?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
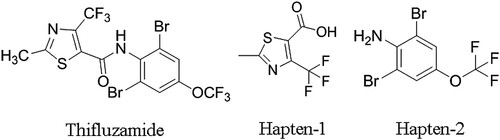
Haptens 2-Methyl-4-(trifluoromethyl)thiazole-5-carboxylic acid and 2,6-Dibromo-4-(trifluoromethoxy)aniline, the two moieties of thifluzamide, were conjugated with carrier proteins for the synthesis of artificial antigens. Two distinct anti-thifluzamide polyclonal antibodies (PAb-1 and PAb-2) were produced from the immunized female Balb/c mice. The indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) in two formats based on the PAbs was developed for thifluzamide analysis. The concentration of 50% inhibition (IC50) of ELISA-1 was 1.39 mg L−1 and its limit of detection (LOD) was 0.082 mg L−1. Meanwhile, ELISA-2 had a similar IC50 of 1.96 mg L−1 and a LOD of 0.074 mg L−1 as ELISA-1. Both the raised PAbs exhibited high specificity to thifluzamide. The recoveries for spiked samples including water and wheat ranged from 72.0% to 128.4%, and the accuracy of ELISA was confirmed by high-performance liquid chromatography. In summary, the ic-ELISA might be a promising tool for simple, sensitive and rapid detection of thifluzamide residues in real samples.
Introduction
Pesticides are widely used in modern agronomic managements to increase the grain yield. Due to the large use of pesticides, weeds and pests are more resistant than before. Moreover, pesticides residues largely exist in the environment and pose a threat to the health of human beings (Abhilash & Singh, Citation2009; Hoferkamp, Hermanson, & Muir, Citation2010; Rekha, Naik, & Prasad, Citation2006). Thifluzamide, as a class of the thiazolylamide fungicide, has been widely applied to agriculture with high systemicity and long-last persistence against numerous plant pathogenic funguses such as Rhizoctonia solani (Chen, Zhang, Wang, Zhang, & Gao, Citation2012). Although detrimental effects were not observed when thifluzamide was sprayed in a certain dose, the residues inevitably exist in edible plants (Wei, Wu, Wang, & Yang, Citation2015) and are released into environment (Gupta & Gajbhiye, Citation2004) with the heavy utilization of this fungicide, which probably jeopardizes customers. In Japan and Korea, the limits for the sum of thifluzamide in rice were formulated at 0.5 and 0.1 mg kg−1, respectively. Therefore, it is significant to develop an effective method to detect and quantify the thifluzamide residues in the environment.
Currently, several chemical detection methods for thifluzamide have been established, including gas chromatograph (GC) (Wu, Wang, Li, & Chen, Citation2006), high-performance liquid chromatography (HPLC) (Guo, Ding, & Xu, Citation2014) and high-performance liquid chromatography-mass spectrometry (HPLC-MS) (Ma, Chen, Zhao, & Zhan, Citation2015). Although these chromatographic techniques perform well with low limits of detection, they require a high dependency on sophisticated apparatus and are not ideal for detecting a large number of samples. Comparatively speaking, enzyme-linked immunosorbent assay (ELISA) is a simple, rapid, economical and sensitive analytical tool to fulfil the needs of quantitative analysis of substance and high-throughput detection. It is not unusual to develop ELISA for pesticide monitoring with the acquisition of special antibody. However, almost all pesticides lack immunogenicity because of their low molecular weight (less than 1000 Da) and the synthesis of artificial complete antigen by conjugating micromolecular pesticide to carrier protein is a prerequisite for ELISA establishment (Dintzis, Okajima, Middleton, Greene, & Dintzis, Citation1989).
Rejeb et al. (Citation2001) had developed immunoaffinity columns to extract thifluzamide from peanuts but peripherally referred to the preparation process of antibodies. To date, there are few reports on ELISA for thifluzamide based on the production of antibody. The moiety, which is the special or typical part structure of the analyte, can be used as hapten when designing the synthesis strategy (Liu et al., Citation2007). Thifluzamide molecule owns no active functional group structurally, whereas 2-Methyl-4-(trifluoromethyl)thiazole-5-carboxylic acid and 2,6-Dibromo-4-(trifluoromethoxy)aniline (two moieties of thifluzamide) bear a carboxyl and an amidogen respectively, which endow them the opportunity to conjugate with proteins more easily. In that, what the present study aims at is preparing specific antibodies against thifluzamide based on the simple synthesis of artificial antigens and developing a new detection method of ELISA for thifluzamide residues’ determination in real samples.
Materials and methods
Regents and equipment
Thifluzamide, 2-Methyl-4-(trifluoromethyl)thiazole-5-carboxylic acid and 2,6-Dibromo-4-(trifluoromethoxy)aniline were obtained from Shuya Chemical Science and Technology Co., Ltd (Shanghai, China); bovine serum albumin (BSA), ovalbumin (OVA) and Freund’s complete and incomplete adjuvants were purchased from Sigma Chemical Co. (St. Louis, USA), N-hydroxysuccinimide (NHS), dicyclohexylcarbodiimide (DCC), N,N′-carbonyldiimidazole (CDI) and 4-dimethylaminopyridine (DMAP) were purchased from Xiya Regent Co., Ltd (Chengdu, China), horseradish peroxidase conjugated goat anti-mouse IgG (HRP-anti-mIgG) was purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, USA), fluazifop-p-butyl, hexaflumuron, acifluorfene and flutolanil were all purchased from Aladdin Regent Co. (Shanghai, China).
Moreover, UV-2700 ultraviolet and visible spectrophotometer (Shimadzu, Japan), 96-well polystyrene microtitre plates (Canada JET Biochemicals Int. Guangzhou, China) and enzyme microplate reader (Prolong, Beijing, China), HPLC with ultraviolet detector (Agilent 1100 series, USA) were used in this study.
Animals and ethics statement
Specefic pathogen Free (SPF) female Balb/c mice (four weeks old) were purchased from the Hubei experimental animal centre (Wuhan, China) and housed in an environment with 12 h light–dark cycle, 20–25°C and 50–70% humidity. Food and water were offered ad libitum. Prior to administration, the mice were quarantined for a week. All experimental procedures were approved by the Office of Scientific Research Management of Central China Normal University, and the application for the use of animals was certified on December 20, 2015 (approval ID: CCNU-IACUC-2016-003).
Synthesis of immunogens and coating antigens
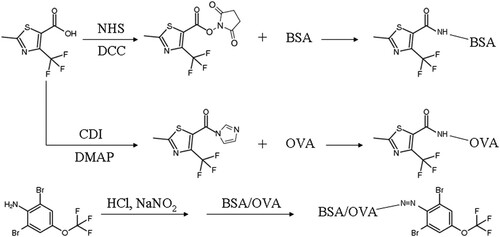
2-Methyl-4-(trifluoromethyl)thiazole-5-carboxylic acid (Hapten-1) and 2,6-Dibromo-4-(trifluoromethoxy)aniline (Hapten-2) were assigned as haptens to perform the conjugation to proteins (). Hapten-1-BSA and Hapten-1-OVA were synthesized by active easter method and CDI/DMAP method, respectively. For Hapten-2, the artificial antigens were both synthesized by diazotization method. The methods were described below and exhibited in . All characterizations of haptens, proteins and the conjugates were confirmed by UV-Vis spectroscopy and SDS-PAGE.
Active easter method (Zhang, Wang, Wen, Shi, & Wang, Citation2015): 44.46 mg Hapten-1 was added to 2.0 mL of DMF containing 23 mg NHS and 41.24 mg DCC. The mixture was stirred for 12 h at 4°C and then centrifuged at 5000 g for 5 min. The collected supernatant was slowly added to 8 mL 0.01 mol L−1 phosphate-buffered saline (PBS) containing 50 mg BSA. The reaction was allowed to proceed overnight at 4°C with gentle stirring. After the content was dialysed in PBS for 72 h (Kong, Liu, Song, Kuang, & Xu, Citation2016), Hapten-1-BSA was obtained and used as immunogen.
CDI/DMAP method (Zhao, He, Pu, & Deng, Citation2008): 44.46 mg Hapten-1 was dissolved in 3 mL DMF followed by the addition of 97.28 mg CDI and 73.3 mg DMAP. The mixture was stirred for hours at room temperature until the colour of liquid changed into brown. The allochroic solution was added drop by drop to 60 mg OVA dissolved in 8 mL borate buffer (pH 9.0) to complete the conjugation under stirring. After an overnight reaction, the solution was centrifuged at 5000 g for 5 min and the supernatant was dialysed in PBS for 72 h to obtain Hapten-1-OVA, which is used as coating antigen.
Diazotization method (Zhang, Wang, & Zhuang, Citation2006): 71 mg Hapten-2 dissolved in 2 mL dioxane and 14 mg NaNO2 were added to 2 mL 0.35 mol L−1 HCl. The mixture was stirred at 4°C and kept for 30 min; subsequently the reaction solution was added to 10 mL 0.2 mol L−1 borate buffer (pH 9.0) containing 60 mg of BSA or OVA and the mixed solution turned orange immediately. The final reaction was continued under constant stirring at 4°C for 2 h. The solution was centrifuged at 10,000 g for 5 min and the collected supernatant was dialysed in PBS for 72 h to remove unconjugated Hapten-2. The obtained Hapten-2-BSA and Hapten-2-OVA were stored at −20°C and used as immunogen and coating antigen, respectively.
Production of polyclonal antisera
Six SPF female Balb/c mice were evenly divided into two groups. Mice of group A were immunized with Hapten-1-BSA and the immunogen used in group B was Hapten-2-BSA. Each mouse was injected subcutaneously at multiple sites on the neck and back with a dose of 100 μg immunogen in 50 μL of PBS, which was emulsified with the same volume of Freund’s complete adjuvant. After four weeks, a booster of the 50 μg immunogen in 50 μL of PBS emulsified with Freund’s incomplete adjuvant (1:1, V/V) was given (Chen, Liu, Song, Kuang, & Xu, Citation2016) and the following three injections with the same dosage were conducted at two-week intervals. Seven days after the last injection, each immunized mice were tail-bled and the polyclonal antisera were collected by centrifugation at 600 g for 10 min.
Screening of the optimal polyclonal antisera dilution ratio
Microplates were coated with 100 μL appropriate concentration of coating antigen (Hapten-1-OVA or Hapten-2-OVA) diluted in 0.05 mol L−1 carbonate buffer (pH 9.6). After incubation overnight at 4°C, the microplates were washed for three times with 0.05% Tween 20 in 0.01 mol L−1 PBS; the unoccupied area of the wells was blocked with 250 μL of PBS containing 3% skim milk powder for 1 h at 37°C to minimize the non-specific adsorption. Then the plates were washed as above, 100 μL of polyclonal antisera diluted in PBS containing 0.1% BSA from 1:1000 to 1:128,000 were added in the wells to react with coating antigen. Following a washing step, the plates were incubated for 1 h at 37°C with diluted HRP-anti-mIgG (1:5000) in PBS containing 0.1% BSA (100 μL well−1). After washing, 100 μL of freshly prepared 0.4 mg mL−1 o-phenylenediamine (OPD) solution (dissolved in 0.05 mol L−1, pH 5.0 citrate-phosphate containing 0.012% H2O2) was added to each well with a following incubation at 37°C for 20 min; the colour development stopped with 50 μL per well of 2 mol L−1 H2SO4. The absorbance was measured using a microplate reader at 492 nm. The standard of selecting the titre of polyclonal antisera was the OD values approximately equal 1.0 to determine the optimal polyclonal antisera dilution ratio (Wang et al., Citation2007).
Indirect competitive ELISA protocol
An indirect competitive ELISA (ic-ELISA) method in two formats was developed for analysing thifluzamide. The procedure was similar as described above except for the competitive step. After microplates were coated with coating antigen and blocked with 3% skim milk powder, 50 μL serial concentrations of thifluzamide (or sample solution) accompanied with 50 μL diluted polyclonal antisera were added to the wells and were incubated for 1 h at 37°C (Yusakul et al., Citation2013). In the next steps, HRP-anti-mIgG and OPD were added successively according to the same handing described above. The reaction was stopped when 50 μL 2 mol L−1 H2SO4 was added in each well, and absorbance was detected by a microplate reader at 492 nm. The percent inhibition was calculated using the following equation:
where ODx represents the absorbance with various concentrations or samples, and ODmax represents the absorbance for the negative control.
Cross-reactivity determination
The specificity of two PAbs was determined by cross-reactivity (CR) value. Several structurally related fluorine-containing pesticides were selected for testing CR. CR was calculated as follows (Zhu, Song, Liu, Kuang, & Xu, Citation2016):
Analysis of samples
Tap water, lake water (derived from the East Lake, Wuhan, China) and wheat were fortified with known concentrations of thifluzamide. The spiked water samples were diluted twice and analysed by ELISA directly. For spiked wheat samples, the pretreatment was conducted by the steps described by Qin et al. (Citation2013). The detected concentrations of spiked samples were calculated from the standard curve running in the same microplate, and each analysis was carried out in triplicate. In addition, the spiked samples were detected by HPLC according to the method described by Guo et al. (Citation2014). The detection data were compared with the ELISA results.
Results and discussion
Selection of thifluzamide haptens
To obtain the antibody of a target analyte, it is vital to complete the synthesis of immunogen and coating antigen. Acting as haptens, pesticides and other low-molecular-weight compounds with active functional groups (such as carboxyl, amidogen and hydroxy) can be combined to carrier proteins directly. On the other hand, activating the intrinsic group of hapten (Abad-Fuentes, Agulló, Esteve-Turrillas, Abad-Somovilla, & Mercader, Citation2014; Fang et al., Citation2011; Wang, Wang, Yang, Wang, & Deng, Citation2012) or introducing a new functional group to the prime structure is also an effective way to obtain the target antibody (Luo et al., Citation2014; Wei et al., Citation2011; Xing et al., Citation2012). Although it is feasible to modify the molecular structure of hapten into a derivative, it calls for proficient research staffs with manipulative skills in the domain of chemical synthesis. Using partial structure rather than the whole molecule as the hapten to couple with proteins is also a simple and viable option for biochemical researchers, from which the corresponding hapten as well as the whole molecule would be recognized by the raised target antibody (Degelmann, Wenger, Niessner, & Knopp, Citation2004; Lee, Ahn, Park, Ko, & Kim, Citation2002; Zhao et al., Citation2006). In brief, approaches to synthesize artificial antigen vary depending on the trait of hapten molecular structure.
It is difficult to couple thifluzamide with carrier protein directly because of lacking active functional group, for which the introduction of an active group to thifluzamide molecule may be proposed as the first option. Nevertheless, such strategy requires researchers to select proper reagents via various chemical processes (such as extraction, drying, purification and identification) to achieve the ideal hapten, which is complicated and laboursome for them. To avoid this, in the present study, the aforementioned two moieties of thifluzamide were acted as haptens instead of remoulding its molecular structure. The process of artificial antigens’ synthesis was simple and efficient since both haptens bearing active groups were conjugated to proteins directly.
Identifications of immunogens and coating antigens
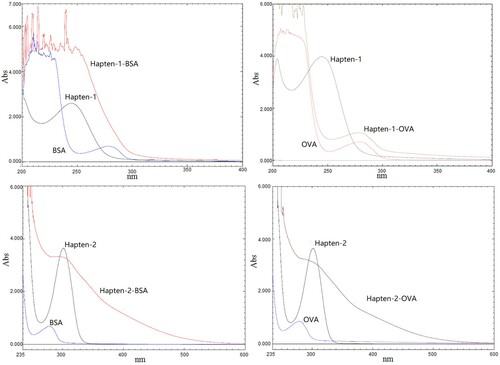
The identification of conjugates was carried out by UV-2700 ultraviolet and visible spectrophotometer. The UV spectra of haptens, carrier proteins and artificial antigens were shown in . It was observed that both the characteristic peaks of BSA and OVA were located at 280 nm, while the characteristic peaks of Hapten-1 and Hapten-2 were at 243 and 303 nm, respectively. Each of the conjugates showed an unlike characteristic compared to the corresponding hapten and carrier protein as well as a different absorption peak. The results preliminarily indicated the successful coupling of the haptens to carrier proteins.
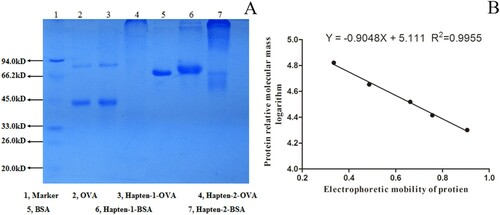
The electrophoretic results ((A)) showed that compared with the band of OVA, Hapten-1-OVA (lane 3) and Hapten-2-OVA (lane 4) lagged behind, showing the tailing phenomenon. Similarly, the bands of Hapten-1-BSA (lane 6) and Hapten-2-BSA (lane 7) also had tailing phenomenon and were behind BSA. All results above indicated that the four artificial antigens were synthesized successfully. According to the standard curve of protein electrophoretic mobility ((B)), it was calculated that the molar ratios of Hapten-1 to OVA and BSA were about 5.86:1 and 14.30:1, respectively; meanwhile the molar ratios of Hapten-2 to OVA and BSA were about 88.21:1 and 103.04:1, respectively.
Antisera screening
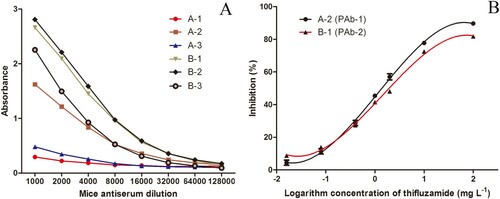
All the antisera from the immunized mice in two groups were obtained to measure the titre and sensitivity to thifluzamide. A preliminary chequerboard titration method was employed to select the befitting concentrations of coating antigen and antiserum. Under this condition, the determined optimal concentrations of two coating antigens (Hapten-1-OVA and Hapten-2-OVA) were both 1 μg mL−1. As shown in (A), the antisera titres of mouse A-2 and all mice in group B were no less than 1:4000. Both the antisera of mice A-2 and B-1, naming as PAb-1 and PAb-2, presented good recognition on thifluzamide ((B)), for which they were selected for establishing ELISA. The optimal antisera dilution ratios of PAb-1 and PAb-2 were 1:4000 and 1:8000, respectively according to their determined titres.
Development of the ic-ELISA
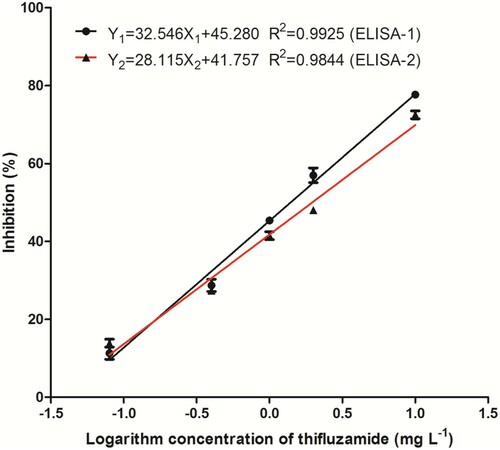
Two ic-ELISA formats (including ELISA-1 based on PAb-1 and ELISA-2 based on PAb-2) were developed for thifluzamide detection. The linear standard curves and linear equations of two formats were exhibited in . When the concentrations of thifluzamide were in the same range of 0.08–10 mg L−1, there was a good linear relationship between the per cent inhibition value and the logarithm of thifluzamide concentration (log C) in both ELISA formats. The concentration of 50% inhibition (IC50) was 1.39 mg L−1 in ELISA-1, and ELISA-2 had a similar IC50 of 1.96 mg L−1. The limit of detection (LOD) calculated at 10% inhibition (IC10) was 0.082 mg L−1 in ELISA-1, and ELISA-2 had an IC10 of 0.074 mg L−1.
It was reported that more hapten molecules conjugating with carrier proteins contribute to improve its immunogenicity (Zheng et al., Citation2016). In this study, the molar ratio of Hapten-2 to BSA was much higher than that of Hapten-1 to BSA, while the IC50 of ELISA-2 based on PAb-2 was similar with ELISA-1 based on PAb-1. We deduced that an appropriate molar ratio of hapten to protein might give the best performance to enhance the immunogenicity of antigen. Researches indicated that a linking arm such as alkyl chain between hapten and protein was considered to be a key point for recognizing hapten by the immune system (Mukunzi, Isanga, Suryoprabowo, Liu, & Kuang, Citation2017; Zhu, Mu, et al., Citation2016). In this study, Hapten-1 and Hapten-2 were conjugated to proteins directly with the formation of amido bond (–CO–NH–) and diazo bond (–N=N–) respectively (), and anti-thifluzamide PAbs were obtained ultimately without the use of a bridging group, which demonstrated that linking arm was not indispensable for antibody generation. The impact of linking arm could probably affect the properties of generated antibodies such as sensitivity and CR, which had been illustrated by previous studies (Grant & Sporns, Citation2005; Wei et al., Citation2011).
Antibody specificity
To test whether two PAbs could react to other analogues, several fluorine-containing pesticides (including fluazifop-p-butyl, hexaflumuron, acifluorfene and flutolanil) were selected as candidates and their CR values were evaluated. The molecular structures of tested compounds, IC50 values and CR values were displayed in . It was showed that PAb-1 had an affinity to Hapten-1 besides thifluzamide, and the CR was 23.36%; similarly, Hapten-2 could be recognized by PAb-2 with a CR of 24.13%. The possible reason behind this phenomenon was that new chemical bonds were produced when haptens were coupled to the carrier proteins; consequently the generated antibodies were more inclined to bond to thifluzamide, which contained identical or function-similar chemical bonds than the haptens. None of the tested pesticides were significantly recognized by PAb-1, and all the CRs were less than 0.01%. For PAb-2, the highest CR was 1.02% for acifluorfene, and the CRs of other compounds were negligible as well. The results indicated that both two raised PAbs exhibited high specificity to the target analyte thifluzamide.
Table 1. CR evaluation of related compounds.
Generally, the generated antibody may show broad specificity to some analogues of the target analyte if the molecular structure of a hapten resides in other compounds. Researchers had prepared polyclonal antibody against cypermethrin; however, the obtained antibody possessed poor specificity due to its high recognition to fenvalerate, cyhalothrin and deltamethrin (Wei, Zhao, Wang, & Wang, Citation2014). Tang et al. (Citation2015) acquired an anti-dimethyl phthalate polyclonal antibody which could detect eight phthalate esters residues in greenhouse soils. Compared with those researches, both two haptens used in this study were representative structures of thifluzamide without sharing by other compounds, hence, the raised two distinct PAbs exhibited high specificity to this fungicide rather than to other pesticides. Our results implied that the specificity of generated antibody was dependent on the uniqueness of molecular structure involved in hapten.
Analysis of spiked samples by ELISA and HPLC
As can be seen from , the recoveries of thifluzamide in all spiked samples ranged from 72.0% to 128.3% determined by ELISA-1, and the highest RSD was 11.1%. For ELISA-2, the recoveries determined were in the range of 97.5–128.4%, and the RSDs were less than 19.8%. Besides, the results were validated by the HPLC method. Several spiked samples (tap water: 1 and 0.5 mg L−1, lake water: 1 and 0.5 mg L−1, wheat: 30 and 0.5 mg kg−1) were analysed by ELISA and HPLC simultaneously. Data between HPLC and ELISA-1 (or ELISA-2) presented a good correlation, and the liner regression equations Y = 0.797X + 0.1356 (R2 = 0.9994) ((A)) and Y = 0.7956X + 0.1934 (R2 = 0.9999) ((B)) were obtained. The results gave the evidence that the developed ELISA had promising accuracy and applicability for thifluzamide residues’ detection.
Figure 7. Comparison of the HPLC and ELISA for the detection of thifluzamide in spiked samples (n = 6).
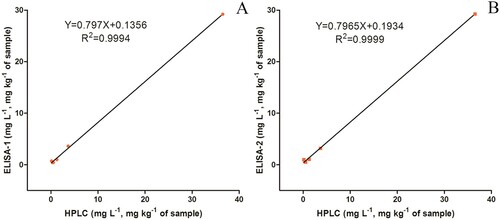
Table 2. Recoveries of thifluzamide in spiked samples determined by ELISA.
Compared with HPLC, the proposed ELISA method is more convenient and economical without complicated skills on chromatographic instrument operation and the high cost of suitable chromatographic columns. Besides, a large number of samples can be tested simultaneously in several 96-well microplates by ELISA (about 30 samples per microplate) with the use of a microplate reader and 4-h waiting, while chromatography technique is time-consuming since only one sample would be detected each time and the chromatographic column need to be washed for several hours after the experiment. Notably, the ic-ELISA has advantages for more simple, inexpensive and rapid detection of thifluzamide residues in environmental samples. However, the finite amount of obtained anti-thifluzamide antibody might be one of drawbacks existing in the study. Fortunately, abundant anti-thifluzamide polyclonal antibodies can be obtained when more or larger animals were immunized using artificial antigens prepared in this preliminary study.
Conclusion
In this study, the ic-ELISA method in two formats for detection of thifluzamide based on two PAbs was developed successfully. The LODs of ELISA-1 and ELISA-2 were 0.082 and 0.074 mg L−1 respectively with a low CR (<1.02%). In conclusion, the proposed ELISA-1 and ELISA-2 could detect the thifluzamide residues simultaneously and used for rapid screening of thifluzamide potentially in multitudinous samples with its sensitivity, accuracy and low costs.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abad-Fuentes, A., Agulló, C., Esteve-Turrillas, F. A., Abad-Somovilla, A., & Mercader, J. V. (2014). Immunoreagents and competitive assays to fludioxonil. Journal of Agricultural and Food Chemistry, 62(13), 2742–2744. doi: https://doi.org/10.1021/jf5001716
- Abhilash, P. C., & Singh, N. (2009). Pesticide use and application: An Indian scenario. Journal of Hazardous Materials, 165(1–3), 1–12. doi: https://doi.org/10.1016/j.jhazmat.2008.10.061
- Chen, Y., Liu, L., Song, S., Kuang, H., & Xu, C. (2016). Establishment of a monoclonal antibody-based indirect enzyme-linked immunosorbent assay for the detection of trimethoprim residues in milk, honey, and fish samples. Food and Agricultural Immunology, 27(6), 830–840. doi: https://doi.org/10.1080/09540105.2016.1183599
- Chen, Y., Zhang, A. F., Wang, W. X., Zhang, Y., & Gao, T. C. (2012). Baseline sensitivity and efficacy of thifluzamide in Rhizoctonia solani. Annals of Applied Biology, 161(3), 247–254. doi: https://doi.org/10.1111/j.1744-7348.2012.00569.x
- Degelmann, P., Wenger, J., Niessner, R., & Knopp, D. (2004). Development of a class-specific ELISA for sulfonylurea herbicides (sulfuron screen). Environmental Science and Technology, 38(24), 6795–6802. doi: https://doi.org/10.1021/es0496266
- Dintzis, R. Z., Okajima, M., Middleton, M. H., Greene, G., & Dintzis, H. M. (1989). The immunogenicity of soluble haptenated polymers is determined by molecular mass and hapten valence. Journal of Immunology, 143(4), 1239–1244.
- Fang, S., Zhang, B., Ren, K., Cao, M., Shi, H., & Wang, M. (2011). Development of a sensitive indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) based on the monoclonal antibody for the detection of the imidaclothiz residue. Journal of Agricultural and Food Chemistry, 59(5), 1594–1597. doi: https://doi.org/10.1021/jf104241n
- Grant, G. A., & Sporns, P. (2005). Effect of the hapten linking arm on cross-reactivity of sulfonamide-specific antibodies in ELISA. Food and Agricultural Immunology, 16(16), 259–272. doi: https://doi.org/10.1080/09540100500288507
- Guo, L. F., Ding, P., & Xu, Y. (2014). Analysis of thifluzamide TC by HPLC. Modern Agrochemicals, 13(4), 28–29.
- Gupta, S., & Gajbhiye, V. T. (2004). Adsorption–desorption, persistence and leaching behavior of thifluzamide in alluvial soil. Chemosphere, 57(6), 471–480. doi: https://doi.org/10.1016/j.chemosphere.2004.06.033
- Hoferkamp, L., Hermanson, M. H., & Muir, D. C. (2010). Current use pesticides in Arctic media; 2000–2007. Science of the Total Environment, 408(15), 2985–2994. doi: https://doi.org/10.1016/j.scitotenv.2009.11.038
- Kong, D., Liu, L., Song, S., Kuang, H., & Xu, C. (2016). Development of ic-ELISA and lateral-flow immunochromatographic assay strip for the detection of folic acid in energy drinks and milk samples. Food and Agricultural Immunology, 27(6), 841–854. doi: https://doi.org/10.1080/09540105.2016.1183600
- Lee, J. K., Ahn, K. C., Park, O. S., Ko, Y. K., & Kim, D. W. (2002). Development of an immunoassay for the residues of the herbicide bensulfuron-methyl. Journal of Agricultural and Food Chemistry, 50(7), 1791–1803. doi: https://doi.org/10.1021/jf011150b
- Liu, W., Zhao, C., Zhang, Y., Lu, S., Liu, J., & Xi, R. (2007). Preparation of polyclonal antibodies to a derivative of 1-aminohydantoin (AHD) and development of an indirect competitive ELISA for the detection of nitrofurantoin residue in water. Journal of Agricultural and Food Chemistry, 55(17), 6829–6834. doi: https://doi.org/10.1021/jf070620k
- Luo, L., Xu, Z. L., Yang, J. Y., Xiao, Z. L., Li, Y. J., Beier, R. C., … Shen, Y. D. (2014). Synthesis of novel haptens and development of an enzyme-linked immunosorbent assay for quantification of histamine in foods. Journal of Agricultural and Food Chemistry, 62(51), 12299–12308. doi: https://doi.org/10.1021/jf504689x
- Ma, L., Chen, J., Zhao, L., & Zhan, X. (2015). Determination of six amide pesticide residues in vegetables and fruits by solid phase extraction-ultra high performance liquid chromatography-tandem mass spectrometry. Chinese Journal of Chromatography, 33(10), 1019–1025. doi: https://doi.org/10.3724/SP.J.1123.2015.05013
- Mukunzi, D., Isanga, J., Suryoprabowo, S., Liu, L., & Kuang, H. (2017). Rapid and sensitive immunoassays for the detection of lomefloxacin and related drug residues in bovine milk samples. Food and Agricultural Immunology, 28(4), 599–611. doi: https://doi.org/10.1080/09540105.2017.1306495
- Qin, X., Sun, Y., Xu, Y. M., Wang, Q., Yao, J. G., & Gao, Y. (2013). Residue determination and degradation of thifluzamide in potato and soil. Journal of Agricultural Resourcesand Environment, 30(6), 83–86.
- Rejeb, S. B., Cléroux, C., Lawrence, J. F., Geay, P. Y., Wu, S., & Stavinski, S. (2001). Development and characterization of immunoaffinity columns for the selective extraction of a new developmental pesticide: Thifluzamide, from peanuts. Analytica Chimica Acta, 432(2), 193–200. doi: https://doi.org/10.1016/S0003-2670(00)01376-3
- Rekha, S. N., Naik, R., & Prasad, R. (2006). Pesticide residue in organic and conventional food-risk analysis. Journal of Chemical Health and Safety, 13(6), 12–19. doi: https://doi.org/10.1016/j.chs.2005.01.012
- Tang, M., Wei, J., Du, H., Zhang, J., Yang, D., & Peng, Y. (2015). Synthesis of an artificial antigen and preparation of a polyclonal antibody for the sensitive determination of phthalate esters by enzyme-linked immunoassay. Analytical Methods, 7(8), 3402–3410. doi: https://doi.org/10.1039/C5AY00079C
- Wang, W. J., Ling, Y., Xu, T., Gao, H. B., Sheng, W., & Li, J. (2007). Development of an indirect competitive ELISA based on polyclonal antibody for the detection of diethylstilbestrol in water samples. Chinese Journal of Chemistry, 25(8), 1145–1150. doi: https://doi.org/10.1002/cjoc.200790214
- Wang, R., Wang, Z., Yang, H., Wang, Y., & Deng, A. (2012). Highly sensitive and specific detection of neonicotinoid insecticide imidacloprid in environmental and food samples by a polyclonal antibody-based enzyme-linked immunosorbent assay. Journal of the Science of Food and Agriculture, 92(6), 1253–1260. doi: https://doi.org/10.1002/jsfa.4691
- Wei, C., Ding, S., You, H., Zhang, Y., Wang, Y., Yang, X., & Yuan, J. (2011). An immunoassay for dibutyl phthalate based on direct hapten linkage to the polystyrene surface of microtiter plates. PLoS One, 6(12), e29196. doi: https://doi.org/10.1371/journal.pone.0029196
- Wei, L. N., Wu, P., Wang, F. R., & Yang, H. (2015). Dissipation and degradation dynamics of thifluzamide in rice field. Water Air and Soil Pollution, 226(5), 1–13. doi: https://doi.org/10.1007/s11270-015-2387-5
- Wei, X., Zhao, Y., Wang, B., & Wang, Y. (2014). Enzyme-linked immunosorbent assay–based two different polyclonal antibodies for the detection of cypermethrin with phenoxybenzene multiresidue. Food and Agricultural Immunology, 25(3), 364–374. doi: https://doi.org/10.1080/09540105.2013.805732
- Wu, H., Wang, G., Li, G., & Chen, T. (2006). Determination of thifluzamide by GC. Pesticide Science and Administration, 27(8), 1–3.
- Xing, Y., Meng, M., Xue, H., Zhang, T., Yin, Y., & Xi, R. (2012). Development of a polyclonal antibody-based enzyme-linked immunosorbent assay (ELISA) for detection of sunset yellow FCF in food samples. Talanta, 99, 125–131. doi: https://doi.org/10.1016/j.talanta.2012.05.029
- Yusakul, G., Udomsin, O., Juengwatanatrakul, T., Tanaka, H., Chaichantipyuth, C., & Putalun, W. (2013). Highly selective and sensitive determination of deoxymiroestrol using a polyclonal antibody-based enzyme-linked immunosorbent assay. Talanta, 114, 73–78. doi: https://doi.org/10.1016/j.talanta.2013.04.011
- Zhang, L., Wang, Z., Wen, Y., Shi, J., & Wang, J. (2015). Simultaneous detection of parathion and imidacloprid using broad-specificity polyclonal antibody in enzyme-linked immunosorbent assay. Analytical Methods, 7(1), 205–210. doi: https://doi.org/10.1039/C4AY01818D
- Zhang, M. C., Wang, Q. E., & Zhuang, H. S. (2006). A novel competitive fluorescence immunoassay for the determination of dibutyl phthalate. Analytical and Bioanalytical Chemistry, 386(5), 1401–1406. doi: https://doi.org/10.1007/s00216-006-0703-z
- Zhao, D., He, L., Pu, C., & Deng, A. (2008). A highly sensitive and specific polyclonal antibody-based enzyme-linked immunosorbent assay for detection of antibiotic olaquindox in animal feed samples. Analytical and Bioanalytical Chemistry, 391(7), 2653–2661. doi: https://doi.org/10.1007/s00216-008-2179-5
- Zhao, J., Yi, G. X., He, S. P., Wang, B. M., Yu, C. X., Li, G., … Li, Q. X. (2006). Development of a monoclonal antibody-based enzyme-linked immunosorbent assay for the herbicide chlorimuron-ethyl. Journal of Agricultural and Food Chemistry, 54(14), 4948–4953. doi: https://doi.org/10.1021/jf060707q
- Zheng, L., Wang, J., Zhang, J., Song, Z., Dong, Y., Wang, Y., … Xi, R. (2016). Preparation of polyclonal antibodies for nateglinide (NTG) and development of a sensitive chemiluminescent immunoassay to detect NTG in tablets and serum. Talanta, 146, 483–489. doi: https://doi.org/10.1016/j.talanta.2015.09.008
- Zhu, D., Mu, Y., Li, Q., Pang, X., Wang, X., Liu, Y., … Chen, H. (2016). A novel method for artificial antigen synthesis and preparation of a polyclonal antibody for the sensitive determination of leucomalachite green in fish samples by enzyme-linked immunoassay. Analytical Methods, 8(32), 6236–6243. doi: https://doi.org/10.1039/C6AY01564F
- Zhu, Y., Song, S., Liu, L., Kuang, H., & Xu, C. (2016). An indirect competitive enzyme-linked immunosorbent assay for acrylamide detection based on a monoclonal antibody. Food and Agricultural Immunology, 27(6), 796–805. doi: https://doi.org/10.1080/09540105.2016.1160369