ABSTRACT
This study was aimed to screen a high-efficient strain for degrading antigenic protein in soybean meal (SBM) and evaluate the effect of fermented soybean meal (FSBM) on growth performance of piglets. A Bacillus subtilis strain BS12 was selected through plate and fermentation experiment, which reduced 92.36% less glycinin and 88.44% less β-conglycinin in SBM. A total of 192 piglets were assigned to receive either a diet of SBM with antibiotics (the control group) or a diet containing 10% FSBM without antibiotics. The average daily gain and feed intake of pigs fed FSBM were superior (p < .10) to those fed the control diet. Reduced (p < .05) mRNA expression of pro-inflammatory cytokines was detected in the jejunum and ileum of pigs fed FSBM. These results demonstrated that a diet containing BS12 FSBM improved growth performance by reducing dietary inflammation in piglets.
Introduction
Soybean meal (SBM) is the most widely used plant protein source in the food and feed industries. Glycinin and β-conglycinin account for about 30% and 40%, respectively, of the total proteins in SBM (Maruyama et al., Citation1999). Soybean allergens (mainly glycinin and β-conglycinin) in the diets of weaned pigs provoked transient hypersensitivity and inflammation associated with abnormal morphology of the small intestine (Li et al., Citation1990; Sun, Li, Dong, Qiao, & Ma, Citation2008). These morphological changes can cause animal malabsorption syndrome, growth depression, and diarrhea (Sun, Li, Dong, et al., Citation2008; Sun, Li, Li, Dong, & Wang, Citation2008). In addition, β-conglycinin was reported to reduce feed intake in younger animals (Hao, Zhan, Guo, Piao, & Li, Citation2009; Nishi, Hara, & Tomita, Citation2003; Xu, Zhou, Wang, & Ai, Citation2010).
Microbial fermentation of animal feed can improve its nutritional quality by eliminating anti-nutritional factors and increasing nutrient bioavailability. Fermented SBM (FSBM) is produced by the addition of various microorganisms to SBM, including Rhizopus oligosporus, Aspergillus oryzae, Lactobacillus brevis, and Bacillus subtilis (Egounlety & Aworh, Citation2003; Feng, Liu, Xu, Lu, & Liu, Citation2007; Hong, Lee & Kim, Citation2004; Refstie, Erland, Grete, & Per, Citation2005). A previous study by Feng et al. (Citation2007) showed that FSBM with a low concentration of glycinin and β-conglycinin could improve feed intake and average daily weight in piglets. However, a diet containing only 9% FSBM with a 40% reduction in glycinin and β-conglycinin did not improve the growth performance of weaned pigs (Kim, Van Heugten, Ji, Lee, & Mateo, Citation2010). Thus, highly efficient allergen degradation strains are essential in the creation of FSBM, in order to improve animal growth performance.
This study was designed to isolate a strain with relatively higher capacity to degrade protein and cellulose and to apply it in the fermentation of SBM. An animal feeding trial was conducted to evaluate the growth performance and immune status in piglets fed a diet based on FSBM.
Materials and methods
SBM and Bacillus strains
In the present study, SBM was obtained from the Cofine Bio-tech Co. Ltd. (Jiaxing, China). Bacillus spp. utilized as the fermentation organism were isolated from preserved vegetables obtained from a local market (Shaoxing, China). The control strains B. subtilis CICC10088, B. subtilis CICC20030, B. subtilis CICC20076, B. subtilis CICC21076, and B. subtilis CICC23741 were obtained from the China Center of Industrial Culture Collection (CICC, Beijing, China). The bacteria were activated twice by culturing in Luria-Bertani medium (1% tryptone, 0.5% yeast extract, and 1% NaCl) at 37°C and 150 rpm for 12 h. The activated culture (i.e. cell pellets at a viable population of 107–8 CFU/mL) was used as the inoculum for SBM fermentation.
Screening and identify of strains
The protein degradation capacity of the strains was determined using protein plates, in which the protein was extracted from the SBM, as described previously (Liu et al., Citation2007). Measurements of the cellulase and amylase activity were performed with a carboxymethylcellulose plate (Hankin & Anagnostakis, Citation1977; Teather & Wood, Citation1982) and a starch plate (Wang et al., Citation2006), respectively. Strains were selected by comparing the ratio of the hydrolysis diameter and strain colony diameter (Wang et al., Citation2006). The strain was purified and cryopreserved at −80°C until sent to CICC and identified with 16S ribosomal RNA gene partial sequencing and gryB sequencing.
SBM fermentation
The SBM was fermented with B. subtilis according to the procedures described by Seo and Cho (Citation2016). Initially, the substrate was sterilized at 105°C for 20 min. After cooling, the SBM was added to 50% (w/v) sterile water and inoculated with 5% (w/v) inoculums at 37°C for 24 h. After fermentation, the SBM was dried by a freeze-dryer and grounded to pass through a 60-mesh screen. The grounded samples were stored at −20°C until further analysis.
Multiple fluorescence labeling and confocal laser scanning microscopy (CLSM)
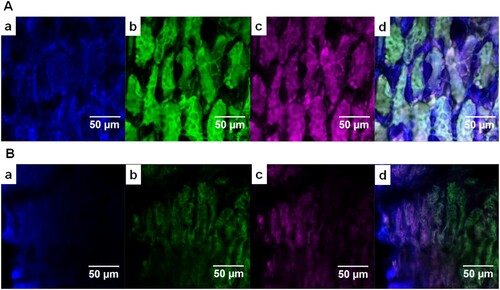
Proteins were labeled with fluorescein isothiocyanate (FITC), cellulose was labeled with calcofluor white (CW), and starch was labeled with Con A (Chen, Lee, & Tay, Citation2007; Adav et al., Citation2010; Wang, Shen, Yu, Ran, & Xu, Citation2012). First, 0.1 M sodium bicarbonate buffer was added to the FSBM sample to keep the amine group in a non-protonated form, after which 10 g/L of the FITC solution was added to the sample for 1 h at room temperature. The mixtures were washed with phosphate-buffered saline twice to remove excess stain and 250 mg/L Con A solution was added and incubated for 30 min. Finally, 300 mg/L CW solution was incubated with the sample and washed with phosphate-buffered saline after 30 min incubation. The stained samples were embedded in Tissue-Tek O.C.T. Compound (Sakura, Finetek USA, Torrance, CA) for observation and frozen at −20°C. Samples were cut into 30-μm sections with cryomicrotome (Cyrotome E, Thermo Shandon Limited, UK), imaged using a ×20 objective, and analyzed with Zen 2010 software (Carl Zeiss, Germany).
Feed experimental design, animals, and diets
All animal procedures were approved by the Institutional Animal Care and Use Committee of Zhejiang University. A 24-d experiment was carried out with 192 pigs (Duroc × Large white × Yorkshire) with body weights of 9.35 ± 0.65 kg. The experiment was a randomized complete block design with 2 dietary treatments, and each treatment was replicated with 6 pens, containing 16 pigs per pen. Pigs were weighed individually and assigned randomly to pens. All pigs were given ad libitum access to feed and water. Room temperature was controlled to about 32°C. Average daily gain and average daily feed intake were measured on day 24; feed gain and diarrhea incidence were also calculated.
The two diets were (1) corn–SBM with 100 mg/kg olaquindox and 50 mg/kg kitasamycin (control, CON); and (2) 10% replacement of protein from SBM in the control diet by protein from FSBM (). All diets were formulated to have similar nutrient content and met or exceeded the nutrient requirements estimated by NRC (Citation2012).
Table 1. Ingredient and composition of experiment basal diet.
Chemical analysis
Dry matter (DM), crude protein (CP), calcium, phosphorus, ether extract, ash, and amino acid composition of SBM, FSBM, and the two diets were analyzed according to AOAC (Citation2000). The trichloroacetic acid-soluble protein (TCA-SP) of the sample was determined using the methods described by Ovissipour et al. (Citation2009). Amounts of β-conglycinin and glycinin were analyzed using an indirect ELISA kit (Longzhoufangke Bio Co., Beijing, China) according to the manufacturer’s protocol.
Animal sampling procedure
At the end of the feeding trial, 12 pigs (6 piglets from each treatment) were selected and humanely killed. Jejunum and ileum samples (0.5 × 0.5 cm) were taken immediately, rinsed in physiological saline, and stored immediately at −20°C until analysis. Digesta samples from the jejunum were collected by massaging the tract from both ends. The digesta samples were stored immediately at −20°C until analysis.
Quantitative real-time PCR
Total RNA of the jejunum and ileum were extracted using TRIzol reagent (Invitrogen, USA). After visualization with the NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, MA, USA), 2 μg mRNA were used in reverse transcription to cDNA. Real-time polymerase chain reaction (PCR) was performed with the StepOne Plus TM system (Applied Biosystems, CA, USA). The primers used for real-time PCR are listed in . Each reaction included 5 μL FastStart Universal SYBR Green Master Mix (Roche, Switzerland). The thermocycler protocol consisted of 10 min at 95°C and 40 cycles of 10 s at 95°C and 35 s at 60°C, and melt curves were added. 18S were used as housekeeping genes. mRNA relative expression was calculated using the 2−ΔΔCt method as described by Yi et al. (Citation2016).
Table 2. List of primers used in this study for real-time PCR.
Western blotting
Total protein of the jejunum and ileum were extracted using a protein extraction kit (KeyGEN, Nanjing, China) and estimated using a BCA kit (KeyGEN, Nanjing, China). Protein supernatant was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a nitrocellulose membrane. After blocking with 5% skimmed milk powder, the membrane was incubated with the appropriate primary antibodies overnight at 4°C, followed by incubation with a horseradish peroxidase-conjugated secondary antibody for 1 h. Bands were detected using ECL (CliNX, Shanghai, China). Band intensity was quantified using Image J software. Primary antibodies for β-actin (Abcam, MA, USA), phos-p65-Nuclear Factor-κB (NF-κB) (Santa Cruz, CA, USA), phos-p38 Mitogen Activated Protein Kinase (MAPK) (Abcam, MA, USA), and phos-IκB-α (Abcam, MA, USA) were used.
Statistical analysis
Statistical analysis was performed using the Student t-test with SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). Differences were considered significant at p < .05. The results were expressed as the means and standard error.
Results
Screening Bacillus spp. for FSBM production
The results of the Bacillus spp. screening showed that three strains (BS9, BS12, and BS21) had larger hydrolysis diameters on the protein plate, and BS21 and BS9 showed weaker cellulase degradation than did BS12 (). We chose five control strains, belonging to B. subtilis, to compare enzyme activity and fermentation efficiency. As shown in (A), BS12 and the control strain CICC20076 showed similar protein degradation activity, but BS12 showed greater cellulase degradation capacity than any of the control strains ((B)). BS12 had starch degradation activity similar to CICC10088 and CICC20076 ((C)). The 16S rDNA sequencing indicated BS12 was close to Bacillus tequilensis KCTC 13622T (AYTO01000043) with 100% identity ((D)), and gryB sequencing indicated BS12 was close to B. subtilis subsp. subtilis BCRC 10255T (DQ309293) with 99% identity ((F)). Thus, BS12 was found to be B. subtilis.
Figure 1. Strain screening with plate assay and identified with hyligenetic tree assay. Note: (A) SBM protein plate; (B) carboxymethylcellulose sodium plate; (C) starch plate; (D) hyligenetic tree of the16S rRNA region of BS12; (F) hyligenetic tree of the gryB region of BS12.
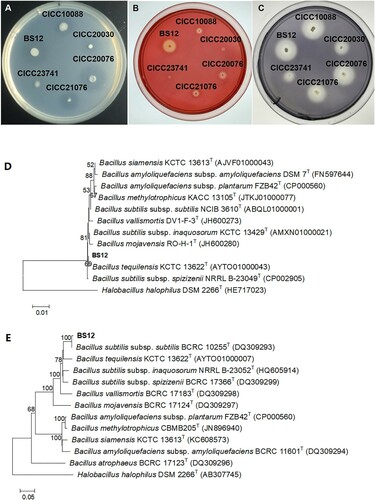
Table 3. Size of the hydrolysis zone formed by the action of enzymes by Bacillus spp.
Biodegradation of soybean antigenic proteins
BS12 and control strains were subsequently cultured on solid-state fermentation using SBM as a sole substrate. The result, shown in , reveals that five subunits of soybean antigenic proteins in SBM, including three bases of β-conglycinin and two bases of glycinin, were separated. Most of α’, α and β base of β-conglycinin and acidic base of glycinin in SBM had been degraded after BS12 fermentation (Fig.2 Lane.2). However subunits of β-conglycinin and glycinin were still remained in other five strains fermentated SBM (Fig. 2 Lane 3 to Lane 7). ELISA analysis indicated that BS12 FSBM had the lowest amounts of glycinin and β-conglycinin (23.36 and 17.26 mg/g, respectively) ().
Figure 2. The resolved proteins from SBM fermented by different strains of Bacillus spp. separated on SDS-PAGE gels. Notes: Proteins were separated on 12% SDS-PAGE gels. The resolved proteins were visualized by Coomassie Brilliant Blue (CBB) staining. Lane 1, nonfermented SBM; Lane 2, BS12 FSBM protein; Lane 3, B. subtilis CICC10088 FSBM protein; Lane 4, B. subtilis CICC20030 FSBM protein; Lane 5, B. subtilis CICC20076 FSBM protein; Lane 6, B. subtilis CICC21076 FSBM protein; and Lane 7, B. subtilis CICC23741 FSBM protein. Molecular weight markers are indicated in kDa.
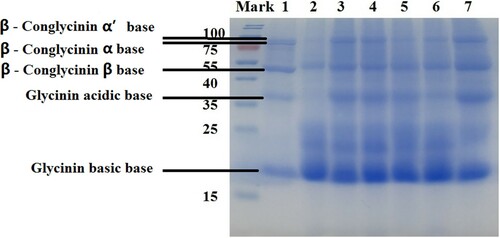
Table 4. ELISA analysis of glycinin and β-conglycinin after fermentation.
Morphology analysis of SBM
The CLSM images of SBM and FSBM are presented in (A,B), respectively. In SBM, cellulose (CW) formed an intact network-like structure, with starch (Con A) combined with the protein body of SBM, and proteins (FITC) embedded in the core of this structure ((A)). After 24 h of fermentation with BS12, the fluorescence intensity of cellulose, protein, and starch decreased and the boundary between cellulose and protein became blurred ((B)).
Chemical analysis of FSBM with BS12
Chemical compositions of SBM and FSBM are shown in . Compared with SBM, FSBM had more CP, ash, and total P. The amount of TCA-SP (<10 kDa) in FSBM was 11.83%; this amount was 4.8-fold higher than SBM. Glycinin decreased from 133.42 in SBM to 10.19 mg/g in FSBM, and β-conglycinin decreased from 113.56 to 13.13 mg/g. Trypsin inhibitor was also decreased after BS12 fermentation. However, there was no obvious difference at lysine and threonine between SBM and FSBM.
Table 5. Analyzed nutrient composition of FSBM.
Pig performance
The growth performance of the piglets is presented in . Over the entire 24-d growth trial, feed intake of piglets fed FSBM was higher than that of piglets fed the CON diet. The average daily gain of piglets in the FSBM group was superior (p < .05). However, there was no difference in feed gain ratio and diarrhea rate.
Table 6. Effects of FSBM on growth performance of piglets.
Small intestine inflammation
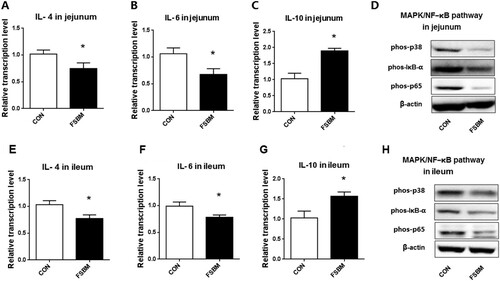
The pigs fed FSBM showed a significantly lower mRNA level of the pro-inflammatory cytokines IL-4 and IL-6 (p < .05) than did pigs in the control group (). In addition, the mRNA level of the cytokine synthesis inhibitory factor IL-10 was significantly higher in the FSBM group (p < .05). The western-blot results indicated that phosphorylation of p38 MAPK, IκB-α, and p65 NF-κB decreased in the jejunum and ileum of pigs fed FSBM.
Figure 4. Reduced inflammation in jejunum and ileum due to diet of FSBM with BS12. Notes: (A–C) Relative mRNA levels of IL-4, IL-6, and IL10 in jejunum of piglets. (D) The phosphorylation of p38 MAPK, IκB-α, and p65 NF-κ B protein in the jejunum of piglets. (E–G) Relative mRNA levels of IL-4, IL-6, and IL10 in the ileum of piglets. (H) The phosphorylation of p38 MAPK, IκB-α, and p65 NF-κB protein in the ileum of piglets. Comparisons were made between the control group, fed nonfermented SBM with antibiotics, and the experimental group, fed a diet that contained 10% FSBM and no antibiotics. Differences were determined by Student t-test. CON, control. Mean ± SEM,*p < .05, n = 6.
Discussion
In the present study, B. subtilis BS12 was screened using protein, cellulose, and starch plate tests. And BS12 was more efficient than five other strains at degrading the antigenic proteins during SBM fermentation. The SBM fermentation with B. subtilis was usually allowed to proceed for 48 or 72 h (Bi et al., Citation2015; Feng et al., Citation2007; Teng et al., Citation2012; Upadhaya & Kim, Citation2015), whereas BS12 fermentation spanned only 24 h in this study. Seo and Cho (Citation2016) investigated changes in the SBM protein profile during B. subtilis KCCM11438P fermentation. And only 50% of trypsin inhibitors were removed and 58% glycinin subunit remaining after 24 h fermentation. Compared to KCCM11438P, BS12 degraded 82.52% less trypsin inhibitors and 92.36% less glycinin in SBM. The CLSM images showed that cellulose, protein, and starch of SBM were degraded during the BS12 fermentation process ((B)). In particular, the cellulose structure of SBM, which protects proteins and starches from enzymatic attack (Wang et al., Citation2012), was destroyed by BS12 fermentation. With the degradation of cellulose, the protein and starch would be degraded easily by protease and amylase of BS12.
FSBM showed increased amounts of DM, CP, Ca, and P in the present study, consistent with previous research on FSBM (Feng et al., Citation2007). This could be explained by the consumption of sucrose and oligosaccharides in SBM during fermentation (Rojas & Stein, Citation2013). TCA-SP consists of small peptides with 2–20 residues. Di- and tripeptides in TCA-SP can be directly absorbed by the animal gut, and AA in the form of small peptides is transported more quickly than constituent AAs in free form (Gilbert, Wong, & Webb, Citation2008). In Bi et al. (Citation2015) research, the soybean peptides were increased 50% after 48 h fermentation by B. subtilis XZI125 had high protease activity. However, the amount of TCA-SP in SBM increased 4.6-fold after 24 h BS12 fermentation in the present study. The result also proved that BS12 exhibited high efficiency in SBM fermentation.
The higher nutritional value of FSBM resulted in better growth performance (feed intake and gain) of piglets, compared with the piglets in the control group, which received nonfermented SBM and antibiotics. Although previous research has shown that pigs fed a diet containing FSBM had a greater average daily gain than control, the fermentation strains and times were different. Feng et al. (Citation2007) FSBM with B. subtilis WB117 only. In the research of Jones et al. (Citation2010), the FSBM was fermented with both B. subtilis and A. oryza. And Yuan et al. (Citation2017) FSBM with Lactobacillus casei (CGMCC1.62), B. subtilis (CGMCC1.504), and Hansenula anomala (CGMCC2.881). All of the FSBM above were fermented for 48 h more than BS12 FSBM for 24 h. Furthermore, the control diet was added with kitasamycin and olaquindox in the present research. Both two antibiotics could limit pathogen proliferation in the gastrointestinal tract of pigets (Ding, Wang, Zhu, & Yuan, Citation2006; Jordan & Knight, Citation1984). The better growth performance of piglets in FSBM group proved fermented feed could contribute to compensate the use of antibiotics in pig production.
The higher feed intake of piglets in this study might due to the low content of β-conglycinin in FSBM diets. β-conglycinin is proved to suppress food intake and gastric emptying by its direct action on mucosal cells and stimulation of cholecystokin release (Nishi et al., Citation2003). It appeared to be more resistant to digestion than glycinin in piglets, leading to lower average daily gain and higher feed-to-gain ratio in piglets (Zhao et al., Citation2008). Low content of β-conglycinin in FSBM contribute to the higher feed intake, which results in better average daily gain.
Moreover, glycinin and β-conglycinin are well-known major causes for immune responses and allergic reactions in piglets (Hao et al., Citation2009; Wu et al., Citation2016). Intestinal mucosal immune responses to glycinin were enhanced by high levels of IL-4 and IL-6 (Sun, Li, Dong, et al., Citation2008). Glycinin and β-conglycinin in SBM induced mouse intestinal epithelial cells to express inflammation factors such as IL-2, IL-6, and IL-8 (Xu et al., Citation2010). In the present study, piglets fed with FSBM exhibited lower mRNA expression of inflammatory cytokines of IL-4 and IL-6 in both the jejunum and ileum than did piglets fed a diet that contained antibiotics. The result indicated that a low content of allergic protein in BS12 FSBM might contribute to a decline in mucosal immune responses.
The MAPK/NF-κB pathway played an important role in regulating inflammatory genes (Fan et al., Citation2011). p38 MAPK was a control factor upstream of the posttranscription and translation of pro-inflammatory cytokines (Newton & Holden, Citation2003).. Phosphorylated IκB-α lead p65 NF-κB activated the transcription of inflammatory cytokine genes (Schottelius & Baldwin, Citation1999). The anti-inflammatory cytokine IL-10 was reported to target the p38 MAPK, causing mRNA destabilization and translational repression (Hao et al., Citation2009). In this research, the decreased phosphorylation of key factor p38 MAPK, IκB-α, and p65 NF-κB may explain the decline in the mRNA level of IL-4 and IL-6. Anti-inflammatory factor IL-10 mRNA was up-regulated in the jejunum and ileum, further confirming that FSBM using BS12 reduced the hypersensitivity caused by antigenic proteins in the diet.
In conclusion, a highly efficient Bacillus strain BS12 was selected to eliminate glycinin and β-conglycinin in SBM through fermentation. The FSBM using BS12 had 92.36% less glycinin and 88.44% less β-conglycinin than did the original SBM. Piglets fed a diet containing 10% FSBM showed improved performance due to reduced levels of gut inflammation. The FSBM exhibited application potential for reduce of antibiotics in feed.
Acknowledgement
We would like to thank Editage (www.editage.cn) for English language editing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Yu Zhang
Yu Zhang is a PhD candidate under the guidance of Dr. Yizhen Wang. She is major in feed fermentation and fermentation strain research.
Changyou Shi
Dr. Changyou Shi is major in feed fermentation and nutrient evaluation. Her recently published articles include: Shi C, He J, Yu J, et al. Physicochemical Properties Analysis and Secretome of Aspergillus niger in Fermented Rapeseed Meal. Plos One, 2016, 11(4):e0153230; Shi C, Zhang Y, Lu Z, et al. Solid-state fermentation of cornsoybean meal mixed feed with Bacillus subtilis and Enterococcus faecium for degrading antinutritional factors and enhancing nutritional value. Journal of Animal Science & Biotechnology, 2017, 8(1):50.
Cheng Wang
Cheng Wang is a PhD candidate under the supervision of Dr. Yizhen Wang. He is major in feed fermentation and nutrient evaluation.
Zeqing Lu
Dr. Zeqing Lu was a PhD candidate under the guidance of Dr. Yizhen Wang. He is major in exploitation and utilization of feeds resources. His recent publications include: Zeqing Lu, Mingliang Jin, Ming Huang et al. Bioactivity of selenium-enriched exopolysaccharides produced by Enterobacter cloacae Z0206 in broilers. Carbohydrate Polymers, 2013, 96: 131–136; Zeqing Lu, Yang Ren, Xihong Zhou et al. Maternal dietary linoleic acid supplementation promotes muscle fibre type transformation in suckling piglets. 2016. Journal of Animal Physiology and Animal Nutrition. Accepted.
Fengqin Wang
Fengqin Wang is a PhD candidate under the supervision of Dr. Yizhen Wang. He is major in Chemical analysis of feed Resources. His recently published works include: Wang, F., Yang, H., & Wang, Y. (2013). Structure characterization of a fucose-containing exopolysaccharide produced by Enterobacter cloacae z0206. Carbohydrate Polymers, 92(1), 503–509; Wang, F., Yang, H., & Wang, Y. (2013). Simultaneous determination of neutral sugars and uronic acid constituents in a novel bacterial polysaccharide using gas chromatography-mass spectrometry. Chinese Journal of Chromatography, 31(1), 53.
Jie Feng
Dr. Jie Feng is a professor of Zhejiang Univsersity University, major in Agricultural and Biological Sciences, Medicine, Biochemistry, Genetics and Molecular Biology, Chemistry, Immunology and Microbiology. Dr. Feng’s recent work includes: Hu Q, Zhao Z, Fang S, et al. Phytosterols improve immunity and exert anti-inflammatory activity in weaned piglets. Journal of the Science of Food & Agriculture, 2017; Fang C L, Sun H, Wu J, et al. Effects of sodium butyrate on growth performance, haematological and immunological characteristics of weanling piglets. Journal of Animal Physiology & Animal Nutrition, 2014, 98(4):680; Sun, H., Liu, X., Wang, Y. Z., Liu, J. X., & Feng, J. (2013). Soybean glycinin- and β-conglycinin-induced intestinal immune responses in a murine model of allergy. Food & Agricultural Immunology, 24(3), 357–369. Sun, H., Liu, X., Wang, Y. Z., Liu, J. X., & Feng, J. (2013). Allergen-specific immunoglobulin, histamine and T-cell responses induced by soybean glycinin and β-conglycinin in balb/c mice of oral sensitisation. Food & Agricultural Immunology, 24(4), 489–501.
Yizhen Wang
Dr. Yizhen Wang is a professor at Zhejiang University and is major in Biochemistry, Genetics and Molecular Biology, Agricultural and Biological Sciences, Medicine, Immunology and Microbiology, Chemistry, Materials Science, Veterinary, Pharmacology, Toxicology and Pharmaceutics, Chemical Engineering, Nursing, Multidisciplinary, Physics and Astronomy, Engineering. Dr. Wang’s Resent research works include: Song, D., Li, X., Cheng, Y., Xiao, X., Lu, Z., & Wang, Y., et al. (2017). Aerobic biogenesis of selenium nanoparticles by enterobacter cloacae z0206 as a consequence of fumarate reductase mediated selenite reduction. Scientific Reports, 7(1), 3239; Shan, T., Liu, J., Wu, W., Xu, Z., & Wang, Y. (2016). Roles of notch signaling in adipocyte progenitor cells and mature adipocytes. Journal of Cellular Physiology, 232(6); Yi, H., Hu, W., Chen, S., Lu, Z., & Wang, Y. (2017). Cathelicidin-wa improves intestinal epithelial barrier function and enhances host defense against enterohemorrhagic escherichia coli o157:h7 infection. Journal of Immunology, 198(4), 1696–1705; Liu, J., Zhu, Y., Luo, G. Z., Wang, X., Yue, Y., & Wang, X., et al. (2016). Abundant DNA 6ma methylation during early embryogenesis of zebrafish and pig. Nature Communications, 7, 13052.
References
- Adav, S. S., Lin, J. C., Yang, Z., Whiteley, C. G., Lee, D. J., & Peng, X. F. (2010). Stereological assessment of extracellular polymeric substances, exo-enzymes, and specific bacterial strains in bioaggregates using fluorescence experiments. Biotechnology Advances, 28(2), 255–280. doi:10.1016/j.biotechadv.2009.08.006
- AOAC, International. (2000). Official methods of analysis (17th edition). Gaithersburg, MD.
- Bi, H., Zhao, H., Lu, F., Zhang, C., Bie, X., & Lu, Z. (2015). Improvement of the nutritional quality and fibrinolytic enzyme activity of soybean meal by fermentation of Bacillus subtilis. Journal of Food Processing and Preservation, 39, 1235–1242. doi:10.1111/jfpp.12340
- Chen, M. Y., Lee, D. J., & Tay, J. H. (2007). Distribution of extracellular polymeric substances in aerobic granules. Applied Microbiology & Biotechnology, 73(6), 1463–1469. doi:10.1007/s00253-006-0617-x
- Ding, M. X., Wang, Y. L., Zhu, H. L., & Yuan, Z. H. (2006). Effects of cyadox and olaquindox on intestinal mucosal immunity and on fecal shedding of Escherichia coli in piglets. Journal of Animal Science, 84(9), 2367–2373. doi:10.2527/jas.2005-564
- Egounlety, M., & Aworh, O. C. (2003). Effect of soaking, dehulling, cooking and fermentation with rhizopus oligosporus, on the oligosaccharides, trypsin inhibitor, phytic acid and tannins of soybean (glycine max, merr.), cowpea (vigna unguiculata, l. walp) and groundbean (macrotyloma geocar). Journal of Food Engineering, 56, 249–254. doi: https://doi.org/10.1016/S0260-8774(02)00262-5
- Fan, Y., Wang, J., Wei, L., He, B., Wang, C., & Wang, B. (2011). Iron deficiency activates pro-inflammatory signaling in macrophages and foam cells via the p38 MAPK-NF-κB pathway. International Journal of Cardiology, 152, 49–55. doi:10.1016/j.ijcard.2010.07.005
- Feng, J., Liu, X., Xu, Z., Lu, Y., & Liu, Y. (2007). Effect of fermented soybean meal on intestinal morphology and digestive enzyme activities in weaned piglets. Digestive Diseases and Sciences, 52, 1845–1850. doi:10.1007/s10620-006-9705-0
- Gilbert, E. R., Wong, E. A., & Webb, K. E. (2008). Peptide absorption and utilization: Implications for animal nutrition and health. Journal of Animal Science, 86, 2135–2155. doi:10.2527/jas.2007-0826
- Hankin, L., & Anagnostakis, S. L. (1977). Solid media containing carboxymethylcellulose to detect CX cellulose activity of micro-organisms. Journal of General Microbiology, 98, 109–115. doi:10.1099/00221287-98-1-109
- Hao, Y., Zhan, Z., Guo, P., Piao, X., & Li, D. (2009). Soybean β-conglycinin-induced gut hypersensitivity reaction in a piglet model. Archives of Animal Nutrition, 63, 188–202. doi:10.1080/17450390902860026
- Hong, K. J, Lee, C. H, & Kim, S. W. (2004). Aspergillus oryzae gb-107 fermentation improves nutritional quality of food soybeans and feed soybean meals. Journal of Medicinal Food, 7(4), gb–107. doi:10.1089/jmf.2004.7.430
- Jones, C. K., DeRouchey, J. M., Nelssen, J. L., Tokach, M. D., Dritz, S. S., & Goodband, R. D. (2010). Effects of fermented soybean meal and specialty animal protein sources on nursery pig performance. Journal of Animal Science, 88(5), 1725–1732. doi:10.2527/jas.2009-2110
- Jordan, F. T., & Knight, D. (1984). The minimum inhibitory concentration of kitasamycin, tylosin and tiamulin for Mycoplasma gallisepticum and their protective effect on infected chicks. Avian Pathology, 13(2), 151–162. doi:10.1080/03079458408418520
- Kim, S., Van Heugten, E., Ji, F., Lee, C., & Mateo, R. (2010). Fermented soybean meal as a vegetable protein source for nursery pigs: I. Effects on growth performance of nursery pigs. Journal of Animal Science, 88, 214–224. doi:10.2527/jas.2009-1993
- Li, D., Nelssen, J., Reddy, P., Blecha, F., Hancock, J., Allee, G., … Klemm, R. (1990). Transient hypersensitivity to soybean meal in the early-weaned pig. Journal of Animal Science, 68, 1790–1799. doi:10.2527/1990.6861790x
- Liu, C., Wang, H., Cui, Z., He, X., Wang, X., Zeng, X., & Ma, H. (2007). Optimization of extraction and isolation for 11S and 7S globulins of soybean seed storage protein. Food Chemistry, 102, 1310–1316. doi:10.1016/j.foodchem.2006.07.017
- Maruyama, N., Sato, R., Wada, Y., Matsumura, Y., Goto, H., Okuda, E., … Utsumi, S. (1999). Structure–physicochemical function relationships of soybean β-conglycinin constituent subunits. Journal of Agricultural and Food Chemistry, 47, 5278–5284. doi:10.1021/jf990360+
- National Research Council. (2012). Nutrient requirements of Swine. (11th revised ed.). Washington, DC: The National Academies Press.
- Newton, R., & Holden, N. (2003). Inhibitors of p38 mitogen-activated protein kinase. BioDrugs, 17, 113–129. doi:10.2165/00063030-200317020-00004
- Nishi, T., Hara, H., & Tomita, F. (2003). Soybean beta-conglycinin peptone suppresses food intake and gastric emptying by increasing plasma cholecystokinin levels in rats. Journal of Nutrition, 133, 352–357. doi: https://doi.org/10.1093/jn/133.2.352
- Ovissipour, M., Abedian, A., Motamedzadegan, A., Rasco, B., Safari, R., & Shahiri, H. (2009). The effect of enzymatic hydrolysis time and temperature on the properties of protein hydrolysates from Persian sturgeon (Acipenser persicus) viscera. Food Chemistry, 115, 238–242. doi:10.1016/j.foodchem.2008.12.013
- Refstie, S., Erland, B., Grete, B., & Per, K. (2005). Lactic acid fermentation eliminates indigestible carbohydrates and antinutritional factors in soybean meal for atlantic salmon (salmo salar). Aquaculture, 246, 331–345. doi: https://doi.org/10.1016/j.aquaculture.2005.01.001
- Rojas, O., & Stein, H. (2013). Concentration of digestible, metabolizable, and net energy and digestibility of energy and nutrients in fermented soybean meal, conventional soybean meal, and fish meal fed to weanling pigs. Journal of Animal Science, 91, 4397–4405. doi:10.2527/jas.2013-6409
- Schottelius, A. J. G., & Baldwin, A. S., Jr. (1999). A role for transcription factor NF-kB in intestinal inflammation. International Journal of Colorectal Disease, 14, 18–28. doi:10.1007/s003840050178
- Seo, S. H., & Cho, S. J. (2016). Changes in allergenic and antinutritional protein profiles of soybean meal during solid-state fermentation with Bacillus subtilis. LWT – Food Science and Technology, 70, 208–212. doi:10.1016/j.lwt.2016.02.035
- Sun, P., Li, D., Dong, B., Qiao, S., & Ma, X. (2008). Effects of soybean glycinin on performance and immune function in early weaned pigs. Archives of Animal Nutrition, 62, 313–321. doi:10.1080/17450390802066419
- Sun, P., Li, D., Li, Z., Dong, B., & Wang, F. (2008). Effects of glycinin on IgE-mediated increase of mast cell numbers and histamine release in the small intestine. The Journal of Nutritional Biochemistry, 19, 627–633. doi:10.1016/j.jnutbio.2007.08.007
- Teather, R. M., & Wood, P. J. (1982). Use of congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Applied & Environmental Microbiology, 43, 777–780.
- Teng, D., Gao, M., Yang, Y., Liu, B., Tian, Z., & Wang, J. (2012). Bio-modification of soybean meal with Bacillus subtilis or Aspergillus oryzae. Biocatalysis and Agricultural Biotechnology, 1, 32–38. doi:10.1016/j.bcab.2011.08.005
- Upadhaya, S. D., & Kim, I. H. (2015). Ileal digestibility of nutrients and amino acids in unfermented, fermented soybean meal and canola meal for weaning pigs. Animal Science Journal, 86, 408–414. doi:10.1111/asj.12305
- Wang, Y., Fuchs, E., daSilva, R. D., Mcdaniel, A., Seibel, J., & Ford, C. (2006). Improvement of Aspergillus niger glucoamylase thermostability by directed evolution. Starch – Stärke, 58, 501–508. doi:10.1002/star.200600493
- Wang, L.-P., Shen, Q.-R., Yu, G.-H., Ran, W., & Xu, Y.-C. (2012). Fate of biopolymers during rapeseed meal and wheat bran composting as studied by two-dimensional correlation spectroscopy in combination with multiple fluorescence labeling techniques. Bioresource Technology, 105, 88–94. doi:10.1016/j.biortech.2011.11.064
- Wu, J. J., Cao, C. M., Meng, T. T., Zhang, Y., Xu, S. L., Feng, S. B., … Wang, X. C. (2016). Induction of immune responses and allergic reactions in piglets by injecting glycinin. Italian Journal of Animal Science, 15, 166–173. doi:10.1080/1828051X.2016.1144488
- Xu, J., Zhou, A., Wang, Z., & Ai, D. (2010). Effects of glycinin and β-conglycinin on integrity and immune responses of mouse intestinal epithelial cells. JournaL of Animal and Plant Sciences, 20, 170–174.
- Yi, H., Zhang, L., Gan, Z., Xiong, H., Yu, C., Du, H., & Wang, Y. (2016). High therapeutic efficacy of Cathelicidin-WA against postweaning diarrhea via inhibiting inflammation and enhancing epithelial barrier in the intestine. Scientific Reports, 6(25679), 1–12. doi:10.1038/srep25679
- Yuan, L., Chang, J., Yin, Q., Lu, M., Di, Y., Wang, P., … Lu, F. (2017). Fermented soybean meal improves the growth performance, nutrient digestibility, and microbial flora in piglets. Animal Nutrition, 3(1), 19–24. doi:10.1016/j.aninu.2016.11.003
- Zhao, Y., Qin, G., Sun, Z., Zhang, X., Bao, N., Wang, T., … Sun, L. (2008). Disappearance of immunoreactive glycinin and β-conglycinin in the digestive tract of piglets. Archives of Animal Nutrition, 62, 322–330. doi:10.1080/17450390802190318