?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
We examined the effects of loach paste (LP) on liver and immune organs of d-galactose (DG)-induced aging mice. The results showed that LP can alleviate the atrophy of spleen and thymus, and restore the blood glucose levels. LP was particularly beneficial to liver. Liver indices and activities of aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase reduced with high doses of LP. Moreover, LP can improve the antioxidant system in liver by increasing the activities of total superoxide dismutase, glutathione peroxidase, and catalase, reducing malondialdehyde concentration. Furthermore, LP can improve immunity and reduce the inflammation of liver. The swelling of liver cells was alleviated and the cell arrangement returned to normal in the LP group. In addition, gene expressions of HSP70 and tumour necrosis factor-α (TNF-α) in liver which can promote inflammatory cytokine decreased significantly. Thus, regular LP intake effectively relieved DG-induced oxidative stress and alleviated liver and immune organs damage.
1. Introduction
Ageing is a complex process of degenerative changes in tissues and organs and a gradual decline in physiological functions. The accumulation of reactive oxygen species (ROS) can damage cell membranes, proteins, lipids, and DNA (Szeto et al., Citation2014), and exacerbate age-related chronic diseases, such as inflammation, atrophy of immune organs, cancer, and heart disease (Zitka et al., Citation2012). Cells have complex endogenous defense mechanisms to eliminate ROS, including enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) (Gong et al., Citation2016). The rates of production and clearing of free radicals from the body maintain a dynamic equilibrium. However, once the balance is disrupted, excess free radicals can destroy the body’s normal functions, and even induce a variety of diseases (Labat-Robert & Robert, Citation2014).
Oxidation resistance usually refers to the ability of a substance to scavenge free radicals (Halliwell, Citation2011). Many food and herbs are rich in antioxidants (Chen et al., Citation2012; Kasote et al., Citation2015). The oxidation-resistant properties of foods have been a topical area of research for several years, and antioxidants intake can reduce the occurrence of immune disease and inflammation (Arunachalam, Parimelazhagan, & Saravanan, Citation2011).
The loach (Misgurnus anguillicaudatus) is an important freshwater fish in East Asian countries, including China, Japan, and Korea. It has been a culinary favourite since ancient times because of its delicious taste and rich nutritional qualities. Traditional Chinese medicine also regards it as an important medicine (You et al., Citation2011). Many substances in the loach have an antioxidant capacity and may have protective effects on human health (You et al., Citation2010a). In modern medicine, the spermidine in loach is thought to be a precursor of arginine, and high arginine intake can reduce the risk of cardiovascular and hepatopathy, reduce ageing, and improve the reproductive capacity (Kumar, Prakash, & Dogra, Citation2011). In recent years, many studies have demonstrated a variety of pharmacological properties in the loach, including anti-inflammatory, anticancer, and hypoglycaemic activities, and an ability to enhance the immune system, in vivo and/or in vitro (You et al., Citation2010b; You et al., Citation2011).
A large number of studies have demonstrated that the long-term injection of d-galactose (DG) causes the accumulation of excessive ROS (Jeong, Liu, & Kim, Citation2017), enhancing oxidative stress, liver and immune organ damaging the body (Wei et al., Citation2005). The aim of the present study was to evaluate the effects of loach meat paste on the physiological functions of mice in which ageing was induced with DG.
2. Materials and methods
2.1. Preparation for loach paste
Loach (M. anguillicaudatus, 15 ± 1.5 g) was purchased from a market in Wuxi City, China. The head and purtenances of the fresh loach were removed, and the fish was cut into small pieces of 2 cm2 and then minced with an RL-22S meat grinder (Wuhan Ruili Food Machinery Co., Ltd, Wuhan, Hubei Province, China). The loach paste (LP) was prepared by grinding the minced loach with a JML-100 colloid mill (Hangzhou Huihe Machinery Co., Ltd, Hangzhou, Zhejiang Province, China), which was then passed through 100 mesh. The LP was then cooked at 100°C for 20 min. Before the animal experiments, the LP was freeze-dried and ground again.
2.2. Animals and drug administration
Male ICR mice were obtained from Suzhou Xinuosai Biological Technology Co., Ltd (Suzhou, Jiangsu Province, China). The mice were aged 9–11 weeks, weighed 37 ± 2 g, and were housed at 24 ± 2°C, with 60 ± 10% humidity, under a 12-h light/dark cycle. The mice had access to feed and water ad libitum. After a 1-week adaptation period, the mice were randomly divided into 4 groups consisting 15 animals each: control group (NC), model group (MG), low LP (L-LP) group, and high LP (H-LP) group. Except for the NC group, the mice were subcutaneously injected with DG at a dose of 500 mg/kg bodyweight once daily for 6 weeks (Salehpour et al., Citation2017). The NC mice were treated with the same volume of physiological saline. At the same time, the L-LP and H-LP groups of mice were administered LP dissolved in physiological saline at doses of 100 and 500 mg/kg bodyweight, respectively, by oral gavage after the injection of DG. The mice in the NC and MG groups were administered the same volume of physiological saline. The mouse bodyweights were recorded at the beginning of the experiment, and just before the mice were killed.
2.3. Blood and tissue sample preparation
At the end of the feeding trial, the mice were fasted overnight. All surgery, including orbital eye bleeding and euthanasia, were performed under anaesthesia induced with a continuous inhalation of 2% isoflurane via a nose cone. The blood samples were collected and centrifuged at 3000 × g at 4°C for 15 min, and the supernatants were stored at −80°C for biochemical and antioxidant analyses. The livers were removed quickly, weighed, and stored at −80 °C until analysis. The whole spleens and thymuses were removed and weighed accurately.
Calculation of liver index: The liver indices, spleen indices, and thymus indices were calculated with the formulae (Huang et al., Citation2012; Peng et al., Citation2014):
2.4. Haematological biochemical assays
One half of each serum sample was analysed for the biochemical parameters: blood glucose, total protein (TP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), blood urea nitrogen (BUN), creatinine, and blood lipid profiles, including triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C), with a biochemical autoanalyzer (Eurolyser, Leuven, Belgium). The other half was stored at −80°C for the later determination of antioxidant-related parameters.
2.5. Determination of antioxidant parameters
The activities of SOD, GSH-Px, and CAT, and the malondialdehyde (MDA) concentration in the liver and serum were examined using commercial kits (Nanjing Jiancheng Bioengineering Research Institute, Nanjing, Jiangsu Province, China) according to the manufacturer’s protocol.
2.6. Histopathology
The mouse liver samples were fixed in 10% neutral formalin buffer for 48 h, dehydrated in ethanol, and embedded in paraffin. Liver sample sections (5 μm thick) were cut with a microtome. The sections were dewaxed, rehydrated, stained with haematoxylin–eosin, and observed under an XSP-13CC optical microscope (Caikang Optical Instrument Co., Ltd, Shanghai, China).
2.7. RNA extraction and gene expressions analysis
The total RNAs from the liver samples were used to analyse the expression of the heat shock protein 70 gene (HSPa1b) and the tumour necrosis factor-α (TNF-α) gene (Tnf-α). Total RNA was extracted from the liver with TRIzol Reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. The RNA concentration and quality were evaluated with spectrophotometry and agarose-gel electrophoresis. The total RNA (3.0 μg) from each sample was reverse transcribed to cDNA with the RevertAid First Strand cDNA Synthesis Kit (TaKaRa Bio Inc., Tokyo, Japan), according to the instructions of the manufacturer.
Real-time quantitative PCR was performed with SYBR Premix Ex Taq™ (TaKaRa Bio Inc.) in a QuantStudio™ 6 Flex Real-time PCR System (Applied Biosystems, Foster City, CA, USA). The primers for real-time amplification are shown in . The PCR cycling programme was: 95°C for 10 min; 40 cycles of 95°C for 10 s and 60°C for 60 s. The quantities of the target transcript relative to the chosen reference gene transcript (Actb) were calculated with the 2−ΔΔCT method (Zhang et al., Citation2017).
Table 1. Primers used for the detection of the expressions of HSPa1b and TNF-α in mice.
2.8. Statistical analysis
Data are expressed as means ± SD and were analysed with the SPSS software (version 20; SPSS Inc., Chicago, IL, USA). Duncan’s multiple range test was used to detect the differences among groups. Statistical significance was set at p < .05.
3. Results and discussion
3.1. Effects of LP on bodyweight
shows that the mouse bodyweights did not differ significantly among the groups throughout the whole experiment. The injection of DG had no effect on the bodyweights of the mice. This is consistent with the results reported by Peng et al. (Citation2014). LP also had no effect on the mouse bodyweights, but the increase in weight was greater in the NC and H-LP groups than in the MG and L-LP groups.
Table 2. Effect of LP on body weight of d-galactose-treated mice (g, n = 15 mice per group).
3.2. Effects of LP on blood glucose and lipid levels
3.2.1. Effect of LP on blood glucose level
The MG group displayed a significant reduction in blood glucose (). However, after treatment with LP by oral gavage for 6 weeks, the blood glucose was higher in the L-LP and H-LP groups than in the MG group. The blood glucose level in the H-LP group differed significantly from that in the MG group, but was the same as that in the NC group.
Figure 1. Effect of LP on blood glucose level of mice. NC, normal control group; MG, model group; L-LP, low dose of loach meat paste; H-LP, high dose of loach paste. Values are mean ± S.D (n = 15); means with different letters (a–d) differ significantly (p < .05).
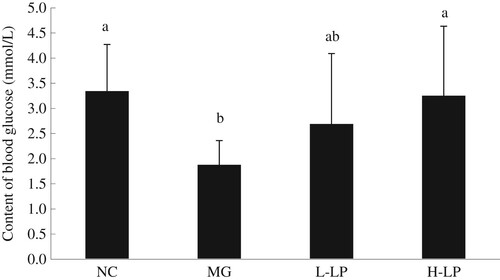
Blood glucose is the main factor providing energy to the body. When subjected to a specific stress stimulus, the body consumes more energy to withstand the external stress (Narne, Pandey, & Phanithi, Citation2017), reducing the blood glucose level. Therefore, prolonged oxidative stress reduces the blood sugar level (Nia, Khorram, Safaiyan, Tarighat-Esfanjani, & Rezazadeh, Citation2017). In this study, the blood glucose level in the MG group was significantly reduced, indicating that the body had consumed a part of its energy supply to resist the stress imposed by DG. However, the blood glucose levels of the LP-treated groups were higher, indicating that LP improves hypoglycaemia.
3.2.2. Effect of LP on serum lipid level
shows that neither DG nor LP had a significant effect on the serum TP, LDL-C, or HDL-C levels in the mice. However, the serum TG levels were significantly reduced in the L-LP and H-LP groups. Similar changes were observed for TC, which were also reduced in the two LP groups. TC was significantly lower in the H-LP groups than in the NC group.
Table 3. Effect of LP on the content of serum protein and lipid of d-galactose-treated mice (n = 15 mice per group).
High serum lipid levels correlate positively with the risk of cardiovascular disease (Kunnen & Van, Citation2012). The serum levels of TC, TG, LDL-C, and HDL-C are commonly used in the diagnosis of diseases associated with lipid metabolic disorders. Abnormally high or low levels of serum TG or TC can contribute to cardiovascular diseases and liver dysfunction (Wooton & Melchior, Citation2017). Our results suggest that LP enhances the lipid metabolism. However, the consumption of LP had no effect on serum TP, LDL-C, or HDL-C.
3.3. Effect of LP on liver, spleen, and thymus
3.3.1. Effect of LP on visceral indices
As shown in (a), compared with the NC mice, the MG mice displayed a significantly increased liver index. The L-LP liver index decreased, but was not significantly different from that of the MG group. However, the liver index was significantly lower in the H-LP mice than in the MG mice. As shown in (b), the MG spleen index did not differ significantly from the NC spleen index. There was no significant difference between the LP-treated and NC groups, but the L-LP and H-LP groups spleen indices were significantly higher than the MG spleen index. As shown in (c), the MG group had a lower thymus index than the NC group. The thymus index was improved in the mice treated with LP, but did not differ significantly from the thymus index in the MG group.
Figure 2. Effect of LP on liver indices (a), spleen indices (b), and thymus indices (c) in each group. NC, normal control group; MG, model group; L-LP, low dose of loach meat paste; H-LP, high dose of loach paste. Values are mean ± S.D (n = 15); means with different letters (a–d) differ significantly (p < .05).
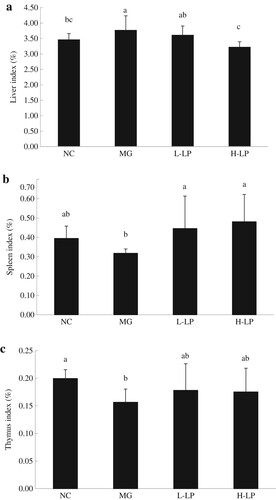
The liver is an important organ for the metabolism (Zhao et al., Citation2017). The invasion of toxic substances can lead to liver cell damage and necrosis, triggering a series of pathological changes in the liver. In this study, the injection of DG damaged the livers of the MG mice by inducing the excessive accumulation of free radicals (Zhang et al., Citation2010). Pathological changes then occurred in their livers, which may have included hyperplasia, liver lumps, and other phenomena, leading to a high liver index. However, the liver index decreased in the LP groups, indicating that LP effectively alleviated the DG-induced damage to the mouse livers. The spleen and thymus are important immune organs in the body, controlling the production of antibodies and immune cells (Liu & Zhao, Citation2017). The spleen and thymus indices directly reflect the body’s immune capacity (Peng et al., Citation2014). In this study, the spleen and thymus indices were restored to normal levels in the LP-treated groups, confirming that LP alleviates oxidative damage and enhances the immunity of mice.
3.3.2. Effect of LP on liver function
As shown in , the same trends in serum ALT, AST, and ALP were observed in the four groups. Compared with the NC group, the three indicators were significantly increased in the MG group, but tended to be lower in the L-LP and H-LP groups than in the MG group. However, the differences in ALT, AST, or ALP between the LP-treated and NC groups were not significant.
Figure 3. Effect of LP on the activities of ALT (a), AST (b), and ALP (c) in serum of mice. NC, normal control group; MG, model group; L-LP, low dose of loach meat paste; H-LP, high dose of loach paste. Values are mean ± S.D (n = 15); means with different letters (a–d) differ significantly (p < .05).
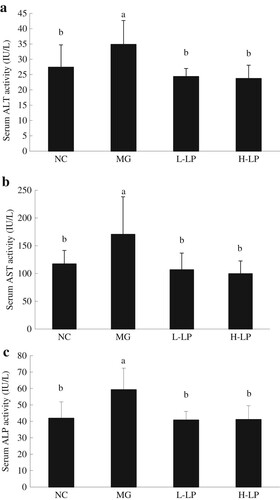
ALT, AST, and ALP are normally present in hepatocytes and their levels in the blood are usually consistent with the degree of liver damage (Zhang, Chen, Sun, & Zhao, Citation2014). During liver inflammation or dysfunction, these enzyme activities increase. In this study, the three enzyme activities increased in the MG group to varying degrees, mainly in response to the damage caused by the accumulation of ROS induced in the liver by DG. The enzyme activities in the LP-treated groups were reduced, probably because the loach polysaccharides, peptides, and other ingredients of LP ameliorate liver injury.
3.3.3. Effects of LP on liver histomorphology
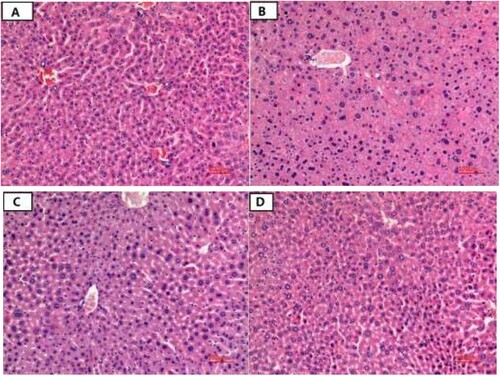
Haematoxylin–eosin-stained liver tissue sections from the mice are shown in . The NC group showed a normal liver tissue structure, with the polygonal, uniformly sized liver cells arranged neatly. The nuclei were round, clear, and located in the cell centres. The cell sizes did not differ greatly and the cytoplasm was evenly stained (red). The liver sinus structure was clear and wide ((a)). In the MG group, the arrangement of the liver cells was disordered, the sizes of the cell nuclei varied, and some of the cell nuclei had dissolved. The swelling of local hepatocytes resulted in the infiltration of inflammatory cells, but no obvious necrosis was detected ((b)). In the L-LP group, the swelling of the liver cells was reduced, the hepatic sinusoids were widened, the cell nuclei still varied in size, and the infiltration of inflammatory cells was detected ((c)). However, in the H-LP group, the swelling of the liver cells was alleviated and the cell arrangement had returned to normal, and did not differ from that of the NC group ((d)).
3.4. Effects of LP on antioxidant ability and immunity of DG-induced ageing mice
3.4.1. Effects of LP on serum and liver antioxidants
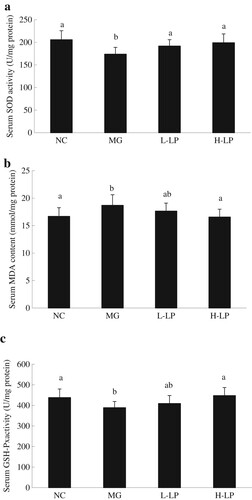
The activity of serum SOD was significantly lower in the MG group than in the NC group ((a)). The serum concentration of MDA was significantly higher in the MG group than in the NC group ((b)). (c) shows that the activity of serum GSH-Px was significantly lower in the MG group than in the NC group. Therefore, the consumption of LP had an impact on the antioxidation indicators in mice insofar as the serum SOD and GSH-Px activities were higher and the MDA contents were lower in the LP groups than in the MG group.
Figure 5. The serum levels of SOD (a), MDA (b), and GSH-Px (c) in each group. NC, normal control group; MG, model group; L-LP, low dose of loach meat paste; H-LP, high dose of loach paste. Values are mean ± SD (n = 15). means with different letters (a–d) differ significantly (p < .05).
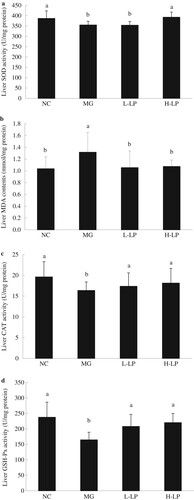
The liver SOD activity was significantly reduced in the MG group compared with the NC group ((a)). The concentration of liver MDA was significantly higher in the MG group than in the NC group ((b)). CAT activity was higher in the livers of the LP groups than in the MG group, but the differences were not significant ((c)). The injection of DG also clearly reduced the GSH-PX level in the MG group ((d)).
Figure 6. The liver levels of SOD (a), MDA (b), CAT (c), and GSH-Px (d) in each group. NC, normal control group; MG, model group; L-LP, low dose of loach meat paste; H-LP, high dose of loach paste. Values are mean ± SD (n = 15). Means with different letters (a–d) differ significantly (p < .05).
SOD, GSH-PX, and CAT are important antioxidant enzymes in the body, with important roles in removing the excess ROS produced by oxidative stress (Castro-Alférez, Polo-López, Marugán, & Fernández-Ibáñez, Citation2017). Reductions in the activities of these three enzymes indicate that the body has been damaged by ROS and its antioxidant capacity has decreased. The accumulation of large numbers of free radicals seriously damages body tissues, further injuring the antioxidant system. Therefore, the status of an animal’s free-radical-scavenging capacity can be evaluated from the activities of SOD, CAT, and GSH-Px. MDA is a direct product of lipid peroxidation, which is closely related to the cell damage caused by oxidative stress (Chowdhury et al., Citation2017). It is often used as an indicator of the degree of peroxidation. When external oxidative stress intensifies, the body’s MDA content increases significantly.
Our results show that LP intake reduced the MDA content and increased the activities of antioxidant enzymes. Therefore, regular LP intake effectively relieved the oxidative stress on the body. This comprehensive analysis of the microstructure of the liver and the changes in antioxidant indicators demonstrate that LP alleviates liver damage and protects the liver.
3.4.2. Effects of LP on HSP70 and TNF-α mRNA expression in liver
As shown in (a), the hepatic expression of HSPa1b was significantly higher in the MG group than in the NC group, whereas the expression of HSPa1b was significantly lower in the LP groups than in the NC group. As shown in (b), compared with the NC group, the expression of Tnf-α in the MG group was significantly elevated, whereas that in the L-LP and H-LP groups was significantly lower than in the MG group, and did not differ significantly from that in the NC group.
Figure 7. Liver genes expression of HSPa1b (a) and TNF-α (b) in each group. NC, normal control group; MG, model group; L-LP, low dose of loach meat paste; H-LP, high dose of loach paste. Values are mean ± SD (n = 15). Means with different letters (a–d) differ significantly (p < .05).
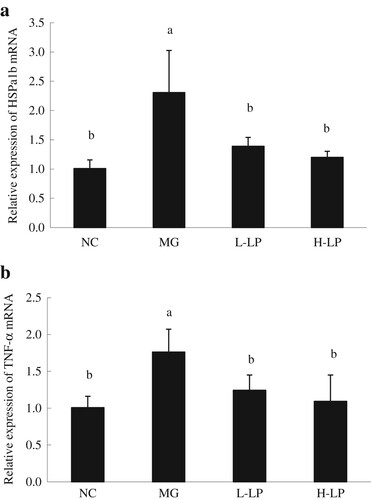
HSP70 is one of the heat shock proteins (HSPs) which plays an important role in the regulation of innate immune response. It is also involved in the immune response under stress, in intracellular trafficking, anti-apoptosis, and antigen processing (Zhang et al., Citation2015). HSP70 can promote the immune system to produce pro-inflammatory cytokines by stimulating the innate immune cells. The HSP70 multigene family contains HSPa1a, HSPa1b, and HSPa1l. HSPa1b plays an important role in folding newly synthesized proteins and refolding denatured proteins (Jiang et al., Citation2012). The expression of HSPa1b changes during liver injury, and was significantly higher in the livers of the MG group than in those of the NC group. However, it was significantly lower in the LP groups than in the MG group, but it did not differ significantly between the LP and NC groups. This indicates that the injection of DG, an exogenous oxidative stimulant, increased HSPa1b expression, whereas LP had an antioxidant function, alleviating the exogenous oxidative effect, improving immunity, and did not stimulate the expression of HSPa1b.
TNF-α is a pro-inflammatory cytokine secreted by T lymphocytes and activated macrophages, which plays an important role in regulating cellular immunity and resisting viral and bacterial invasion. It is also involved in tumour progression, ageing, and other pathophysiological processes (Vimala, Hussain, & Wan Nazaimoon, Citation2012). In this study, a significant increase in TNF-α gene (Tnf-α) expression in the MG group showed that the injection of DG caused the accumulation of large amounts of ROS, which induced the excessive expression of Tnf-α. Our results show that LP eliminated some of the ROS, maintaining the balance of the body’s oxidative system, improving immunity, and thus restoring the stable expression of Tnf-α.
4. Conclusion
The biological activities of LP were studied in a DG-induced ageing mouse model. Our results indicate that LP has antioxidative activity in vivo and alleviates the liver damage caused by oxidative stress. The loach has been a favourite Chinese traditional medicine and food for thousands of years. This study provides new evidence of its biological activities.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Arunachalam, K., Parimelazhagan, T., & Saravanan, S. (2011). Phenolic content and antioxidant potential of Sarcostigma kleinii wight. & Arn. Food and Agricultural Immunology, 22(2), 161–170. doi: https://doi.org/10.1080/09540105.2010.549211
- Castro-Alférez, M., Polo-López, M. I., Marugán, J., & Fernández-Ibáñez, P. (2017). Mechanistic model of the Escherichia coli, inactivation by solar disinfection based on the photo-generation of internal ROS and the photo-inactivation of enzymes: CAT and SOD. Chemical Engineering Journal, 318(15), 214–223. doi: https://doi.org/10.1016/j.cej.2016.06.093
- Chen, J., Sun, H. N., Sun, A. D., Lin, Q. H., Wang, Y., & Tao, X. Y. (2012). Studies of the protective effect and antioxidant mechanism of blueberry anthocyanins in a CC14-induced liver injury model in mice. Food and Agricultural Immunology, 23(4), 352–362. doi: https://doi.org/10.1080/09540105.2011.634378
- Chowdhury, M. I., Hasan, M., Islam, M. S., Sarwar, M. S., Amin, M. N., Uddin, S. M. N., … Hussain, M. S. (2017). Elevated serum MDA and depleted non-enzymatic antioxidants, macro-minerals and trace elements are associated with bipolar disorder. Journal of Trace Elements in Medicine and Biology, 39, 162–168. doi: https://doi.org/10.1016/j.jtemb.2016.09.012
- Gong, Y. S., Guo, J., Hu, K., Gao, Y. Q., Xie, B. J., Sun, Z. D., … Hou, F. L. (2016). Ameliorative effect of lotus seedpod proanthocyanidins on cognitive impairment and brain aging induced by D-galactose. Experimental Gerontology, 74, 21–28. doi: https://doi.org/10.1016/j.exger.2015.11.020
- Halliwell, B. (2011). Free radicals and antioxidants – quo vadis? Trends in Pharmacological Sciences, 32(3), 125–130. doi: https://doi.org/10.1016/j.tips.2010.12.002
- Huang, Q., Zhang, S., Zheng, L., He, M., Huang, R., & Lin, X. (2012). Hepatoprotective effects of total saponins isolated from Taraphochlamys affinis against carbon tetrachloride induced liver injury in rats. Food and Chemical Toxicology, 50(3), 713–718. doi: https://doi.org/10.1016/j.fct.2011.12.009
- Jeong, H., Liu, Y. N., & Kim, H. S. (2017). Dried plum and chokeberry ameliorate D-galactose-induced aging in mice by regulation of Pl3k/Akt-mediated Nrf2 and Nf-kB pathways. Experimental Gerontology, 95, 16–25. doi: https://doi.org/10.1016/j.exger.2017.05.004
- Jiang, J. L., Shi, Y., Shan, Z. J., Yang, L. Y., Wang, X. R., & Shi, L. L. (2012). Bioaccumulation, oxidative stress and HSP70 expression in Cyprinus carpio, L. exposed to microcystin-LR under laboratory conditions. Comparative Biochemistry & Physiology Part C Toxicology & Pharmacology, 155(3), 483–490. doi: https://doi.org/10.1016/j.cbpc.2011.12.008
- Kasote, D. M., Katyare, S. S., Hegde, M. V., & Bae, H. (2015). Significance of antioxidant potential of plants and its relevance to therapeutic applications. International Journal of Biological Sciences, 11(5), 982–991. doi: https://doi.org/10.7150/ijbs.12096
- Kumar, A., Prakash, A., & Dogra, S. (2011). Protective effect of curcumin (Curcuma longa) against D-galactose-induced senescence in mice. Food and Agricultural Immunology, 13(1), 42–55.
- Kunnen, S., & Van, E. M. (2012). Lecithin: Cholesterol acyltransferase: Old friend or foe in atherosclerosis. Journal of Lipid Research, 53, 1783–1799. doi: https://doi.org/10.1194/jlr.R024513
- Labat-Robert, J., & Robert, L. (2014). Longevity and aging. Role of free radicals and xanthine oxidase. A review. Pathologie Biologie, 62(2), 61–66. doi: https://doi.org/10.1016/j.patbio.2014.02.009
- Liu, X., & Zhao, X. H. (2017). Immune potentials of the mucor-fermented Mao-tofu and especially its soluble extracts for the normal mice. Food and Agricultural Immunology, 28(5), 859–875. doi: https://doi.org/10.1080/09540105.2017.1318834
- Narne, P., Pandey, V., & Phanithi, P. B. (2017). Interplay between mitochondrial metabolism and oxidative stress in ischemic stroke: An epigenetic connection. Molecular and Cellular Neuroscience, 82, 176–194. doi: https://doi.org/10.1016/j.mcn.2017.05.008
- Nia, B. H., Khorram, S., Safaiyan, A., Tarighat-Esfanjani, A., & Rezazadeh, H. (2017). The effects of natural clinoptilolite and nano-sized clinoptilolite supplementation on glucose levels and oxidative stress in rats with streptozotocin-induced diabetes. Canadian Journal of Diabetes, 82, 176–194.
- Peng, X. Y., Kong, B. H., Yu, H. Y., & Diao, X. P. (2014). Protective effect of whey protein hydrolysates against oxidative stress in D-galactose-induced ageing rats. International Dairy Journal, 34(1), 80–85. doi: https://doi.org/10.1016/j.idairyj.2013.08.004
- Salehpour, F., Ahmadian, N., Rasta, S. H., Farhoudi, M., Karimi, P., & Sadigh-Eteghad, S. (2017). Transcranial low-level laser therapy improves brain mitochondrial function and cognitive impairment in D-galactose-induced aging mice. Neurobiology of Aging, 58, 140–150. doi: https://doi.org/10.1016/j.neurobiolaging.2017.06.025
- Szeto, Y.T., Tse, R.S.C., Benzie, I.F.F., Kalle, W., & Pak, S.C. (2014). An in vitro study of Sanchi (Panax pseudoginseng) for its DNA protective effect. Food and Agricultural Immunology, 25(3), 332–340. doi: https://doi.org/10.1080/09540105.2013.794202
- Vimala, B., Hussain, H., & Wan Nazaimoon, W. M. (2012). Effects of edible bird’s nest on tumour necrosis factor-alpha secretion, nitric oxide production and cell viability of lipopolysaccharide-stimulated RAW 264.7 macrophages. Food and Agricultural Immunology, 23(4), 303–314. doi: https://doi.org/10.1080/09540105.2011.625494
- Wei, H., Li, L., Song, Q., Ai, H., Chu, J., & Li, W. (2005). Behavioural study of the D-galactose induced aging model in C57BL/6J mice. Behavioural Brain Research, 157(2), 245–251. doi: https://doi.org/10.1016/j.bbr.2004.07.003
- Wooton, A. K., & Melchior, L. M. (2017). Obesity and type 2 diabetes in our youth: A recipe for cardiovascular disease. The Journal for Nurse Practitioners, 13(3), 222–227. doi: https://doi.org/10.1016/j.nurpra.2016.08.035
- You, L., Zhao, M., Liu, R. H., & Regenstein, J. M. (2011). Antioxidant and antiproliferative activities of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. Journal of Agricultural & Food Chemistry, 59(14), 7948–7953. doi: https://doi.org/10.1021/jf2016368
- You, L., Zhao, M., Regenstein, J. M., & Ren, J. Y. (2010a). Changes in the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates during a simulated gastrointestinal digestion. Food Chemistry, 120(3), 810–816. doi: https://doi.org/10.1016/j.foodchem.2009.11.018
- You, L., Zhao, M., Regenstein, J. M., & Ren, J. (2010b). Purification and identification of antioxidative peptides from loach (Misgurnus anguillicaudatus) protein hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Research International, 43(4), 1167–1173. doi: https://doi.org/10.1016/j.foodres.2010.02.009
- Zhang, S., Chen, J., Sun, A., & Zhao, L. Y. (2014). Protective effects and antioxidant mechanism of bamboo leaf flavonoids on hepatocytes injured by CCl4. Food and Agricultural Immunology, 25(3), 386–396. doi: https://doi.org/10.1080/09540105.2013.810709
- Zhang, C. N., Li, X. F., Tian, H. Y., Zhang, D. D., Jiang, G. Z., Lu, K. L., … Liu, W. B. (2015). Effects of fructooligosaccharide on immune response, antioxidant capability and HSP70 and HSP90 expressions of blunt snout bream (Megalobrama amblycephala) under high ammonia stress. Fish Physiology and Biochemistry, 41(1), 203–217. doi: https://doi.org/10.1007/s10695-014-0017-6
- Zhang, T. T., Lu, Q. Q., Su, C. L., Yang, Y. R., Hu, D., & Xu, Q. S. (2017). Mercury induced oxidative stress, DNA damage, and activation of antioxidative system and Hsp70 induction in duckweed (Lemna minor). Ecotoxicology and Environmental Safety, 143, 46–56. doi: https://doi.org/10.1016/j.ecoenv.2017.04.058
- Zhang, Z. F., Lu, J., Zheng, Y. L., Hu, B., Fan, S. H., Wu, D. M., … Liu, C. M. (2010). Purple sweet potato color protects mouse liver against D-galactose-induced apoptosis via inhibiting caspase-3 activation and enhancing PI3 K/Akt pathway. Food and Chemical Toxicology, 48(8–9), 2500–2507. doi: https://doi.org/10.1016/j.fct.2010.06.023
- Zhao, X. Y., Wang, L. Q., Zhang, H., Zhang, D. D., Zhang, Z. H., & Zhang, J. (2017). Protective effect of artemisinin on chronic alcohol induced-liver damage in mice. Environmental Toxicology and Pharmacology, 52, 221–226. doi: https://doi.org/10.1016/j.etap.2017.04.008
- Zitka, O., Skalickova, S., Gumulec, J., Masarik, M., Adam, V., Hubalek, J., … Kizek, R. (2012). Redox status expressed as GSH: GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncology Letters, 4, 1247–1253. doi: https://doi.org/10.3892/ol.2012.931