ABSTRACT
Chlorpyrifos (CPF) has been widely used around the world as a pesticide for both agricultural and residential application. Although various studies have reported toxicity and health-related effects from CPF exposure, the potential threat and the molecular mechanism of CPF toxicity to human liver have not been well-characterized. In this study, we identify cytotoxicity of CPF to human normal liver cells in vitro. We demonstrate that the viability of QSG7701 cells is inhibited by CPF in a time- and concentration-dependent manner. Intracellular biochemical assays showed that CPF-induced apoptosis of QSG7701 cells concurrent with a decrease in the mitochondrial membrane potential, the release of cytochrome c into the cytosol, up-regulate the expression level of Bax/Bcl-2 and a marked activation of caspase-9/-3. These results indicate that CPF has a potential risk to human liver that can induce apoptosis of human liver cells through caspase-dependent mitochondrial pathways.
1. Introduction
Chlorpyrifos (O,O'-diethyl-O-3,5,6-trichloro-2-pyridyl phosphorothionate; CPF) is a broad spectrum organophosphate insecticide that is widely used for agricultural pest control and managing residential indoor pests since its registration (Jang et al., Citation2015; Wang et al., Citation2013 ). CPF mainly functions as a neurotoxin via inhibition of acetylcholinesterase (AChE), and it is generally considered that CPF has low toxicity for healthy adults because AChE-reactive neurons are restricted to the mammalian central nervous system (Bagley et al., Citation1989; Moser, Citation2000). However, more and more examples in recent years have illustrated that chemical substances of anthropogenic origin potentially affect the health of humans through long-term accumulation (Bolognesi et al., Citation1994). Besides, CPF is a lipophilic molecule and it can easily pass through the cell membrane into the cytoplasm (Ki, Park, Lee, Shin, & Koh, Citation2013). Therefore, it poses a potential risk for the health of humans.
Experimental studies have demonstrated that CPF exposure results in inhibition of DNA synthesis, induces apoptosis and leads to adverse neurodevelopmental problems in the central nervous system, and the mechanism proposed for toxicity of CPF is oxidative stress which may be via production of reactive oxygen species (ROS) from mitochondria (Cho, Nakamura, & Lipton, Citation2010; Lee, Park, Shin, & Koh, Citation2012; Moser, Citation2000; Saulsbury, Heyliger, Wang, & Johnson, Citation2009). ROS produced during oxidative stress have been reported to initiate signalling cascades leading to apoptosis (Yu et al., Citation2008). Recent studies showed that CPF has adverse effects on non-target organisms including liver of rats and fish, but it has emerged that reports revealed the underlying mechanism in the development of liver toxicity induced by CPF to humans (Ahmed & Zaki, Citation2009; Xing et al., Citation2014). Compared to the central nervous system, CPF is more potent to cause toxic effects in human liver associated with eating. In our present work, a study on cytotoxic and apoptotic effects of human liver cells was carried out to evaluate the safety of the widely used insecticide, and we found that CPF has significant cytotoxicity.
Apoptosis is a genetically programmed type of cell death mechanism for multicellular organisms (Elmore, Citation2007). It is essential to eliminate dead or abnormal cells and plays a vital role in maintaining the stability of the internal environment and maintaining the balance of development in multicellular organisms (Kerr, Wyllie, & Currie, Citation1972; Taylor, Cullen, & Martin, Citation2008). Despite a wide range of inducing signals, some common pathways exist in apoptosis (Nagata, Citation1997). For instance, in the intrinsic pathway, mitochondria irreversibly commit cells to apoptosis by releasing death factors into cytosol (Kroemer et al., Citation1995). The apoptosis process is strictly regulated and the Bcl-2 gene families play a key role in the process. The Bcl-2 family mainly promotes the apoptosis gene (such as Bax) and anti-apoptotic genes (such as Bcl-2) (Miyashita et al., Citation1994). Among them, the Bax/Bcl-2 is the key factor in regulating apoptosis. Many researchers think of it as the prognosis of apoptosis and tumour markers (Perfettini et al., Citation2004). Cytochrome c, a death factor, can form a complex with Apaf-1 in the presence of dATP in cytosol. This is followed by activation of caspase-9 and caspase-3, which results in the activation of a cascade of caspases (Petronilli et al., Citation1999). Finally, these activated caspases degrade key structural and nuclear proteins and irreversibly commit the cells to death (Kaufmann et al., Citation1993; Tang & Kidd, Citation1998). If cells are under abnormal environment pressure, they will undergo apoptosis, and when apoptosis continuance to organism, formation harm cannot be estimated more.
In the present study, QSG7701 cells were used as a model for typical human normal liver cells to evaluate the toxicological effects of CPF on human liver, and we found that CPF has significant cytotoxicity to it in vitro. We used different methods to verify the mechanism of action of CPF against QSG7701 cells, which showed that it can induce apoptosis. Our results indicate that it can down-regulate mitochondrial membrane potential (MMP) level, make cytochrome c release, up-regulate the expression level of Bax/Bcl-2 and activate caspase-9 and caspase-3. In conclusion, this evidence shows that the QSG7701 cells were undergoing caspase-dependent mitochondrial apoptosis.
2. Materials and methods
2.1. Chemicals and reagents
CPF (98% purity) was purchased from Shanghai Pesticide Research Institution (Shanghai, China). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) and Rhodamine123 (Rh-123) were obtained from Sigma–Aldrich (St. Louis, MO, USA). Most antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA) except for horseradish peroxidase-conjugated anti-rabbit IgG was from Sangon Biotech Co., Ltd. (Shanghai, China). The BCA Protein Assay Kit was obtained from Pierce (Rockford, IL, USA). The caspase-3 and caspase-9 Activity Assay Kit and the Mitochondria/Cytosol Fractionation Kit were obtained from Beyotime Institute of Biotechnology (Shanghai, China). Other reagents and chemicals used were of analytical grade and purchased locally.
2.2. Cell culture
The human normal liver cell line QSG7701 was obtained from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). It was cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, Logan, Utah, USA) supplemented with 10% heat-inactivated foetal bovine serum (FBS, Gibco, Grand Island, NY, USA), streptomycin (100 μg/mL) and penicillin (100 U/mL) (Hyclone, Logan, Utah, USA), and incubated at 37°C in a 5% CO2 incubator. The medium was exchanged once per two days. After treatment, the QSG7701 cells were harvested by 0.25% Trypsin-EDTA (Gibco, Grand Island, NY, USA).
2.3. Cell viability assay
Cell viability, as a testing endpoint of cytotoxicity, was determined by MTT assays (Zhang et al., Citation2015). This assay measures the conversion of MTT to purple formazan by succinate dehydrogenase of the intact mitochondria of living cells. QSG7701 cells were seeded into 96-well plates (150 µL/well) at a density of 1 × 106 cells per millilitre in DMEM (10% FBS) incubated for 12 h. After that the cells were exposed to 800, 400, 200, 100, 50 and 25 μM CPF, and 0.1% DMSO was used as negative control. After 24 and 48 h of treatment, 20 μL of MTT (5 mg/mL) was added to each well. To dissolve the formazan crystals, the culture media was discarded and 150 μL of DMSO was added after 4 h of incubation. Then, the absorbances were measured at 492 and 630 nm by a Synergy H1 microplate reader (Bio-Teck, Winooski, VT, USA). Each experiment was repeated at least three times in quadruplicate. The inhibitory rates of the cells were calculated by the following formula: % inhibitory rate = [1 − (mean (OD492–OD630) in test wells)/(mean absorbency in control wells)] × 100%.
2.4. Apoptosis assay
Apoptosis-associated changes were examined by cytofluorometry. The Invitrogen™ Alexa Fluor 488 Annexin V/Dead cell apoptosis kit was used to assess apoptosis in the early and later events of cellular cytotoxicity. QSG7701 cells were cultured in a 6-well culture plate (1 × 106 cells/mL) in 2 mL of complete DMEM medium. After 24 h, cells were treated with four CPF concentrations (50, 100, 200 and 400 μM), and 0.1% DMSO was used as negative control. For the apoptosis assay, cells were centrifuged at 100×g for 5 min and washed twice with cold PBS (pH 7.4) to remove CPF. Then, the cells were labelled with Annexin V-FITC and PI for 15 min at room temperature (25°C) in the dark. Finally, cells were analysed using a flow cytometer (B.D. FACS Calibur). Data analysis was performed using the Flowjo software program.
2.5. MMP (△Ψm) analysis
MMP (▵Ψm) is an important parameter of mitochondrial function. It is used as an early apoptotic marker in cells (Koya et al., Citation2000). The changes in MMP (ΔΨm) were determined on the basis of mitochondrial retention of the fluorescent dye Rhodamine123(Rh-123) (Scaduto & Grotyohann, Citation1999). Rh-123 is a positively charged molecule which accumulates in the energized mitochondria. A decrease in the fluorescence intensity of Rh-123 indicates a decline in MMP. After being treated with CPF at concentrations of 50, 100, 200 and 400 μM for 6 h, QSG7701 cells were harvested, washed twice with PBS (pH 7.4) and stained with Rh-123 at 37°C for 20 min in the dark. After incubation, the cells were washed twice by PBS (pH 7.4) to remove extracellular Rh-123. The fluorescence of the CPF-treated cells was examined by fluorescence microscopy. The fluorescence intensity was analysed using ImageJ software, and 200 stained cells from each treatment group were counted. Each experiment was conducted three times, and the results were reported as the mean of the three experiments.
2.6. Western blot analysis
Total protein from CPF-treated cells were extracted in RIPA lysis buffer (Sigma–Aldrich, St. Louis, MO, USA) with 1 mM Phenylmethylsulfonyl fluoride (PMSF, Sigma–Aldrich, St. Louis, MO,USA). Mitochondrial and cytosolic proteins were isolated using the Mitochondria/Cytosol Fractionation Kit according to the manufacturer’s instructions, and quantified by using the BCA method. Equal amounts of protein (30–50 μg) were separated by 8–15% SDS-PAGE and electrophoretically transferred to the polyvinylidene difluoride membrane (Millipore Corp, Atlanta, GA, USA). The blots were blocked in Tris-buffered saline-Tween (10 mM Tris·HCl, pH 7.5/150 mM NaCl/0.1% Tween-20) with 5% non-fat dry milk at room temperature for 1 h. Then they were processed for immunoblotting with primary antibodies for cytochrome c, Bcl-2, Bax and β-actin (diluted 1:300; 1:300; 1:300; 1:1000, respectively) and HRP-conjugated secondary antibodies were incubated respectively. Signals were visualized after treatment by enhanced chemiluminescence reagent (Pierce, Rockford, IL, USA). All the protein bands were scanned and integrated density values were quantified by ImageJ software and normalized to that of β-actin.
2.7. Caspase-3 and caspase-9 activity assay
Either caspase-3 or caspase-9 activity is an early marker of cells undergoing apoptosis. The activities of caspase-3 and caspase-9 were determined using the caspase-3 activity kit and caspase-9 activity kit separately. To investigate whether caspase-3 is involved, QSG7701 cells were treated with CPF and then the cells were extracted with extraction buffer at 4°C for 20 min. The soluble extracts were collected by centrifugation at 12,000×g and 4°C for 15 min. The supernatants were collected and protein concentration was determined by BCA protein assay. Cellular extracts were then incubated with 20 ng of Ac-DEVD-pNA (caspase-3 activity) or Ac-LEHD-pNA (caspase-9 activity) in a 96-well plate for 4 h at 37°C. Absorbances were measured at 405 nm.
2.8. Statistical analysis
At least three individual experiments were conducted for each experiment and a satisfactory correlation was achieved between the results of each individual experiment. Differences between groups were analysed using one-way analysis of variance followed by Dunnet’s test with a p ≤ .05 considered as statistically significant. Data are expressed as the mean ± SD. All statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Inhibition of QSG7701 cells’ viability by CPF
To evaluate the cytotoxic effect of CPF on QSG7701 cells the MTT assay was performed. As shown in , the CPF inhibited QSG7701 cells’ viability in a time- and concentration-dependent manner. The proliferation inhibition rates of QSG7701 cells were 8.54 ± 1.24%, 10.94 ± 0.83%, 18.60 ± 2.71%, 51.16 ± 4.35%, 55.81 ± 0.60% and 85.24 ± 0.68% after 24 h of treatment with CPF at the concentrations of 25, 50, 100, 200, 400 and 800 μM, which finally reached 27.27 ± 3.53%, 31.62 ± 8.10%, 35.52 ± 7.96%, 60.27 ± 0.89%, 68.57 ± 1.62% and 88.04 ± 0.74% after 48 h of treatment. IC50 values for CPF treatments of 24 and 48 h are given in .
Figure 1. Cytotoxicity of CPF on QSG7701 cells. Cell viability of QSG7701 cells treated with 25, 50, 100, 200, 400 and 800 μM CPF for 24 and 48 h. The cell viability is expressed as the mean values (±SD) of three independent experiments. *p < .05 and **p < .01 represent significant differences relative to negative control.
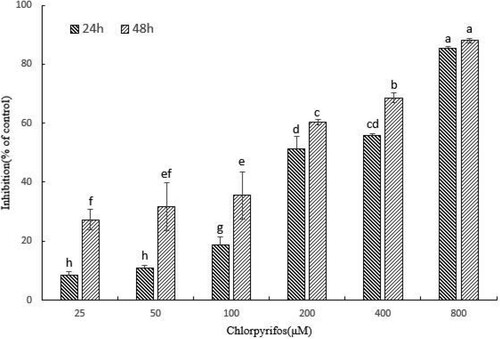
Table 1. The half maximal inhibitory concentration (IC50) of QSG7701 cells exposed to CPF.
3.2. CPF-induced apoptosis in QSG7701 cells
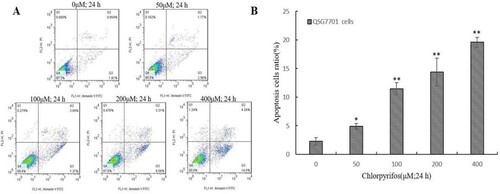
After treatment with 50, 100, 200 and 400 μM of CPF for 24 h, an increasing number of cells began to shrink, float from the bottom of the plates (images not shown) and undergo fragmentation. The effect of CPF on QSG7701 cells was then determined by flow cytometry. We further demonstrated the occurrence of CPF-induced apoptosis by Annexin V-PI staining methods ((A)). The flow cytometry assay showed that the ratio of apoptotic cells increased from 2.30 ± 0.64% in untreated cells to 4.88 ± 0.50%, 11.46 ± 1.04%, 14.40 ± 2.43% and 19.58 ± 0.85% in 50, 100, 200 and 400 μM CPF-treated cells, respectively, which indicated that the apoptotic rate in QSG7701 cells increased in a dose-dependent manner ((B)).
Figure 2. CPF-induced apoptosis in QSG7701 cells. Representative flow cytometric analysis conducted for cells stained with Anexin V-PI following treatment with 50, 100, 200, 400 μM CPF and 0.1% DMSO used as a control for 24 h (A). The lower right panel shows the early apoptotic cells and the upper right panel shows the late apoptotic cells or cells undergoing necrosis. Quantitative data are shown in the right panel (B). The data represent the means ± SD values of three experiments in triplicate. *p ≤ .05 and **p ≤ .01 vs. the negative control.
3.3. Mitochondrial dysfunction induced by CPF
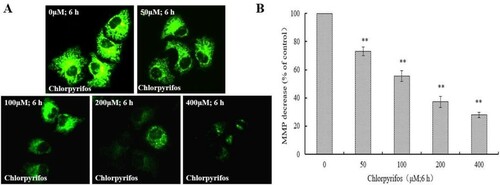
MMP regulates mitochondrial membrane selectivity and permeability to various substances in order to maintain normal mitochondrial morphology and function (Chen, Citation1988). A reduction in MMP plays a key role in triggering apoptosis. Compared to cytochrome c and other mitochondrial factors, loss of MMP is considered the earlier event in the apoptosis cascade (Ly, Grubb, & Lawen, Citation2003). Thus, an attempt was made to assess the effect of CPF on MMP in QSG7701 cells. Using fluorescent microscopy, we determined that exposure of QSG7701 cells ((A)) to 50, 100, 200 and 400 μM CPF for 6 h resulted in a gradual decrease in green fluorescence intensity in the mitochondria in a concentration-dependent manner ((A,B)). The results indicated that the reduction of MMP was induced by CPF treatment in QSG7701 cells.
Figure 3. Decrease of MMP in CPF-treated QSG7701 cells. QSG7701 cells were pre-treated with 0, 50, 100, 200 and 400 μM of CPF for 6 h and then loaded with Rh-123. The quantification of Rh-123 accumulation in mitochondria (green fluorescence) was analysed by flow microscopy (200×) (A). Data were considered significant at **p < .01 vs. the negative control (B). Data are the means ± SD of three independent experiments and expressed as the percentage of the control cells.
3.4. Effects of CPF on the level of apoptosis-related proteins in QSG7701 cells
To clarify the mechanism of CPF-induced apoptosis, we examined the expression of apoptosis-related proteins in QSG7701 cells following treatment with CPF at various concentrations for indicated times. Cytochrome c is a basic protein which is anchored in the inner mitochondrial membrane by its association with anionic phospholipids (Ott, Robertson, Gogvadze, Zhivotovsky, & Orrenius, Citation2002). It is an essential factor in a mitochondrial-mediated apoptosis pathway and plays a key role in the apoptosis (Li et al., Citation2000). Extracted cytosolic fractions of QSG7701 cells treated with 50, 100, 200 and 400 μM CPF were analysed by western blot assay. As shown in (A), in treated QSG7701 cells, cytochrome c increased with CPF treatment in a concentration-dependent manner in the cytosolic fractions.
Figure 4. Effect of CPF on the expression of apoptosis-related proteins in QSG7701 cells. Cells were fractionized following treatment with 50, 100, 200, 400 μM CPF for 6 h and cyt c release was detected (A). Cyto denote cytosolic fractions. Whole cell extracts were prepared for cells treated with 50, 100, 200 and 400 μM CPF for 6 h and the expressions of pro-apoptotic proteins as Bax and anti-apoptotic proteins as Bcl-2 were detected (B). β-actin was used as an equal loading control. The densitometric analysis results are shown in the right panel (C). Effect of CPF on the activation of caspase-3/-9 in QSG7701 cells (D). Data are represented as means ± SD from three independent experiments. *p ≤ .05 and **p < .01 vs. the negative control.
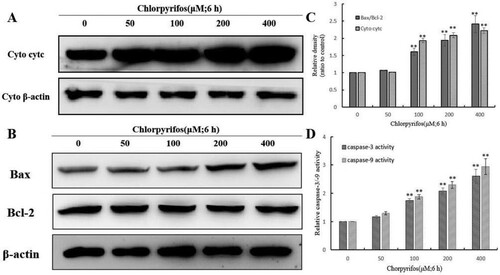
Bcl-2 family members including the pro-apoptotic protein (e.g. Bax) and the anti-apoptotic protein (e.g. Bcl-2) are important apoptosis-regulatory factors and constitute critical control points in the mitochondrial apoptotic pathway (Cao et al., Citation2001; Susin et al., Citation1996). The protein expression levels of anti-apoptotic protein Bcl-2, and pro-apoptotic protein Bax, were measured by western blot assay. In QSG7701 cells without CPF treatment, they presented a high expression level of Bcl-2 and only a slight expression of Bax. As shown in (B,C), the expression level of Bax/Bcl-2 was up-regulated in a concentration-dependent manner after CPF treatment.
3.5. Effects of CPF on caspase-3 and caspase-9 activation in QSG7701 cells
In response to pro-apoptotic signals, caspases propagate apoptosis. Activated caspase-9 directly cleaves and activates the executioner caspase-3, consequently leading to cell apoptosis (Morishima, Nakanishi, Takenouchi, Shibata, & Yasuhiko, Citation2002). The CPF-treated QSG7701 cells were investigated for the activation of caspase-9/-3 by colorimetric enzymatic assay. As shown in (D), the activation of both caspase-9 and caspase-3 increased in a concentration-dependent manner after CPF treatment. Our results indicated that the CPF-induced apoptosis was mediated through the activation of caspase-9/-3.
4. Discussion
Pesticide pollution in the environment has been increasing due to their extensive use in agriculture. And the liver in the human organism is primarily responsible for the metabolism of toxic substances, including pesticide residues in food. Therefore, the effect of CPF on the maintenance of the human hepatocyte function is very important. In our study, we investigated if the CPF has significant cytotoxicity to human normal liver cells and whether the action mechanism of it relies on the mitochondrial apoptotic pathway. This would not only supply evidence for identifying the safety of CPF to human beings, but also provide a theoretical basis for understanding its mechanisms of toxicity.
To evaluate the cytotoxic effects of CPF, we firstly tested the effect of different concentrations of CPF on the viability of QSG7701 cells by MTT assay. The result showed that the cell viabilities were inhibited in a time- and concentration-dependent manner upon CPF treatment (). Our further study verified whether CPF inhibits QSG7701 cells’ proliferation by inducing cell apoptosis. Flow cytometry analysis results indicated that the proportions of apoptotic cells obviously increased at different concentrations of CPF for 24 h ((A,B)). This demonstrates that CPF has significant cytotoxicity to human liver cells in vitro, which might be the foundation of its potential risk to the maintenance of the human hepatocyte function.
As is known, apoptosis relies on two major pathways, the cell death receptor-mediated extrinsic pathway and the mitochondrial-mediated intrinsic pathway (Evan, Citation1997). The two most important characteristics of the mitochondrial-mediated apoptotic pathway are loss of MMP and release of cytochrome c (Garrido et al., Citation2006). The disruption of MMP is an important means of turning on/off apoptosis and it is recognized as the key step in the apoptotic cascade (Hüttemann et al., Citation2011). The results of the fluorescence microscopy experiments show CPF can induce the disruption of MMP in a concentration-dependent manner ((B)). When the MMP reduced, cytochrome c is released from mitochondria into the cytosol (Chauvin et al., Citation2001). Cytochrome c, a basic protein, is anchored in the inner mitochondrial membrane, and initiates caspase activation when released from the mitochondrial during apoptosis (Liu, Kim, Yang, Jemmerson, & Wang, Citation1996). Western blotting indicated an increase of cytochrome c release to the cytosol in CPF-treated QSG7701 cells ((A)). The results of the MMP collapse and cytochrome c release suggested that mitochondrial-dependent intrinsic pathway potential contributed to CPF-induced apoptosis in QSG7701 cells.
Bcl-2 family members are considered to relate to the release of cytochrome c (Gross, McDonnell, & Korsmeyer, Citation1999). Previous studies have shown that the anti-apoptotic protein Bcl-2 primarily suppresses the release of cytochrome c and the pro-apoptotic protein Bax induces redistribution of cytochrome c to the cytosol (Antonsson, Montessuit, Sanchez, & Martinou, Citation2001; Zhang et al., Citation2000), where it causes activation of caspase proteases (e.g.caspase-9 and caspase-3), and subsequently cell death (Susin et al., Citation1999). Our data showed that CPF greatly increases the ratio of Bax/Bcl-2 ((C)), which may increase the release of cytochrome c from the mitochondrial into the cytoplasm and then activate caspase-9 and caspase-3.
Caspases are a family of cysteine proteases that play a key role in modulating apoptosis. Caspases begin as inactive precursors, but upon receiving an apoptotic signal, the pro-caspase will undergo proteolytic processing to generate an active enzyme. After the death receptor, located on the cell membrane, receives stimulation signals, the initial caspase is activated by the extrinsic pathway (extrinsic) and intrinsic pathway (mitochondrial pathway). Finally, the executed caspase is activated to make the relevant substrate degradation and induce apoptosis (Fuchs & Steller, Citation2011; Zhang et al., Citation2015). Among them, caspase-3 is the major effector protein of apoptosis that is activated by an initiator caspase as caspase-9. In our system, both the initiator caspase-9 and the effector caspase-3 were activated ((D)), suggesting that each of these caspases contributed to CPF-induced QSG7701 cell apoptosis.
In conclusion, we demonstrate that CPF has the ability to induce apoptosis of QSG7701 cells. CPF is regarded as a substance of high environmental safety based on its specificity targets, but the potential to affect the health of humans through long-term accumulation was ignored. Unlike the very well-reported mechanisms of toxicity in the nervous system, literature on the action of CPF in a non-target system is still very limited. Our results demonstrate that CPF induces the death of human normal liver cell line QSG7701 via the caspase-dependent mitochondrial apoptotic pathways. This will contribute to a better understanding of the apoptotic process induced by CPF and promote the awareness of CPF as a widely used insecticide with human health effects. However, we could not demonstrate whether the induction of apoptosis of the human hepatocyte is also related to other pathways induced by CPF in vitro with our present data. This will be our focus in future studies.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahmed, M. M., & Zaki, N. I. (2009). Assessment the ameliorative effect of pomegranate and rutin on Chlorpyrifos-ethyl-induced oxidative stress in rats. Nature & Science of Sleep, 7, 49–61.
- Antonsson, B., Montessuit, S., Sanchez, B., & Martinou, J.-C. (2001). Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. Journal of Biological Chemistry, 276, 11615–11623. doi: https://doi.org/10.1074/jbc.M010810200
- Bagley, C., Rodberg, G., Wellings, D., & Young, L. (1989). Topography of beta NGF receptor-positive and AChE-reactive neurons in the central nervous system. EXS, 57, 50–58.
- Bolognesi, C., Peluso, M., Degan, P., Rabboni, R., Munnia, A., & Abbondandolo, A. (1994). Genotoxic effects of the carbamate insecticide, methyomyl. II. In vivo studies with pure compound and the technical formulation, “Lannate 25.” Environmental & Molecular Mutagenesis, 24, 235–242. doi: https://doi.org/10.1002/em.2850240313
- Cao, G., Minami, M., Pei, W., Yan, C., Chen, D., O’Horo, C., … , Chen, J. (2001). Intracellular Bax translocation after transient cerebral ischemia: Implications for a role of the mitochondrial apoptotic signaling pathway in ischemic neuronal death. Journal of Cerebral Blood Flow & Metabolism, 21, 321–333. doi: https://doi.org/10.1097/00004647-200104000-00001
- Chauvin, C., De Oliveira, F., Ronot, X., Mousseau, M., Leverve, X., & Fontaine, E. (2001). Rotenone inhibits the mitochondrial permeability transition-induced cell death in U937 and KB cells. Journal of Biological Chemistry, 276, 41394–41398. doi: https://doi.org/10.1074/jbc.M106417200
- Chen, L. B. (1988). Mitochondrial membrane potential in living cells. Annual Review of Cell Biology, 4, 155–181. doi: https://doi.org/10.1146/annurev.cb.04.110188.001103
- Cho, D.-H., Nakamura, T., & Lipton, S. A. (2010). Mitochondrial dynamics in cell death and neurodegeneration. Cellular & Molecular Life Sciences, 67, 3435–3447. doi: https://doi.org/10.1007/s00018-010-0435-2
- Elmore, S. (2007). Apoptosis: A review of programmed cell death. Toxicologic Pathology, 35, 495–516. doi: https://doi.org/10.1080/01926230701320337
- Evan, G. (1997). Cancer – A matter of life and cell death. International Journal of Cancer, 71, 709–711. doi: https://doi.org/10.1002/(SICI)1097-0215(19970529)71:5<709::AID-IJC2>3.0.CO;2-V
- Fuchs, Y., & Steller, H. (2011). Programmed cell death in animal development and disease. Cell, 147, 1640. doi: https://doi.org/10.1016/j.cell.2011.11.045
- Garrido, C., Galluzzi, L., Brunet, M., Puig, P. E., Didelot, C., & Kroemer, G. (2006). Mechanisms of cytochrome c release from mitochondria. Cell Death & Differentiation, 13, 1423–1433. doi:10.1038/sj.cdd.4401950
- Gross, A., McDonnell, J. M., & Korsmeyer, S. J. (1999). BCL-2 family members and the mitochondria in apoptosis. Genes & Development, 13, 1899–1911. doi: https://doi.org/10.1101/gad.13.15.1899
- Hüttemann, M., Pecina, P., Rainbolt, M., Sanderson, T. H., Kagan, V. E., Samavati, L., … , Lee, I. (2011). The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: From respiration to apoptosis. Mitochondrion, 11, 369–381. doi: https://doi.org/10.1016/j.mito.2011.01.010
- Jang, Y., Lee, A. Y., Jeong, S.-H., Park, K.-H., Paik, M.-K., Cho, N.-J., … Cho, M.-H. (2015). Chlorpyrifos induces NLRP3 inflammasome and pyroptosis/apoptosis via mitochondrial oxidative stress in human keratinocyte HaCaT cells. Toxicology, 338, 37–46. doi: https://doi.org/10.1016/j.tox.2015.09.006
- Kaufmann, S. H., et al. (1993). Specific proteolytic cleavage of poly(ADP-ribose) polymerase: An early marker of chemotherapy-induced apoptosis. Cancer Research, 53, 3976–3985.
- Kerr, J. F. R., Wyllie, A. H., & Currie, A. R. (1972). Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. British Journal of Cancer, 26, 239–257. doi: https://doi.org/10.1038/bjc.1972.33
- Ki, Y.-W., Park, J. H., Lee, J. E., Shin, I. C., & Koh, H. C. (2013). JNK and p38 MAPK regulate oxidative stress and the inflammatory response in Chlorpyrifos-induced apoptosis. Toxicology Letters, 218, 235–245. doi: https://doi.org/10.1016/j.toxlet.2013.02.003
- Koya, R. C., Fujita, H., Shimizu, S., Ohtsu, M., Takimoto, M., Tsujimoto, Y., & Kuzumaki, N. (2000). Gelsolin inhibits apoptosis by blocking mitochondrial membrane potential loss and cytochrome c release. Journal of Biological Chemistry, 275, 15343–15349. doi: https://doi.org/10.1074/jbc.275.20.15343
- Kroemer, G., Petit, P., Zamzami, N., Vayssiere, J. L., & Mignotte, B. (1995). The biochemistry of programmed cell death. Faseb Journal Official Publication of the Federation of American Societies for Experimental Biology, 9, 1277–1287. doi: https://doi.org/10.1096/fasebj.9.13.7557017
- Lee, J. E., Park, J. H., Shin, I. C., & Koh, H. C. (2012). Reactive oxygen species regulated mitochondria-mediated apoptosis in PC12 cells exposed to Chlorpyrifos. Toxicology & Applied Pharmacology, 263, 148–162. doi: https://doi.org/10.1016/j.taap.2012.06.005
- Li, K., Li, Y., Shelton, J. M., Richardson, J. A., Spencer, E., Chen, Z. J., … Williams, R. S. (2000). Cytochrome c deficiency causes embryonic lethality and attenuates stress-induced apoptosis. Cell, 101, 389–399. doi: https://doi.org/10.1016/S0092-8674(00)80849-1
- Liu, X., Kim, C. N., Yang, J., Jemmerson, R., & Wang, X. (1996). Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell, 86, 147–157. doi: https://doi.org/10.1016/S0092-8674(00)80085-9
- Ly, J. D., Grubb, D. R., & Lawen, A. (2003). The mitochondrial membrane potential (delta psi(m)) in apoptosis; an update. Apoptosis, 8, 115–128. doi: https://doi.org/10.1023/A:1022945107762
- Miyashita, T., Krajewski, S., Krajewska, M., Wang, H. G., Lin, H. K., Liebermann, D. A., & Hoffman, B. (1994). Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene, 9, 1799–1805.
- Morishima, N., Nakanishi, K., Takenouchi, H., Shibata, T., & Yasuhiko, Y. (2002). An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Journal of Biological Chemistry, 277, 34287–34294. doi: https://doi.org/10.1074/jbc.M204973200
- Moser, V. C. (2000). Dose–response and time-course of neurobehavioral changes following oral Chlorpyrifos in rats of different ages. Neurotoxicology & Teratology, 22, 713–723. doi: https://doi.org/10.1016/S0892-0362(00)00087-8
- Nagata, S. (1997). Apoptosis by death factor. Cell, 88, 355–365. doi: https://doi.org/10.1016/S0092-8674(00)81874-7
- Ott, M., Robertson, J. D., Gogvadze, V., Zhivotovsky, B., & Orrenius, S. (2002). Cytochrome c release from mitochondria proceeds by a two-step process. Proceedings of the National Academy of Sciences of the United States of America,99, 1259-1263. doi: https://doi.org/10.1073/pnas.241655498
- Perfettini, J. L., Roumier, T., Castedo, M., Larochette, N., Boya, P., Raynal, B., … , Kroemer, G. (2004). NF-κB and p53 are the dominant apoptosis-inducing transcription factors elicited by the HIV-1 envelope. Journal of Experimental Medicine, 199, 629–640. doi: https://doi.org/10.1084/jem.20031216
- Petronilli, V., Miotto, G., Canton, M., Brini, M., Colonna, R., Bernardi, P., & Di Lisa, F. (1999). Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophysical Journal, 76, 725–734. doi: https://doi.org/10.1016/S0006-3495(99)77239-5
- Saulsbury, M. D., Heyliger, S. O., Wang, K., & Johnson, D. J. (2009). Chlorpyrifos induces oxidative stress in oligodendrocyte progenitor cells. Toxicology, 259, 1–9. doi: https://doi.org/10.1016/j.tox.2008.12.026
- Scaduto, R. C., & Grotyohann, L. W. (1999). Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophysical Journal, 76, 469–477. doi: https://doi.org/10.1016/S0006-3495(99)77214-0
- Susin, S. A., Lorenzo, H. K, Zamzami, N., Marzo, I., Snow, BE., Brothers, GM., & Kroemer, G. (1999). Molecular characterization of mitochondrial apoptosis-inducing factor. Nature International Weekly Journal of Science, 397, 441–446.
- Susin, S. A., Zamzami, N., Castedo, M., Hirsch, T., Marchetti, P., & Macho, A. (1996). Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. Journal of Experimental Medicine, 184, 1331–1341. doi: https://doi.org/10.1084/jem.184.4.1331
- Tang, D., & Kidd, V. J. (1998). Cleavage of DFF-45/ICAD by multiple caspases is essential for its function during apoptosis. Journal of Biological Chemistry, 273, 28549–28552. doi: https://doi.org/10.1074/jbc.273.44.28549
- Taylor, R. C., Cullen, S. P., & Martin, S. J. (2008). Apoptosis: Controlled demolition at the cellular level. Nature Reviews Molecular Cell Biology, 9, 231–241. doi: https://doi.org/10.1038/nrm2312
- Wang, X., Xing, H., Jiang, Y., Wu, H., Sun, G., Xu, Q., & Xu, S. (2013). Accumulation, histopathological effects and response of biochemical markers in the spleens and head kidneys of common carp exposed to atrazine and Chlorpyrifos. Food & Chemical Toxicology An International Journal Published for the British Industrial Biological Research Association, 62, 148–158. doi: https://doi.org/10.1016/j.fct.2013.08.044
- Xing, H., Zhang, Z., Yao, H., Liu, T., Wang, L., Xu, S., & Li, S. (2014). Effects of atrazine and Chlorpyrifos on cytochrome P450 in common carp liver. Chemosphere, 104, 244–250. doi: https://doi.org/10.1016/j.chemosphere.2014.01.002
- Yu, F., Wang, Z., Ju, B., Wang, Y., Wang, J., Bai, D. (2008). Apoptotic effect of organophosphorus insecticide Chlorpyrifos on mouse retina in vivo via oxidative stress and protection of combination of vitamins C and E. Experimental & Toxicologic Pathology, 59, 415–423. doi: https://doi.org/10.1016/j.etp.2007.11.007
- Zhang, H., Huang, Q.H., Ke, N., Matsuyama, S., Hammock, B., Godzik, A., & Reed, J.C. (2000). Drosophila pro-apoptotic Bcl-2/Bax homologue reveals evolutionary conservation of cell death mechanisms. Journal of Biological Chemistry, 275, 27303–27306.
- Zhang, B., Xu, Z., Zhang, Y., Shao, X., Xu, X., Cheng, J., & Li, Z. (2015). Fipronil induces apoptosis through caspase-dependent mitochondrial pathways in Drosophila S2 cells. Pesticide Biochemistry & Physiology, 119, 81–89. doi: https://doi.org/10.1016/j.pestbp.2015.01.019
