 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Spinosad, a neurotoxic insecticide, is widely used for crop protection. In order to elucidate the effects of spinosad on oxidative stress and genotoxicity in Sf9 cells, the levels of lipid peroxidation, the activity of antioxidative enzymes, and DNA damage were measured. The results showed that spinosad caused a time-dependent increase in the formation of malondialdehyde and decrease in the activity of superoxide dismutase and catalase. Further studies confirmed that spinosad induced 8-oxoguanine accumulation in Sf9 cells, which is accompanied by increased expression of DNA repair enzymes (OGG1 and MTH1). The neutral comet assay revealed that spinosad induced significant time-related increases of DNA double-strand breaks in Sf9 cells. Our results indicate that spinosad effectively induced oxidative stress and DNA damage in Sf9 cells.
1. Introduction
Spinosad, a neurotoxic insecticide, is produced by fermentation of soil actinomycete Saccharopolyspora spinosa (Sparks, Dripps, Watson, & Paroonagian, Citation2012). Owing to its highly selective toxicological profile, spinosad is widely used for control of crop and amenity plants, public hygiene, and veterinary pests (Böhm et al., Citation2014; Mandal, Singh, Battu, & Singh, Citation2013; Markussen & Kristensen, Citation2012). There is considerable evidence that spinosad may have oxidative damage by compromise antioxidant systems, excess reactive oxygen species (ROS) production and induce lipid peroxidation (Ahmed Citation2012; Pérez-Pertejo, Reguera, Ordóñez, & Balaña-Fouce, Citation2008; Piner & Üner, Citation2013, Citation2014). Furthemore, several studies have reported that spinosad exposure may cause DNA fragmentation and chromosomal aberration in rats (Ahmed Citation2012; Mansour, Mossa, & Heikal, Citation2008).
Oxidative stress can be defined as the state of imbalance between prooxidant challenge and antioxidant systems, attributing to the overproduction of ROS that exceed the capacity of antioxidant defense systems (Baysal et al., Citation2016; Li, Jiang, Cao, Xie, & Huang, Citation2017). Various environmental stresses are known to enhance the ROS production, such as ultraviolet radiation, heavy metals and pesticide remnant, disturbing the cellular redox homeostasis (Ma et al., Citation2014; Shahid et al., Citation2014; Thévenod, Friedmann, Katsen, & Hauser, Citation2000). Increase of ROS elicits a wide variety of physiological and cellular events, including lipid peroxidation, antioxidants’ depletion, and DNA damage (Chen et al., Citation2012; She et al., Citation2016; Wang, Martínez, et al., Citation2016). The cellular antioxidant enzymes mainly include superoxide dismutase (SOD), catalase (CAT), and glutathione S-transferases (GSTs), which play a crucial role in scavenging ROS, but overexposure causes their depletion, leading to disruption of core metabolic and regulatory process (Marin & Taranu, Citation2012; Mejdoub, Fahde, Loutfi, & Kabine, Citation2017). They are involved in a number of different defense mechanisms against oxidative stress. For example, SOD converts superoxide radical () to peroxide (H2O2), and CAT detoxify peroxide to oxygen and water (Li et al., Citation2016). GSTs may catalyze the conjugate reaction of glutathione (GSH) to a large variety of electrophilic compounds, thereby protecting the cell components from oxidative damage (Townsend et al., Citation2009).
ROS-mediated oxidative DNA damage primarily includes base lesions and DNA strand breaks (Yeh et al., Citation2016). Among the various types of oxidative lesions, 8-oxoguanine (8-oxoG) is one of the major oxidized base in both nucleotide pools and polymerized DNA, which is strongly mutagenic as it can mispair with adenine residues instead of the cysteine residues, leading to GC to AT transversion mutations (Leon et al., Citation2016). 8-oxoG-DNA glycosylase (OGG1) excises 8-oxoG paired with cytosine in DNA, and 8-oxoG nucleoside triphosphatase (MTH1) hydrolyzes 8-oxo–dGTP in nucleotide pools, avoid incorporation of 8-oxo-dGMP into DNA, thus preventing the accumulation of oxidative DNA damage (Sheng et al., Citation2012). DNA damage that is characterized by DNA strand breaks and chromosomal instability can lead to chromosome mis-segregation during mitosis and generate micronuclei (Bakhoum, Kabeche, Murnane, Zaki, & Compton, Citation2014).
Our previous studies showed that spinosad exposure induced mitochodrial dysfunction, ROS overproduction, and chromatin condensation in Sf9 cells (Yang et al., Citation2017). The aims of this study were to examine the effects of spinosad on lipid peroxidation and antioxidant defensive system, including SOD, CAT, and GST. To gain insight into the mechanism of oxidative DNA damage, we analyzed the accumulation of 8-oxoG and the expression of the DNA repairase. Additionally, the genotoxicity of spinosad in Sf9 cells was studied by the comet assay. The results show that spinosad induces antioxidant responses in Sf9 cells, which was accompanied by increased DNA damage.
2. Materials and methods
2.1. Chemicals and reagents
Spinosad (98% purity), 4′,6-diamidino-2-phenylindole (DAPI), N,N,N′,N′-tetramethylethylenediamine (TEMED), dimethylsulfoxide (DMSO), 2-amino-2-(hydroxymethyl)-1,3-propanediol (Tris) were purchased from Sigma-Aldrich Co., LLC. (St. Louis, MO, USA). Polyacrylamide (30%) was obtained from Shanghai Sangon Biotech Co., Ltd. (Shanghai, China). Stock solution of spinosad was prepared in DMSO and diluted into the culture media to the desired concentrations. The final concentration of DMSO in the cell culture media was <0.1%.
SFX-insect cell culture medium, fetal bovine serum (FBS), penicillin–streptomycin were purchased from Thermo Fisher Scientific Inc (Grand Island, NY, USA). 8-oxoG antibody was purchased from Abcam (Cambridge, MA, USA), and OGG1, MTH1 were purchased from Abgent (Suzhou, China). Antibody against β-Actin was purchased from Absci (Boston, MA, USA). Secondary antibodies conjugated with horseradish peroxidase (HRP) were obtained from Sangon (Shanghai, China). Enhanced chemiluminescence substrate solution was obtained from Tanon Science & Technology Co., Ltd. (Shanghai, China). All other reagents and chemicals used were of analytical grade and purchased locally.
2.2. Cell culture conditions
Sf9 cells were cultured at 28°C in SFX-insect cell culture medium supplemented with 10% FBS, penicillin (100 U/mL), and streptomycin (100 μg/mL). The cells were maintained in exponential and asynchronous phase of growth by replacing with fresh culture media every two to three days.
2.3. Biochemical analysis
Sf9 cells were treated with 60 μM spinosad for 0, 6, 12, 18, and 24 h. The cells were collected and lysed in RIPA lysis buffer, the lysate were centrifuged at 12,000 g for 10 min at 4°C to precipitate cell debris, and the supernatants were collected and stored at −20°C. The protein concentrations were determined using BCA Protein Assay Kit (Pierce, Rockford, IL, USA).
The content of malondialdehyde (MDA) was measured with a thiobarbituric acid (TBA) substances assay kit (Beyotime, Jiangsu, China). MDA could react with TBA to form a red adduct, which has a maximum absorption at 535 nm. The content of MDA was determined according to a standard curve.
The SOD activity was asssyed using the SOD assay kit by Beyotime Biotechnology (Jiangsu, China). The assay uses nitroblue tetrazolium chloride (NBT) for detection of superoxide anion radicals generated by xanthine oxidase. The reduction of NBT to diformazan was examined following the increase in absorption at 560 nm. One unit of SOD activity was defined as the amount of enzyme that exhibited half dismutation of the superoxide anion radical.
The CAT activity was determined using H2O2 as a substrate, following the protocol of commercial kits (Beyotime, Jiangsu, China). Briefly, the excess of H2O2 was decomposed by catalase for 5 min, and residual H2O2 coupled with a substrate was catalyzed by hydrogen peroxidase to generate a product (N-4-antipyryl-3-chloro-5-sulfonate-p-benzoquinonemonoimine) that absorbs maximally at 520 nm. Absorbance of the product vs. concentraion of H2O2 standard curve provides catalase activity.
The determination of GST activity was revised according to the method described in the paper by Goutzourelas et al. (Citation2015). In short, 5 µl of GSH (10 mM) and 50 µl of 1-chloro-2,4-dinitrobenzene (CDNB) was mixed in 25 µl of phosphate buffer (100 mM, pH 7.4). This was followed by the addition of 20 μl of cytosolic lysate. The changes in absorbance were recorded at 340 nm for 5 min. The GST activity was expressed as micromole of CDNB conjugate produced per minute per milligram of total protein.
2.4. Immunofluorescence assay
Immunofluorescence staining was performed to identify the DNA oxidative lesions (Ohno, Oka, & Nakabeppu, Citation2009). Sf9 cells were seeded in glass bottom dishes incubation for 24 h. After treatment with 60 μM spinosad for different durations, cells were fixed with 4% formaldehyde in PBS for 15 min, washed thrice with PBS, and permeabilized with 1% Triton X-100 for 15 min, blocked with 5% BSA in PBS for 1 h and incubated with anti-8-oxoG antibody (1:100) overnight at 4°C. After washing, cells were incubated with Alexa Fluor 488-conjugated secondary antibody (1:1000) for 1 h at room temperature. After washing again in PBS, cells were stained with DAPI (1 μg/mL in PBS) (Sigma), and localization of 8-oxoG and nuclei was performed by fluorescence microscopy, using rhodamine, FITC and UV filters, respectively.
2.5. Western blot analysis
To explore the underlying mechanisms of spinosad-induced DNA oxidative lesions, OGG1 and MTH1 protein expressions were analyzed using western blot analysis. After treatment with 60 μM spinosad for different durations, cells were washed twice with PBS and lysed in RIPA lysis buffer containing protease inhibitor cocktail (Sigma, St. Louis, USA). Equal amounts of protein were separated on SDS-PAGE gels and transferred to polyvinylidene-difluoride (PVDF) membranes. After blocking for 2 h with 5% nonfat milk in TBST, the membranes were incubated overnight at 4°C with primary antibodies. Target proteins were detected using the appropriate HRP-conjugated secondary antibodies (1:5000 dilution) and enhanced chemiluminescence reagent (Tanon, Shanghai, China).
2.6. Neutral comet assay
The neutral comet assay described by Wakabayashi, Ishii, Hatakeyama, Inoue, and Tanaka (Citation2010) was modified for detecting DNA double-strand breaks. Briefly, Sf9 cells were treated with 60 μM spinosad for various durations, and the cells were washed and resuspended in PBS. Microscope slides were precoated with 0.8% normal melting point agarose and solidified at 4°C. Cells suspension were mixed with 1% low melting point agarose and layered on the precoated microscopic slides. After solidification, the slides were immersed in the lysis buffer (100 mmol/L Na2EDTA, 2.5 mol/L NaCl, 10 mmol/L Tris-HCl, 1% Triton X-100 and 10% DMSO, pH 10) for 2 h at 4°C in the dark. The slides were washed gently with distilled water and equilibrated in electrophoresis buffer (1 × TAE (Tris base, Acetic acid and EDTA)) for 20 min. An electric field was then applied at 20 V (1 V/cm) and 300 mA for 10 min. Slides were washed and stained with 20 μg/mL PI solution. Single cell images were captured with a fluorescence microscope (Leica, Wetzlar and Mannheim, Gemany). At least 100 cells were randomly selected from each group and analyzed using the Comet Assay Software Project (CASP).
2.7. Statistical analysis
Data were shown as averages ± standard deviation of the three independent experiments. Data were analyzed by one-way analysis of variance (ANOVA) followed by a t-test, and p-values less than .05 were considered statistically significant.
3. Results
3.1. Effect of spinosad on MDA content and SOD, CAT and GST activities
MDA is one of the final products of polyunsaturated fatty acids, which is widely used as an indicator of lipid peroxidation (Zhang, Chen, Sun, & Zhao, Citation2014). In Sf9 cells under treatment with 60 μM spinosad, the MDA concentration increased within 6 h, peaked at 12 h after spinosad exposure, and thereafter started gradually decreasing ((A)). These results indicated that spinosad enhanced lipid peroxidation, and MDA was eliminated along with the extending of time.
Figure 1. Effect of spinosad on the content of MDA (A) and the activities of SOD (B), CAT (C) and GST (D) in Sf9 cells. The data shown are the mean from three independent experiments. Each value is the mean ± SD of three determinations. *p < .05, **p < .01 vs. control group.
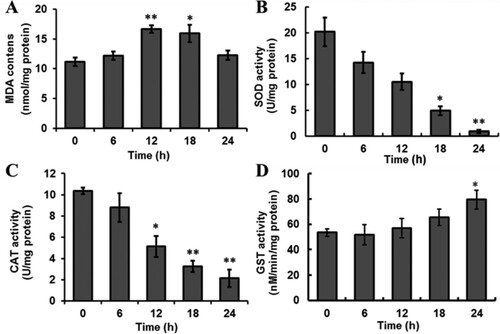
The activitives of antioxidant enzymes (including SOD, CAT and GSTs) are indirect indicators of oxidative stress. The activity of SOD and CAT were decreased in a time-dependent manner in the Sf9 cells treated with spinosad ((B,C)). The results indicated that treatment with spinosad stimulated the response of antioxidant defenses and resulted in the oxidative damage of biomacromolecule.
The effect of spinosad on the GSTs activity of Sf9 cells is shown in (D). After exposure to spinosad, all treatments showed an increase in GSTs activity in comparison to the control; however, these changes were not statistically significant (p > .05) until after 24 h spinosad exposure.
3.2. Spinosad exposure increased accumulation of 8-oxoG and the expression of DNA repair proteins
In order to evaluate the extent of oxidative damage of DNA, we used an anti-8-oxoG antibody to perform immunofluorescence microscopy. As shown in (A), the Sf9 cells were treated with 60 μM spinosad for indicated durations, which clearly demonstrate that spinosad can indeed induce 8-oxoG accumulation. With increasing incubation times, the fluorescence was weakened, indicating that the accumulation of 8-oxoG in the genomic DNA was reduced.
Figure 2. Spinosad exposure increased accumulation of 8-oxoG and the expression of DNA repair proteins. Sf9 cells were treated with 60 μM spinosad at the indicated durations, (A) anti-8-oxoG monoclonal antibody was used to detect 8-oxoG and DAPI was for nuclei staining. (B) Expression of OGG1 and MTH1 in Sf9 cells after exposure to spinosad, β-actin was used for loading control. (C) Densitometry evaluation of three independent experiments was carried out. The data shown are the mean from three independent experiments. Each value is the mean ± SD of three determinations. *p < .05, **p < .01 vs. control group.
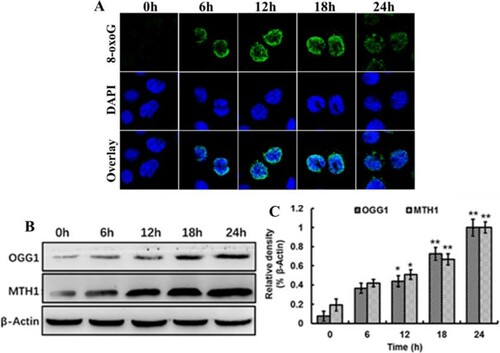
DNA damage caused by free radicals is associated with mutation, which is repaired mainly via the base excision repair mechanize with many repair enzymes. The OGG1 and MTH1 proteins are well-studied repair enzymes that hydrolyses oxidized bases, thus preventing their incorporation into DNA (Carter et al., Citation2015). Treatment of cells with spinosad resulted in a time-dependent accumulation of OGG1 and MTH1 protein, which was positively related to 8-oxoG contents ((B)). These results indicated that OGG1 and MTH1 proteins are responsible for the excision of 8-oxoG.
3.3. Spinosad induces double-strand breaks
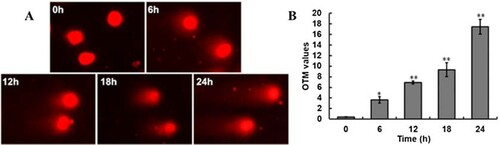
The comet assay is a rapid, sensitive, and relatively simple method for analyzing DNA damage at the level of individual cell (She et al., Citation2016). The amount of DNA that migrates away from the nuclei is used to assess the extent of DNA damage. Treatment of Sf9 cells with 60μM spinosad resulted in an extremely significant increase in DNA migration, the DNA fragments diffused in the agarose, forming a tail in comet tail. (A) shows the comet images for Sf9 cells; the comet tails were observed to be lengthened in a time-dependent manner. The control group cells showed a nucleoid core with zero or minimal DNA migration. The olive tail moment (OTM) values of neutral comet assay in Sf9 cells showed significant increases (p < .01) after 12 h, compared to the control ((B)).
Figure 3. Spinosad induces DNA damage in Sf9 cells. (A) Spinosad-induced double DNA strand break in Sf9 cells. The migration of DNA fragments were measured by the neutral comet assay; (B) the OTM values of neutral comet assay. The data are presented as mean ± SD of three independent experiments performed with triplicate measurements.*, **p < .05, .01 vs. control of the same treatment group.
4. Discussion
Spinosad was previously thought to be a reduced-risk biopesticide by United States Environmental Protection Agency due to its selective toxicity to non-target species and the lower potential for environmental damage, which is widely used in agriculture and stockbreeding (Kirst, Citation2010). Until now, spinosad has been shown to be non-target toxicity, the molecular mechanism has been paying more and more attention (Biondi et al., Citation2012). Our previous study has found that spinosad could cause mitochodrial dysfunction and ROS overproduction in Sf9 cells. In this study, spinosad could disturb redox status, enhance 8-oxoG and DNA oxidation impairment repair enzymes’ production, and lead to DNA strand break in Sf9 cells.
Under conditions of oxidative stress, ROS altered the chemical properties of biomolecules, which causes oxidative insults such as proteins denaturation, lipids peroxidation and nucleic acids’ oxidation (Lee, Huang, & Shyur, Citation2013). Cells present a variety of defense mechanisms to neutralize the oxidative damage; antioxidant enzymes SOD and CAT constitute the first line of defense against the toxic effects of superoxide radicals (Zhang, Wang, Sun, Fang, & Tang, Citation2016). In spinosad-treated Sf9 cells, we observed a significant decrease in SOD and CAT activities ((B,C)). The inhibition of the SOD or CAT activity by pesticides has been reported in various studies (Du et al., Citation2015; Wang, Wang, Wang, Zhu, & Wang, Citation2016). Histidine and arginine are often found in the active sites of enzymes, which have an unpaired electron each and are susceptible to radical damage (Asha, Mathew, & Lakshmanan, Citation2012). In our case, free radical damage of the active sites of antioxidant enzymes might be a possible cause for the decline in enzymes’ activity of spinosad-treated Sf9 cells. GST is a key enzyme of cellular detoxification systems, which is important for antioxidant protection and xenobiotic metabolism (Gweshelo, Muswe, & Mukanganyama, Citation2016). In the present study, the GST activity was increased in Sf9 cells that were exposed to both spinosad concentrations ((D)). Similarly, spinosad-induced decrease in the activity of GST was observed in vivo and in vitro (Pérez-Pertejo et al., Citation2008; Piner & Üner, Citation2014). Lipid peroxidation is considered to be a indicator of the oxidative damage of the cellular components. The data presented here show that the short-term exposure of the cells to spinosad promoted an increase of MDA in the cells ((A)), which probably can be ascribed to excessive ROS production.
The mechanism causing DNA damage may be associated with the generation of free radicals as a result of oxidative stress. The radicals can directly react with DNA, or may indirectly act as signaling molecules to mediate DNA damage. Due to its low redox potential, guanine is the primary target of oxidation in DNA, which is a well-characterized marker for oxidative stress-induced DNA damage (Lee et al., Citation2013). We observed that increased 8-oxoG levels in the DNA of Sf9 cells after exposure to spinosad, and accompanied by upregulation of OGG1 and MTH1 expression(). This observation suggests that spinosad induces oxidative stress, resulting in oxidative DNA damage in Sf9 cells; both OGG1 and MTH1 proteins are required to maintain genetic integrity.
Several investigations had proved that spinosad treatments can induce obvious DNA damage, such as chromosomal aberration, micronucleus frequency, and DNA fragmentation (Ahmed Citation2012; Mansour et al., Citation2008; Uggini & Suresh, Citation2013). DNA strand breaks are considered to be critical primary lesions, which can result in genomic instability and eventually cell death (Halaby et al., Citation2013). In this study, we observed spinosad-induced DNA double-strand breaks in Sf9 cells ((A)). The mechanism causing DNA damage maybe associated with the generation of free radicals as a result of oxidative stress. The radicals can directly react with DNA, or may indirectly act as signaling molecules to mediate DNA damage.
In conclusion, our results demonstrated that spinosad induced oxidative stress with subsequent DNA damage and chromosomal breakage in Sf9 cells, suggesting that spinosad might be genotoxic.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Wenping Xu
Wenping Xu is a Ph.D. in Microbiology. Presently, he is working as a teacher and researcher at the School of Pharmacy of the East China University of Science and Technology in P.R. China. His research interest is in microbial pesticides discovery.
Mingjun Yang
Mingjun Yang is a Ph.D. in Toxicology. Presently, he is working as a post doctoral at Shanghai Institute of Planned Parenthood Research in P.R. China. His research interest is in drug toxicology.
Jufang Gao
Jufang Gao is a Ph.D. in Physiology. Presently, she is working as a teacher and researcher at the College of Life and Environmental Sciences of the Shanghai Normal University in P.R. China. Her research interest is in the structure and function of bioactive substances.
Yang Zhang
Yang Zhang is a Ph.D. in Toxicology. Presently, he is working as a post doctoral at the School of Pharmacy of the East China University of Science and Technology in P.R. China. His research interest is in pesticide toxicology
Liming Tao
Liming Tao is a Ph.D. in Zoology. Presently, he is working as a teacher and researcher at the School of Pharmacy of the East China University of Science and Technology in P.R. China. His research interest includes Biopesticide Innovation and Pesticide Toxicology.
References
- Ahmed, M. A.-E. (2012). Hepatoprotective effects of antioxidants against non-target toxicity of the bio-insecticide spinosad in rats. African Journal of Pharmacy and Pharmacology, 6(8), 550–559.
- Asha, K. K., Mathew, S., & Lakshmanan, P. T. (2012). Flavonoids and phenolic compounds in two mangrove species and their antioxidant property. Indian Journal of Geo-Marine Sciences, 41(3), 259–264.
- Bakhoum, S. F., Kabeche, L., Murnane, J. P., Zaki, B. I., & Compton, D. A. (2014). DNA-damage response during mitosis induces whole-chromosome missegregation. Cancer Discovery, 4(11), 1281–1289. doi:10.1158/2159-8290.CD-14-0403
- Baysal, E., Gulsen, S., Aytac, I., Celenk, F., Ensari, N., Taysi, S., … Kanlikama, M. (2016). “Oxidative stress in otosclerosis.” Redox Report, 1–5.
- Böhm, C., Schnyder, M., Thamsborg, S. M., Thompson, C. M., Trout, C., Wolken, S., & Schnitzler, B. (2014). Assessment of the combination of spinosad and milbemycin oxime in preventing the development of canine Angiostrongylus vasorum infections. Veterinary Parasitology, 199(3–4), 272–277. doi:10.1016/j.vetpar.2013.10.024
- Biondi, A., Mommaerts, V., Smagghe, G., Viñuela, E., Zappalà, L., & Desneux, N. (2012). The non-target impact of spinosyns on beneficial arthropods. Pest Management Science, 68(12), 1523–1536. doi:10.1002/ps.3396
- Carter, M., Jemth, A.-S., Hagenkort, A., Page, B. D. G., Gustafsson, R., Griese, J. J., … Stenmark, P. (2015). Crystal structure, biochemical and cellular activities demonstrate separate functions of MTH1 and MTH2. Nature Communications, 6, 7871. doi:10.1038/ncomms8871
- Chen, J., Sun, H., Sun, A., Hua Lin, Q., Wang, Y., & Tao, X. (2012). Studies of the protective effect and antioxidant mechanism of blueberry anthocyanins in a CC14-induced liver injury model in mice. Food and Agricultural Immunology, 23(4), 352–362. doi:10.1080/09540105.2011.634378
- Du, L., Li, G., Liu, M., Li, Y., Yin, S., Zhao, J., & Zhang, X. (2015). Evaluation of DNA damage and antioxidant system induced by di-n-butyl phthalates exposure in earthworms (Eisenia fetida). Ecotoxicology and Environmental Safety, 115, 75–82. doi:10.1016/j.ecoenv.2015.01.031
- Goutzourelas, N., Stagos, D., Housmekeridou, A., Karapouliou, C., Kerasioti, E., Aligiannis, N., … Kouretas, D. (2015). Grape pomace extract exerts antioxidant effects through an increase in GCS levels and GST activity in muscle and endothelial cells. International Journal of Molecular Medicine, 36(2), 433–441. doi: https://doi.org/10.3892/ijmm.2015.2246
- Gweshelo, D., Muswe, R., & Mukanganyama, S. (2016). In vivo and in vitro inhibition of rat liver glutathione transferases activity by extracts from Combretum zeyheri (Combretaceae) and Parinari curatellifolia (Chrysobalanaceae). BMC Complementary and Alternative Medicine, 16(1), 2. doi:10.1186/s12906-016-1235-5
- Halaby, M. J., Hakem, A., Li, L., El Ghamrasni, S., Venkatesan, S., Hande, P. M., … Hakem, R. (2013). Synergistic interaction of Rnf8 and p53 in the protection against genomic instability and tumorigenesis. PLoS Genetics, 9(1), e1003259. doi:10.1371/journal.pgen.1003259
- Kirst, H. A. (2010). The spinosyn family of insecticides: Realizing the potential of natural products research. The Journal of Antibiotics, 63(3), 101–111. doi:10.1038/ja.2010.5
- Lee, W.-L., Huang, J.-Y., & Shyur, L.-F. (2013). Phytoagents for cancer management: Regulation of nucleic acid oxidation, ROS, and related mechanisms. Oxidative Medicine and Cellular Longevity, 2013, 925804. doi:10.1155/2013/925804
- Leon, J., Sakumi, K., Castillo, E., Sheng, Z., Oka, S., & Nakabeppu, Y. (2016). 8-Oxoguanine accumulation in mitochondrial DNA causes mitochondrial dysfunction and impairs neuritogenesis in cultured adult mouse cortical neurons under oxidative conditions. Scientific Reports, 6, 111. doi:10.1038/srep22086
- Li, W., Jiang, B., Cao, X., Xie, Y., & Huang, T. (2017). Protective effect of lycopene on fluoride-induced ameloblasts apoptosis and dental fluorosis through oxidative stress-mediated Caspase pathways. Chemico-Biological Interactions, 261, 27–34. doi:10.1016/j.cbi.2016.11.021
- Li, Y., Wei, L., Cao, J., Qiu, L., Jiang, X., Li, P., … Diao, X. (2016). Oxidative stress, DNA damage and antioxidant enzyme activities in the pacific white shrimp (Litopenaeus vannamei) when exposed to hypoxia and reoxygenation. Chemosphere, 144, 234–240. doi:10.1016/j.chemosphere.2015.08.051
- Mandal, K., Singh, S., Battu, R. S., & Singh, B. (2013). An overview of persistence of spinosad in biotic and abiotic components of the environment and advances in its estimation techniques. Bulletin of Environmental Contamination and Toxicology, 90(4), 405–413. doi:10.1007/s00128-012-0913-3
- Mansour, S. A., Mossa, A. H., & Heikal, T. M. (2008). Cytogenetic and hormonal alteration in rats exposed to recommended “safe doses” of spinosad and malathion insecticides. International Journal of Agriculture and Biology, 10(1), 9–14.
- Marin, D. E., & Taranu, I. (2012). Overview on aflatoxins and oxidative stress. Toxin Reviews, 31(3-4), 32–43. doi:10.3109/15569543.2012.730092
- Markussen, M. D., & Kristensen, M. (2012). Spinosad resistance in female Musca domestica L. from a field-derived population. Pest Management Science, 68(1), 75–82. doi:10.1002/ps.2223
- Ma, H., Wallis, L. K., Diamond, S., Li, S., Canas-Carrell, J., & Parra, A. (2014). Impact of solar UV radiation on toxicity of ZnO nanoparticles through photocatalytic reactive oxygen species (ROS) generation and photo-induced dissolution. Environmental Pollution, 193, 165–172. doi:10.1016/j.envpol.2014.06.027
- Mejdoub, Z., Fahde, A., Loutfi, M., & Kabine, M. (2017). Oxidative stress responses of the mussel Mytilus galloprovincialis exposed to emissary’s pollution in coastal areas of Casablanca. Ocean & Coastal Management, 136, 95–103. doi:10.1016/j.ocecoaman.2016.11.018
- Ohno, M., Oka, S., & Nakabeppu, Y. (2009). Quantitative analysis of oxidized guanine, 8-oxoguanine, in mitochondrial DNA by immunofluorescence method. In J. A. Stuart (Ed.), Mitochondrial DNA: Methods and protocols (pp. 199–212). Totowa, NJ: Humana Press.
- Piner, P., & Üner, N. (2013). Oxidative stress and apoptosis was induced by bio-insecticide spinosad in the liver of Oreochromis niloticus. Environmental Toxicology and Pharmacology, 36(3), 956–963. doi:10.1016/j.etap.2013.08.009
- Piner, P., & Üner, N. (2014). Organic insecticide spinosad causes in vivo oxidative effects in the brain of Oreochromis niloticus. Environmental Toxicology, 29(3), 253–260. doi:10.1002/tox.21753
- Pérez-Pertejo, Y., Reguera, R. M., Ordóñez, D., & Balaña-Fouce, R. (2008). Alterations in the glutathione-redox balance induced by the bio-insecticide spinosad in CHO-K1 and vero cells. Ecotoxicology and Environmental Safety, 70(2), 251–258. doi:10.1016/j.ecoenv.2007.06.009
- Shahid, M., Pourrut, B., Dumat, C., Nadeem, M., Aslam, M., & Pinelli, E. (2014). Heavy-metal-induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants. In D. M. Whitacre, Reviews of environmental contamination and toxicology (Vol. 232, pp. 1–44). Cham: Springer.
- Sheng, Z., Oka, S., Tsuchimoto, D., Abolhassani, N., Nomaru, H., Sakumi, K., … Nakabeppu, Y. (2012). 8-Oxoguanine causes neurodegeneration during MUTYH-mediated DNA base excision repair. Journal of Clinical Investigation, 122(12), 4344–4361. doi:10.1172/JCI65053
- She, X., Wang, F., Ma, J., Chen, X., Ren, D., & Lu, J. (2016). In vitro antioxidant and protective effects of corn peptides on ethanol-induced damage in HepG2 cells. Food and Agricultural Immunology, 27(1), 99–110. doi:10.1080/09540105.2015.1079597
- Sparks, T. C., Dripps, J. E., Watson, G. B., & Paroonagian, D. (2012). Resistance and cross-resistance to the spinosyns – A review and analysis. Pesticide Biochemistry and Physiology, 102(1), 1–10. doi:10.1016/j.pestbp.2011.11.004
- Thévenod, F., Friedmann, J. M., Katsen, A. D., & Hauser, I. A. (2000). Up-regulation of multidrug resistance P-glycoprotein via nuclear factor-κB activation protects kidney proximal tubule cells from cadmium- and reactive oxygen species-induced apoptosis. Journal of Biological Chemistry, 275(3), 1887–1896. doi:10.1074/jbc.275.3.1887
- Townsend, D. M., Manevich, Y., He, L., Hutchens, S., Pazoles, C. J., & Tew, K. D. (2009). Novel role for glutathione S-transferase π: Regulator of protein S-glutathionylation following oxidative and nitrosative stress. Journal of Biological Chemistry, 284(1), 436–445. doi:10.1074/jbc.M805586200
- Uggini, G. K., & Suresh, B. (2013). Genotoxic effects of two different classes of insecticide in developing chick embryos. Toxicological and Environmental Chemistry, 95(6), 992–1005. doi:10.1080/02772248.2013.828888
- Wakabayashi, M., Ishii, C., Hatakeyama, S., Inoue, H., & Tanaka, S. (2010). ATM and ATR homologes of Neurospora crassa are essential for normal cell growth and maintenance of chromosome integrity. Fungal Genetics and Biology, 47(10), 809–817. doi:10.1016/j.fgb.2010.05.010
- Wang, X., Martínez, M. A., Wu, Q., Ares, I., Martínez-Larrañaga, M. R., Anadón, A., & Yuan, Z. (2016). Fipronil insecticide toxicology: Oxidative stress and metabolism. Critical Reviews in Toxicology, 46(10), 876–899. doi:10.1080/10408444.2016.1223014
- Wang, J., Wang, J., Wang, G., Zhu, L., & Wang, J. (2016). DNA damage and oxidative stress induced by imidacloprid exposure in the earthworm Eisenia fetida. Chemosphere, 144, 510–517. doi:10.1016/j.chemosphere.2015.09.004
- Yang, M., Wang, B., Gao, J., Zhang, Y., Xu, W., & Tao, L. (2017). Spinosad induces programmed cell death involves mitochondrial dysfunction and cytochrome C release in Spodoptera frugiperda Sf9 cells. Chemosphere, 169, 155–161. doi:10.1016/j.chemosphere.2016.11.065
- Yeh, Y.-T., Hsu, Y.-N., Huang, S.-Y., Lin, J.-S., Chen, Z.-F., Chow, N.-H., … Su, S.-J. (2016). Benzyl isothiocyanate promotes apoptosis of oral cancer cells via an acute redox stress-mediated DNA damage response. Food and Chemical Toxicology, 97, 336–345. doi:10.1016/j.fct.2016.09.028
- Zhang, S., Chen, J., Sun, A., & Zhao, L. (2014). Protective effects and antioxidant mechanism of bamboo leaf flavonoids on hepatocytes injured by CCl4. Food and Agricultural Immunology, 25(3), 386–396. doi:10.1080/09540105.2013.810709
- Zhang, J., Wang, Y., Sun, K.-M., Fang, K., & Tang, X. (2016). A study of oxidative stress induced by two polybrominated diphenyl ethers in the rotifer Brachionus plicatilis. Marine Pollution Bulletin, 113(1–2), 408–413. doi:10.1016/j.marpolbul.2016.10.032
