ABSTRACT
The aim of this study was to investigate the effects of route of inoculation and dose on production and avidity of IgY antibodies in chickens. The animals were inoculated with 20 µg of human IgG by intramuscular, intradermal, or subcutaneous routes, or with 2, 20, or 200 µg of human IgG via IM to evaluate the effect of dose. All routes led to significant levels of specific antibodies after the fifth inoculation. There was a significant difference in the levels of antibodies produced with 2 and 200 µg of antigen after the third and seventh inoculation. It was observed that there is no influence of route of inoculation or dose on the avidity of the antibodies produced. Furthermore, the index of avidity is influenced directly by the number of inoculations. These results are important to help future studies where antibody production of IgY from laying hens is necessary.
KEYWORDS:
1. Introduction
In avians, three classes of antibodies are found, IgY, IgM, and IgA (Spillner et al., Citation2012). IgY is the only class found in the egg yolk and provides immunity to the foetus before it is able to generate its own humoral immune response (Carlander, Citation2002). It is estimated that 100 mg of IgY can be recovered from one egg yolk (Dias da Silva & Tambourgi, Citation2010).
In recent years studies have shown the feasibility of application of IgY antibodies in human and veterinary immunotherapy for cystic fibrosis by Pseudomonas aeruginosa (Thomsen et al., Citation2016), diarrhoea by rotavirus (Motoi et al., Citation2005), gastritis by Helicobacter pylori (Wang et al., Citation2014), dental caries by Streptococcus mutans (Bachtiar, Afdhal, Meidyawati, Soejoedono, & Poerwaningsih, Citation2016), diarrhoea in pigs (Kweon et al., Citation2000; da Rosa et al., Citation2015) and canine Parvovirus (Van Nguyen, Umeda, Yokoyama, Tohya, & Kodama, Citation2006).
A major advantage of using IgY antibodies in relation to antibodies produced in mammals is that IgY production is less invasive, as there is no need for blood or even the death of the animal to obtain large amounts of antibodies (Schade et al., Citation1996). Other advantages are the high yield of specific antibodies (Pauly et al., Citation2009; Sheng et al., Citation2015); and the larger immunogenicity compared to antigens of mammals due to phylogenetic distance and easy purification of IgY antibody from egg yolk (De Meulenaer & Huyghebaert, Citation2001; Dias da Silva & Tambourgi, Citation2010). In addition, these antibodies have several important biological properties for immunotherapy and immunodiagnosis as there is no activation of the complement system of mammals, nor interaction with the receptors of the Fc portion of immunoglobulins present on the surface of mammalian cells (Dias da Silva & Tambourgi, Citation2010; Kumar, Abbas, Fausto, & Aster, Citation2010).
In an attempt to improve the production of IgY, several studies have investigated the route of inoculation and antigen dose (Schade et al., Citation2005). These studies show that the levels of production of IgY can be influenced by the inoculation route chosen and antigen dose (Eto et al., Citation2012; Schade et al., Citation2005; Schwarzkopf, Staak, Behn, & Erhard, Citation2000). For example, studies show that the subcutaneous (SC) route results in higher levels of antibodies than other routes, such as the intramuscular (IM) route, but this effect may be dependent on the type of antigen used (Chang, Ou-Yang, Chen, & Chen, Citation1999; Erhard et al., Citation2000). Furthermore, there is a wide range of doses of antigens being used for the production of IgY (Dong, Liu, Xiao, & Li, Citation2008; de Andrade et al., Citation2013). However, doses as small as 1 µg of antigen are apparently enough to trigger significant IgY antibody production (Larsson, Carlander, & Wilhelmsson, Citation1998). Although there are studies on the influence of these factors on the production of antibodies, there is a lack of studies on the influence of these on the avidity of the antibodies produced (Schade et al., Citation2005). Avidity is an important feature of antibodies and directly impacts on the ability of antibodies to neutralise the antigen.
Therefore, this study aimed to investigate the effects of using different routes of inoculation and doses of antigen on production and avidity of IgY antibodies in laying hens.
2. Material and methods
2.1. Animals
White leghorn chickens, 20 weeks old, were kept in the warehouses of the farm school of the Universidade Estadual de Londrina, in cages of galvanised wire, with one animal/cage, at room temperature, with 17 h of light, receiving clean water and laying ration ad libitum. The ration was formulated to meet the minimum requirements proposed by Rostagno et al. (Citation2005). The study was approved by the Animal Experimentation Ethics Committee of the Universidade Estadual de Londrina, process number: 11976.
2.2. Experimental procedure
2.2.1. Effects of inoculation on production and avidity of IgY antibodies
For the analysis of the effect of inoculation route on production and avidity of IgY antibodies, 24 animals were randomly placed into 4 groups. The animals from the control group (CG, n = 6) were inoculated intramuscularly with phosphate-buffered saline (PBS) pH 7.4 in aluminium hydroxide (AH). The remaining laying hens were inoculated with 200 µL containing 20 µg/animal of human IgG (Sigma®, batch: 20H8990) in AH by IM (n = 6), intradermal (ID, n = 6) or SC (n = 6) routes. Additional inoculations were performed on days 15, 30, 60, 105, 150, and 195 after the first inoculation. Before the first inoculation and 7 days after inoculation serum samples were collected and stored at −20°C until the moment of use.
2.2.2. Effects of antigen dose on production and avidity of IgY antibodies
To evaluate the effect of antigen dose on production and avidity of IgY antibodies, 24 animals were randomly divided into 4 groups. In the control group (CG, n = 6) laying hens were inoculated IM with 200 µL of PBS in AH. The remaining animals were inoculated with 200 µL containing human IgG (Sigma®, batch: 20H8990) in AH by IM route in the following concentrations: 2 µg/animal (2 µg, n = 6), 20 µg/animal (20 µg, n = 6), and 200 µg/animal (200 µg, n = 6). Additional inoculations were performed on days 15, 30, 60, 105, 150, and 195 after the first inoculation. Serum samples were obtained and stored as described above.
2.3. Analysis of production and antibody avidity in laying hens
Determination of the levels of antibody production IgY anti-human IgG was performed in polystyrene plates with flat bottoms (Costar®) sensitized with human IgG solution (1.25 µg/mL) in carbonate-sodium bicarbonate buffer (pH 9.6), followed by overnight incubation at 4°C. The plates were washed with PBS 1 x, and blocked with 5% Skim Milk in PBS for 2 h at 37°C. After 3 washes with PBS-0.05% Tween 20, chicken serum (1:1000) was added. The plate was incubated for 1 h at 37°C and washed, as described earlier. Goat anti-Chicken IgY-Fc Fragment Antibody horseradish peroxidase (HRP) Conjugated (Bethyl®, batch: A30-104P-28, 1:40,000) was added and incubated for a further 1 h at 37°C. After a new washing with PBS-0.05% Tween 20, the substrate/chromogen (H2O2/TMBZ) was added. After 5 min at 37°C the reaction was stopped with 2 N H2SO4 and the optical density was determined at 450 nm. All procedures were carried out in automatic equipment for ALISEI immunoassays (Radim Diagnostics, Pomezia, Italy).
Estimation of the avidity of the IgY antibodies anti-human IgG was performed as described previously by de Andrade et al. (Citation2013). After sensitisation of the plaques, as described above, serum samples (1:1000) were incubated for 1 h at 37°C and washed 3 times with PBS-0.05% Tween 20. For each serum sample, two wells were treated with 100 µL of 2 M magnesium chloride (Nuclear, Diadema, SP, Brazil) and two with 100 µL of saline (NaCl 0,9%). The plate was incubated for 30 min at 37°C and washed as described earlier. Next, Goat anti-Chicken IgY-Fc Fragment Antibody HRP Conjugated was added and the other steps followed the protocol described above. The avidity index of IgY antibodies was determined by the relationship between the absorbance obtained at 450 nm in the presence or absence of 2 M magnesium chloride.
2.4. Statistical analysis
Statistical analyses were carried out in the program GraphPad Prism 5. The data were submitted to homogeneity (Levene’s) and normality (Kolmogorov–Smirnov) tests. According to these tests, the ANOVA or Kruskal–Wallis test was applied, followed by a post hoc Bonferroni or Dunn tests, for parametric or nonparametric data, respectively. P values <.05 were considered significant.
3. Results and discussion
Several factors affect the production of antibodies in chickens. Studies with chicken strains have shown that the genetic basis of the animal has a direct effect on its ability to produce antibodies (Carlander, Wilhelmson, & Larsson, Citation2003; Emam et al., Citation2014). This effect is related to the presence of polymorphisms associated with genes involved in the production of antibodies (Gehad, Mashaly, Siegel, Dunnington, & Siegel, Citation1999). Another important factor is nutrition. Several nutrients have an influence on the production of antibodies because they provide essential elements for the immune response (Du et al., Citation2016; Forte et al., Citation2016; Perween et al., Citation2016). One of the most important factors for the production of antibodies is the use of adjuvants. The most commonly used adjuvants are based on aluminium salts and oil emulsions (Heegaard, Fang, & Jungersen, Citation2016). The immunostimulatory effect of adjuvants is associated with antigen retention ability and interaction with receptors for molecular patterns that recognise microbial molecules (He, Zou, & Hu, Citation2015). In addition, several studies have shown the effect of the antigen route and dose on antibody production.
3.1. Routes of inoculation and IgY production
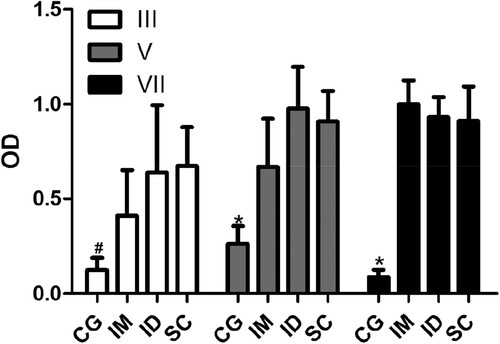
The most common routes for antigen inoculation are IM, SC, and ID routes (Erhard et al., Citation2000; Eto et al., Citation2012; Murphy, Tavares, & Walport, Citation2010). In laying hens, the IM route is the most commonly used for production of antibodies (Schade et al., Citation2005). The results showed that the ID and SC routes, but not the IM route, reached significant levels of specific antibodies after the third inoculation of the antigen. Furthermore, after the fifth inoculation, all routes resulted in significant levels of specific antibodies (). These results are in agreement with Erhard et al. (Citation2000), who using different antigens, including human IgG, showed that antigen inoculation via SC resulted in antibody levels greater than via IM (Erhard et al., Citation2000). A similar result was obtained by Mayo, Persdotter-Hedlund, Tufvesson, and Hau (Citation2003) when analysing the IgY antibody levels anti-bovine serum albumin. However, the effect of routes is apparently dependent on the type of antigen. Studies using complex antigens such as microorganisms or cells show that the IM route can be more efficient than SC. Chang et al. (Citation1999), using S. mutans to compare the effects of IM and SC routes, showed that the production of IgY via IM was almost 10 times greater than through SC. Furthermore Eto et al. (Citation2012), using sheep red blood cells as the antigen observed that the intravenous route resulted in a better response when compared with IM and SC, although there were no significant differences in the levels of antibodies produced by these two routes.
Figure 1. Effects of the inoculation of antigen on the production of specific antibodies. The levels of antibody (IgY) anti-human IgG from serum collected 7 days after the third (III), fifth (V), and seventh inoculation (VII) from animals without inoculation (CG) and inoculated through intramuscular (IM), intradermal (ID), or subcutaneous routes (SC) were determined by indirect enzyme-linked immunosorbent assay (ELISA). The data are shown as mean and standard deviation. #Significant difference between the CG and ID and SC groups (Dunn, P < .007). *Significant difference between CG and IM, ID, and SC groups (Bonferroni, P < .0001).
3.2. Routes of inoculation and IgY avidity
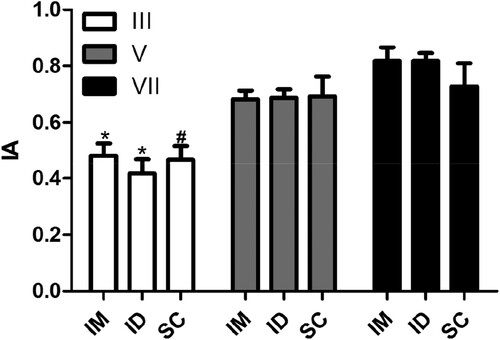
When we investigated the effect of inoculation route on the avidity of the IgY antibodies, no significant differences were observed (). However, we did observe a significant increase in the index of avidity after the fifth inoculation. This effect was observed in all the routes used (). Our results are in accordance with the work performed by de Andrade et al. (Citation2013), who investigated the anti-bothropic and anti-crothalic IgY antibody avidity after seven inoculations. In this study, IgY antibodies presented a significant increase in avidity after the fifth immunisation and this effect remained over time, as in our study.
Figure 2. Influence of route of inoculation of antigen on the avidity index of specific antibodies. The avidity index (AI) was determined by indirect ELISA using the relationship between the serum sample treated with chaotrope (MgCl2 2 M) and the same sample treated with saline. Serum samples collected 7 days after the third (III), fifth (V), and seventh (VII) inoculation of the antigen were analysed from animals without inoculation or inoculated through intramuscular (IM), intradermal (ID), or subcutaneous routes (SC). The data are shown as mean and standard deviation. *Significant difference between samples collected after the third, fifth, and seventh inoculations (IM, Bonferroni, P = .003; ID, Bonferroni, P < .0001). #Significant difference between samples collected after the third and seventh inoculations (Bonferroni, P = .0025).
3.3. Dose of antigen on IgY antibody production
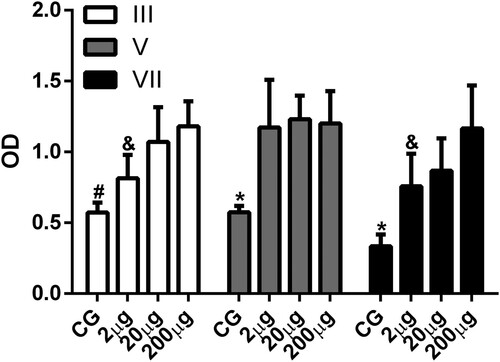
With respect to antigen dose the majority of studies on the production of IgY antibodies use doses between 50 and 200 µg of the antigen in each inoculation by the IM route to induce significant production of IgY antibodies (Camenisch et al., Citation1999; Erhard et al., Citation2000; Matsuda et al., Citation1999; Murphy et al., Citation2010; Zolfagharian & Dounighi, Citation2015). In this work we used doses of 2, 20, and 200 µg of human IgG to investigate the effects of antigen dose on IgY antibody production. Analysis of the levels of specific antibodies produced showed that after the third inoculation there were significant levels of specific antibodies with doses of 20 and 200 µg, but not with 2 µg of antigen (). After the fifth inoculation of antigen there was a significant level of specific antibodies with all doses (). Furthermore, the results obtained showed that there was a significant difference between the levels of specific antibodies produced after the third and seventh inoculation of the 2 and 200 µg doses of antigen (). These results are in line with the work carried out by Erhard et al. (Citation2000) who observed that doses ranging from 0.5 to 1000 µg can lead to significant levels of antibody production depending on the type of antigen used, adjuvant type, and route of inoculation.
Figure 3. Antigen dose effect on the production of specific antibodies. The levels of serum antibody (IgY) anti-human IgG were determined by indirect ELISA. Serum samples collected 7 days after the fifth (V) and seventh (VII) inoculation of animals without inoculation (CG) or inoculated with 2 µg (2 µg), 20 µg (20 µg), and 200 µg (200 µg) of human IgG were analysed. The data are shown as mean and standard deviation. #Significant difference between CG, 20 µg, and 200 µg groups (Bonferroni, P < .0001). *Significant difference between the CG, 2, 20, and 200 µg groups (V, Dunn, P = .0045; VII, Bonferroni, P < 0.0001). &Significant difference between the 2 and 200 µg groups (Bonferroni = P < .0001).
3.4. Dose of antigen on IgY antibody avidity
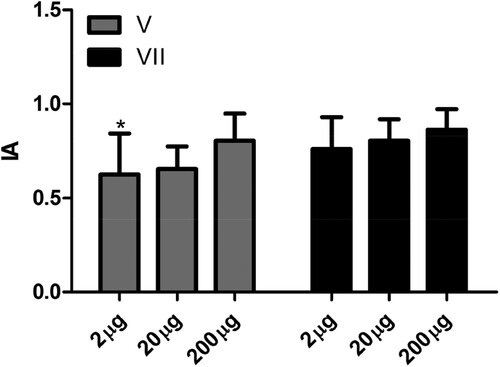
With respect to avidity, in our study we did not observe an effect related to inoculation of different doses of antigen (). With 2 µg of antigen there was a significant increase in IgY antibody avidity between the fifth and seventh inoculation (). There are few studies on the avidity of IgY antibodies. An increase in IgY antibody avidity raised against specific antigens has been observed in different studies only after multiple doses of antigen (Bollen, Crowley, Stodulski, & Hau, Citation1996; de Andrade et al., Citation2013). The results obtained in the present study show that doses as small as 2 µg of antigen are sufficient to produce high levels of specific antibodies with avidity equal to that achieved by inoculation with 200 µg of antigens. These results support the recommendation made by the European Center for the Validation of Alternative Methods (Schade et al., Citation1996) for use of low doses of antigens to obtain significant levels of specific antibodies. In addition, it is important to note that in this study we used AH as an adjuvant, and not Freund’s complete adjuvant (FCA) or Freund’s incomplete adjuvant (FIA). With the use of this adjuvant it was possible to obtain significant levels of specific antibodies with doses of antigens such as 2 µg. It is important to note that aluminium-based adjuvants, such as that used in this study, are the most frequently used in poultry vaccines (Heegaard et al., Citation2016). Their use is associated with less tissue damage when compared with FCA or FIA. However, studies show that this adjuvant results in lower levels of specific antibodies when compared with FCA or FIA. Its mechanisms of action probably involve the deposition effect, a pro-phagocytic action, and activation of the inflammatory response (He et al., Citation2015).
Figure 4. Influence of dose of antigen on the avidity index of specific antibodies. The avidity index (AI) was determined by indirect ELISA using the relationship between the serum sample treated with chaotrope (MgCl2 2 m) and the same sample treated with saline. Serum samples collected 7 days after the fifth (V) and seventh (VII) inoculation of the antigen were analysed in the animals in the control group (CG) without inoculation and inoculated with 2 µg (2 µg), 20 µg (20 µg), and 200 µg (200 µg) of human IgG. The data are shown as medium and standard deviation. *Significant difference between the fifth and seventh inoculation in animals from the 2 µg group (Paired t-test P = .0064).
4. Conclusions
The results obtained in this study show that the dose used for the production of specific antibodies against purified antigens can be over 25–100 times smaller than the doses normally used for this purpose. It is important to note that this was achieved using AH as an adjuvant instead of the commonly used FCA or FIA. This represents a significant reduction in the amount of antigen required for the production of specific antibodies. Whereas the antigen is a limiting factor for production of specific antibodies, we believe that the present study shows the feasibility of production of specific antibodies even with small amounts of antigen. In addition, this study showed that the route of inoculation does not significantly influence the levels or the eagerness of antibodies produced against purified antigens. Therefore, the route of inoculation used in a study may be set by the authors based on variables such as volume and adjuvant used. In addition, the results obtained show that the number of antigen doses significantly influenced the avidity index of specific antibodies produced.
These results are important for allowing the design of protocols for specific IgY antibody production for immunotherapy or immunodiagnosis using low doses of antigens and by an inoculation route defined by variables such as type and volume of the antigen.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bachtiar, E. W., Afdhal, A., Meidyawati, R., Soejoedono, R. D., & Poerwaningsih, E. (2016). Effect of topical anti-Streptococcus mutans IgY gel on quantity of S. mutans on rats’ tooth surface. Acta Microbiologica et Immunologica Hungarica, 63(2), 159–169. doi: https://doi.org/10.1556/030.63.2016.2.2
- Bollen, L. S., Crowley, A., Stodulski, G., & Hau, J. (1996). Antibody production in rabbits and chickens immunized with human IgG A comparison of titre and avidity development in rabbit serum, chicken serum and egg yolk using three different adjuvants. Journal of Immunological Methods, 191, 113–120. doi.org/10.1016/0022-1759(96)00010-5
- Camenisch, G., Tini, M., Chilov, D., Kvietikova, I., Srinivas, V., Caro, J., … Gassmann, M. (1999). General applicability of chicken egg yolk antibodies: The performance of IgY immunoglobulins raised against the hypoxia-inducible factor 1alpha. The FASEB Journal, 13(1), 81–88. Retrieved from http://www.fasebj.org/content/13/1/81.full.pdf+html doi: https://doi.org/10.1096/fasebj.13.1.81
- Carlander, D. (2002). Avian IgY antibody. In vitro and in vivo (Unpublished master’s thesis). Acta Universitatis Upsaliensis, Uppsala.
- Carlander, D., Wilhelmson, M., & Larsson, A. (2003). Immunoglobulin Y levels in egg yolk from three chicken genotypes. Food and Agricultural Immunology, 15(1), 35–40. doi: https://doi.org/10.1080/0954010031000138087
- Chang, H. M., Ou-Yang, R. F., Chen, Y. T., & Chen, C. C. (1999). Productivity and some properties of immunoglobulin specific against Streptococcus mutans serotype c in chicken egg yolk (IgY). Journal of Agricultural and Food Chemistry, 47(1), 61–66. doi: https://doi.org/10.1021/jf980153u
- da Rosa, D. P., Vieira, M. M., Kessler, A. M., de Moura, T. M., Frazzon, A. P. G., McManus, C. M., … Ribeiro, A. M. L. (2015). Efficacy of hyperimmunized hen egg yolks in the control of diarrhea in newly weaned piglets. Food and Agricultural Immunology, 26(5), 622–634. doi: https://doi.org/10.1080/09540105.2014.998639
- de Andrade, F. G., Eto, S. F., Ferraro, A. C. N. S., Marito, D. T. G., Vieira, N. J., Cheirubim, A. P., … Venancio, E. J. (2013). The production and characterization of anti-bothropic and anti-crotalic IgY antibodies in laying hens: A long term experiment. Toxicon, 66, 18–24. doi: https://doi.org/10.1016/j.toxicon.2013.01.018
- De Meulenaer, B., & Huyghebaert, A. (2001). Isolation and purification of chicken egg yolk immunoglobulins: A review. Food and Agricultural Immunology, 13(4), 275–288. doi: https://doi.org/10.1080/09540100120094537
- Dias da Silva, W., & Tambourgi, D. V. (2010). Igy: A promising antibody for use in immunodiagnostic and in immunotherapy. Veterinary Immunology and Immunopathology, 135(3-4), 173–180. doi: https://doi.org/10.1016/j.vetimm.2009.12.011
- Dong, D., Liu, H., Xiao, Q., & Li, R. (2008). Affinity purification of egg yolk immunoglobulins (IgY) with a stable synthetic ligand. Journal of Chromatography B, 870(1), 51–54. doi: https://doi.org/10.1016/j.jchromb.2008.05.036
- Du, E., Wang, W., Gan, L., Li, Z., Guo, S., & Guo, Y. (2016). Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. Journal of Animal Science and Biotechnology, 7, 19. doi:10.1186/s40104-016-0079-7. eCollection 2016.
- Emam, M., Mehrabani-Yeganeh, H., Barjesteh, N., Nikbakht, G., Thompson-Crispi, K., … Mallard, B. (2014). The influence of genetic background versus commercial breeding programs on chicken immunocompetence. Poultry Science, 93(1), 77–84. doi: https://doi.org/10.3382/ps.2013-03475
- Erhard, M. H., Mahn, K., Schmidt, P., Oltmer, S., Preisinger, R., Zinsmeister, P., & Stangassinger, M. (2000). Evaluation of various immunisation procedures in laying hens to induce high amounts of specific egg yolk antibodies. Alternatives to Laboratory Animals: ATLA, 28, 63–80. Retrieved from http://www.atla.org.uk/evaluation-of-various-immunisation-procedures-in-laying-hens-to-induce-high-amounts-of-specific-egg-yolk-antibodies/
- Eto, S. F., Andrade, F. G., Pinheiro, J. W., Balarin, M. R., Ramos, S. P., & Venancio, E. J. (2012). Effect of inoculation route on the production of antibodies and histological characteristics of the spleen in laying hens. Brazilian Journal of Poultry Sciencie, 14(1), 63–66. Retrieved from http://www.scielo.br/pdf/rbca/v14n1/a11v14n1.pdf doi: https://doi.org/10.1590/S1516-635X2012000100011
- Forte, C., Moscati, L., Acuti, G., Mugnai, C., Franciosini, M. P., Costarelli, S., … Trabalza-Marinucci, M. (2016). Effects of dietary Lactobacillus acidophilus and Bacillus subtilis on laying performance, egg quality, blood biochemistry and immune response of organic laying hens. Journal of Animal Physiology and Animal Nutrition, 100(5), 977–987. doi:10.1111/jpn.12408. Epub 2015 Nov 28.
- Gehad, A. E., Mashaly, M. M., Siegel, H. S., Dunnington, E. A., & Siegel, P. B. (1999). Effect of genetic selection and MHC haplotypes on lymphocyte proliferation and interleukin-2 like activity in chicken lines selected for high and low antibody production against sheep red blood cells. Veterinary Immunology and Immunopathology, 68(1), 13–24. doi.org/10.1016/S0165-2427(99)00008-2
- He, P., Zou, Y., & Hu, Z. (2015). Advances in aluminum hydroxide-based adjuvant research and its mechanism. Human Vaccines & Immunotherapeutics, 11(2), 477–488. doi:10.1080/21645515.2014.10040260 doi: https://doi.org/10.1080/21645515.2014.1004026
- Heegaard, P. M., Fang, Y., & Jungersen, G. (2016). Novel adjuvants and immunomodulators for veterinary vaccines. Methods in Molecular Biology, 1349, 63–82. doi: https://doi.org/10.1007/978-1-4939-3008-1_5
- Kumar, V., Abbas, A. K., Fausto, N., & Aster, J. C. (2010). Bases patológicas das doenças. Rio de Janeiro: Elsevier.
- Kweon, C. H., Kwon, B. J., Woo, S. R., Kim, J. M., Woo, G. H., … Lee, Y. S. (2000). Immunoprophylactic effect of chicken egg yolk immunoglobulin (Ig Y) against porcine epidemic diarrhea virus (PEDV) in piglets. Journal of Veterinary Medical Science, 62(9), 961–964. doi.org/10.1292/jvms.62.961
- Larsson, A., Carlander, D., & Wilhelmsson, M. (1998). Antibody response in laying hens with small amounts of antigen. Food and Agricultural Immunology, 10, 29–36. doi.org/10.1080/09540109809354966
- Matsuda, H., Mitsuda, H., Nakamura, N., Furusawa, S., Mohri, S., & Kitamoto, T. A. (1999). A chicken monoclonal antibody with specificity for the N-terminal of human prion protein. FEMS Immunology & Medical Microbiology, 23(3), 189–194. doi: https://doi.org/10.1111/j.1574-695X.1999.tb01238.x
- Mayo, S. L., Persdotter-Hedlund, G., Tufvesson, M., & Hau, J. (2003). Systemic immune response of young chickens orally immunized with bovine serum albumin. In Vivo, 17(3), 261–268. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/?term=Systemic+immune+response+of+young+chickens+orally+immunized+with+bovine+serum+albumin
- Motoi, Y., Sato, K., Hatta, H., Morimoto, K., Inoue, S., & Yamada, A. (2005). Production of rabies neutralizing antibody in hen’s eggs using a part of the G protein expressed in Escherichia coli. Vaccine, 23(23), 3026–3032. doi.org/10.1016/j.vaccine.2004.11.071
- Murphy, K., Tavares, P., & Walport, M. (2010). Imunobiologia de Janeway. Porto Alegre: Artmed.
- Pauly, D., Dorner, M., Zhang, X., Hlinak, A., Dorner, B., & Schade, R. (2009). Monitoring of laying capacity, immunoglobulin Y concentration, and antibody titer development in chickens immunized with ricin and botulinum toxins over a two-year period. Poultry Science, 88(2), 281–290. doi: https://doi.org/10.3382/ps.2008-00323
- Perween, S., Kumar, K., Chandramoni, Kumar, S., Singh, P. K., … Dey, A. (2016). Effect of feeding different dietary levels of energy and protein on growth performance and immune status of Vanaraja chicken in the tropic. Veterinary World, 9(8), 893–899. doi: https://doi.org/10.14202/vetworld.2016.893-899
- Rostagno, H. S., Albino, L. F. T., Donzele, J. L., Gomes, P. C., Oliveira, R. F., Lopes, D. C., … Barreto, L. S. T. (2005). Tabelas Brasileiras para aves e suínos. Composição de alimentos e exigências nutricionais (2nd ed.). Viçosa: Universidade Federal Viçosa, 186 p.
- Schade, R., Calzado, E. G., Sarmiento, R., Chacana, P. A., Porankiewicz-Asplund, J., & Terzolo, H. R. (2005). Chicken egg yolk antibodies (IgY-technology): a review of progress in production and use in research and human and veterinary medicine. Alternatives to Laboratory Animals: ATLA, 33(2), 129–154. Retrieved from http://www.atla.org.uk/chicken-egg-yolk-antibodies-igy-technology-a-review-of-progress-in-production-and-use-in-research-and-human-and-veterinary-medicine/
- Schade, R., Staak, C., Hendriksen, C., Erhard, M., Hugl, H., Koch, G., & Straughan, D. (1996). The production of avian (egg yolk) antibodies: IgY. Alternatives to Laboratory Animals: ATLA, 24, 925–934. Retrieved from http://altweb.jhsph.edu/pubs/ecvam/ecvam21.html
- Schwarzkopf, C., Staak, C., Behn, I., & Erhard, M. (2000). Immunisation. In Chicken egg yolk antibodies, production and application: IgY technology (pp. 25–64). Berlin: Springer Lab Manuals.
- Sheng, Y., Ni, H., Zhang, H., Li, Y., Wen, K., & Wang, Z. (2015). Production of chicken yolk IgY to sulfamethazine: Comparison with rabbit antiserum IgG. Food and Agricultural Immunology, 26(3), 305–316. doi: https://doi.org/10.1080/09540105.2014.914468
- Spillner, E., Braren, I., Greunke, K., Seismann, H., Blank, S., & du Plessis, D. (2012). Avian IgY antibodies and their recombinant equivalents in research, diagnostics and therapy. Biologicals, 40(5), 313–322. doi: https://doi.org/10.1016/j.biologicals.2012.05.003
- Thomsen, K., Christophersen, L., Bjarnsholt, T., Jensen, P. Ø., Moser, C., & Høiby, N. (2016). Anti-Pseudomonas aeruginosa IgY antibodies augment bacterial clearance in a murine pneumonia model. Journal of Cystic Fibrosis, 15(2), 171–178. doi: https://doi.org/10.1016/j.jcf.2015.08.002
- Van Nguyen, S., Umeda, K., Yokoyama, H., Tohya, Y., & Kodama, Y. (2006). Passive protection of dogs against clinical disease due to Canine parvovirus-2 by specific antibody from chicken egg yolk. Canadian Journal of Veterinary Research, 70(1), 62–64. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1325096/
- Wang, B., Yang, J., Cao, S., Wang, H., Pan, X., Zhu, J., … Li, M. (2014). Preparation of specific anti-Helicobacter pylori yolk antibodies and their antibacterial effects. International Journal of Clinical and Experimental Pathology, 7(10), 6430–6437. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4230139/
- Zolfagharian, H., & Dounighi, N. M. (2015). Study on development of Vipera lebetina snake anti-venom in chicken egg yolk for passive immunization. Human Vaccines & Immunotherapeutics, 11(11), 2734–2739. doi: https://doi.org/10.4161/21645515.2014.985492
