadeh, H. R., Reilly, T. P., Bourdi, M., … Martin, J. L. (2002). Protection against acetaminophen-induced liver injury and lethality by interleukin 10: Role of inducible nitric oxide synthase. Hepatology, 35(2), 289–298. doi: https://doi.org/10.1053/jhep.2002.30956
- Prior, R. L., & Wu, X. (2006). Anthocyanins: Structural characteristics that result in unique metabolic patterns and biological activities. Free Radical Research, 40(10), 1014–1028. doi: https://doi.org/10.1080/10715760600758522
- Reuter, S., Gupta, S. C., Chaturvedi, M. M., & Aggarwal, B. B. (2010). Oxidative stress, inflammation, and cancer: How are they linked? Free Radical Biology and Medicine, 49, 1603–1616. doi: https://doi.org/10.1016/j.freeradbiomed.2010.09.006
- Rufino, M. do S. M., Alves, R. E., de Brito, E. S., Pérez-Jiménez, J., Saura-Calixto, F., & Mancini-Filho, J. (2010). Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chemistry, 121(4), 996–1002. doi: https://doi.org/10.1016/j.foodchem.2010.01.037
- Sarban, S., Kocyigit, A., Yazar, M., & Isikan, U. E. (2005). Plasma total antioxidant capacity, lipid peroxidation, and erythrocyte antioxidant enzyme activities in patients with rheumatoid arthritis and osteoarthritis. Clinical Biochemistry, 38(11), 981–986. doi: https://doi.org/10.1016/j.clinbiochem.2005.08.003
- Schauss, A. G., Clewell, A., Balogh, L., Szakonyi, I. P., Financsek, I., Horváth, J., … Hirka, G. (2010). Safety evaluation of an açai-fortified fruit and berry functional juice beverage (MonaVie Active®). Toxicology, 278(1), 46–54. doi: https://doi.org/10.1016/j.tox.2010.04.017
- Schauss, A. G., Wu, X., Prior, R. L., Ou, B., Huang, D., Owens, J., … Shanbrom, E. (2006). Antioxidant capacity and other bioactivities of the freeze-dried Amazonian palm berry, Euterpe oleraceae mart. (acai). Journal of Agricultural and Food Chemistry, 54(22), 8604–8610. doi: https://doi.org/10.1021/jf0609779
- Sousa De Brito, E., De Araújo, M. C. P., Alves, R. E., Carkeet, C., Clevidence, B. A., & Novotny, J. A. (2007). Anthocyanins present in selected tropical fruits: Acerola, jambolão, jussara, and guajiru. Journal of Agricultural and Food Chemistry, 55(23), 9389–9394. doi: https://doi.org/10.1021/jf0715020
- Tilg, H., Kaser, A., & Moschen, A. R. (2006). How to modulate inflammatory cytokines in liver diseases. Liver International, 26(9), 1029–1039. doi: https://doi.org/10.1111/j.1478-3231.2006.01339.x
- Virgili, F., & Marino, M. (2008). Regulation of cellular signals from nutritional molecules: A specific role for phytochemicals, beyond antioxidant activity. Free Radical Biology and Medicine, 45, 1205–1216. doi: https://doi.org/10.1016/j.freeradbiomed.2008.08.001
- Wang, Y. J., Lee, C. C., Chang, W. C., Liou, H. B., & Ho, Y. S. (2001). Oxidative stress and liver toxicity in rats and human hepatoma cell line induced by pentachlorophenol and its major metabolite tetrachlorohydroquinone. Toxicology Letters, 122(2), 157–169. doi: https://doi.org/10.1016/S0378-4274(01)00361-7
- Youdim, K. A., Martin, A., & Joseph, J. A. (2000). Incorporation of the elderberry anthocyanins by endothelial cells increases protection against oxidative stress. Free Radical Biology and Medicine, 29(1), 51–60. doi: https://doi.org/10.1016/S0891-5849(00)00329-4
- Zhang, H., Liu, R., & Tsao, R. (2016). Anthocyanin-rich phenolic extracts of purple root vegetables inhibit pro-inflammatory cytokines induced by H2O2 and enhance antioxidant enzyme activities in Caco-2 cells. Journal of Functional Foods, 22, 363–375. doi: https://doi.org/10.1016/j.jff.2016.01.004
- Zhang, L., Miao, L., Han, F., & Dou, X. (2011). Cytokine levels in serum of patients with chronic hepatitis C and its significance. Chinese Journal of Cellular and Molecular Immunology, 27(3), 301–303.
- Zhang, M., Pan, L.-J., Jiang, S.-T., & Mo, Y.-W. (2016). Protective effects of anthocyanins from purple sweet potato on acute carbon tetrachloride-induced oxidative hepatotoxicity fibrosis in mice. Food and Agricultural Immunology, 27(2), 157–170. doi: https://doi.org/10.1080/09540105.2015.1079589
- Zhou, Z., Chang, X., Shao, C., Wu, Q., Wu, Q., & Huang, M. (2009). Pyrrolidine dithiocarbamate attenuates paraquat-induced lung injury in rats. Journal of Biomedicine and Biotechnology, 2009. doi: https://doi.org/10.1155/2009/619487
