?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
An enzyme-linked immunosorbent assay to measure prednisolone have been developed. An antibody was raised in rabbits using prednisolone-21-hemisuccinate (PSL-21-HS) conjugated to bovine serum albumin. Similarly, PSL-21-HS was conjugated to horseradish peroxidase to prepare enzyme conjugate. The developed assay has been validated for sensitivity and effective displacement at 50% of the assay were 0.078 and 2.64 ng/mL, respectively. This assay showed cross-reaction with only 4 steroids – i.e. progesterone 1.76%, 17α-OH progesterone 5.89%, cortisol 7.69% and prednisone 1.13%, out of 55 analogous steroids. The percent recovery of prednisolone from the exogenously spiked human serum pools was in the range of 94.84–100.17%. The intra-assay and inter-assay coefficients of variation ranged from 5.79% to 8.00% and from 3.23% to 8.63%, respectively. The serum prednisolone values obtained by this method correlated well with the commercially available kit and found to be 0.93.
1. Introduction
The prednisolone is a synthetic analogue of endogenous cortisol, having potent glucocorticoid and low mineralocorticoid activity, and is used in the treatment and management of a broad range of ailments, including severe asthma, rheumatic arthritis, gastrointestinal and hematological disorders (Fiel & Vincken, Citation2006; Thrower, Citation2009). Although the actions of this agent is largely palliative rather than curative, it remain the first-line option for many physicians in the treatment of disease (Schimmer & Parker, Citation2001). According to Indian Pharmacopoeia, in India it is officially permitted to use in treatment of Addison’s disease, in organ transplant and drug-resistant diseases (Cushing’s syndrome) (Tripathi, Citation2008). Glucocorticoid belongs to steroid family, particularly of pregnane class containing C-21 derivatives. Glucocorticoids have important functions upon carbohydrate, protein and calcium metabolism; it also possesses potent anti-inflammatory and immunosuppressive activities (Beotra et al., Citation2009; Deventer, Thuyne, Mikulcikova, Van Eenoo, & Delbeke, Citation2007; Ramsay et al., Citation2003; Uchiyama, Hanajiri, Kawahara, & Goda, Citation2009). The activity of glucocorticoids largely depends upon the substituent attached to the nucleus. It has been found that presence of Δ1, 2 in corticosteroids enhances anti-inflammatory activity and decreases salt-retaining activity (Delgado & Remers, Citation1998). PSL is often misused in sports due to its anti-inflammatory effect, which leads to a decrease in pain and increases an athlete’s ability (Shobha et al., Citation2012). Similarly PSL improves pulmonary function in horse and is being used in equine sports to increase athlete horse performance (Fidani et al., Citation2012). PSL is also being used illegally as a growth promoter in veal calves, bulls and old cows (Nebbia et al., Citation2014).
Different techniques have been developed and employed for the measurement of prednisolone such as Radioimmunoassay (RIA) (Syedanaglbashl, Mizuchl, Yotsumoto, & Mlyachl, Citation1980), which requires expensive and sophisticated instruments, causes health hazard, requires regulatory approval, radioactive counting of large number of samples is time-consuming and a large amount of scintillation fluid is required for 3H counting. The gas chromatography-mass spectroscopy (GC-MS) (Amendola, Garribbam, & Botre, Citation2003; Shibasakia et al., Citation2008), liquid chromatography-mass spectrometry (LC-MS) (van der Hoeven et al., Citation1997), liquid chromatography coupled to tandem mass spectrometry (LC/MS-MS) (Frerichs & Tornatore, Citation2004; Leporati et al., Citation2013; Vincenti et al., Citation2012), reversed-phase high-performance liquid chromatography (RP-HPLC) (Cho, Shin, & Yoo, Citation2003; Kurakula, Mohd, Samhuidrom, & Diwan, Citation2011), high-performance liquid chromatography (HPLC) (Chen et al., Citation2014; Doppenschmitt, Scheidel, Harrison, & Surmann, Citation1995; Jusko, Pyszczynski, Bushway, D’Ambrosio, & Mis, Citation1994; Sher, Fatima, Perveen, & Siddiqui, Citation2016), ultra-high-performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS) (McWhinney, Briscoe, Ungerer, & Pretorius, Citation2010) and capillary separation of glucocorticoids coupled to various detectors are the standard methods used for the measurement (Rao, Petersen, Bissell, Okorodudu, & Mohammad, Citation1999). The limitation of GC-MS method is the requirement of derivatization of analyte before it is render volatile for detection (Amendola et al., Citation2003; Shibasakia et al., Citation2008). The LC-MS, UHPLC-MS/MS and LC-MS/MS are the most widely used methods available for measuring prednisolone and cortisol in serum, plasma, saliva and urine (Frerichs & Tornatore, Citation2004; Leporati et al., Citation2013; McWhinney et al., Citation2010; Vincenti et al., Citation2012). These methods are sensitive and specific, but they require expensive equipment, large volumes of solvents, and highly trained individuals for operating complicated instruments. The RP-HPLC are suffering from their own drawbacks such as retention times equalling 6.4 min for prednisolone, which ultimately leads to less number of samples processing per day and solvents use are expensive etc. (Kurakula et al., Citation2011). The measuring of glucocorticoids by HPLC is a laboratory-intensive procedure (Jusko et al., Citation1994), sample processing time for each sample is about 40 min or more, which include extraction, centrifugation and chromatography (Doppenschmitt et al., Citation1995), and uses of ultraviolet (UV) detection, leads to inadequate limits of quantitation (LOQ) for the anticipated clinical concentrations (Chen et al., Citation2014; Doppenschmitt et al., Citation1995; Jusko et al., Citation1994). HPLC coupled with fluorescence detection involves extraction of analyte followed by derivatization, which makes this technique cumbersome, sophisticated and expensive but the detection limit is 0.1 ng/mL (Chen et al., Citation2014).
Compared with physico-chemical methods, Enzyme-linked immunosorbent assay (ELISA) is rapid, simple, effective and needs less or no sample preparation. Therefore, ELISA is very common as a biochemical and clinical analytical method. There is no literature available for direct estimation of prednisolone in serum by enzyme-linked immunosorbent assay (ELISA). Although commercial ELISA kits are available from different companies for the measurement of prednisolone in serum, but their know-how is not published. Enzyme immunoassays represent the most rapidly growing nonisotopic methods in the diagnostics industry. Hence, the present study was designed to develop an ELISA method for simple, rapid, direct, sensitive and accurate estimation of prednisolone in serum, using indigenously prepared antibody, PSL-21-HS-BSA-immunogen and PSL-21-HS-HRP-enzyme conjugate.
2. Materials and methods
The experimental protocols were approved by the Institutional Animal Ethics Committee (IAEC) of National Institute of Health and Family Welfare (NIHFW), New Delhi, India. All animal experimentation was performed in accordance with the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India.
2.1. Materials
All chemicals, reagents, salts and solvents used in this study were of high purity analytical grade. N-hydroxysuccinimide (NHS), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDAC), Dioxan, dimethyl formamide (DMF), Tetramethylbenzidine (TMB), Hydrogen peroxide urea (H2O2.U)/carbamide peroxide, Bovine serum albumin (BSA), Freund’s complete/incomplete adjuvant and thimerosal were procured from Sigma Chemical Company (St. Louis, MO, USA). Horseradish peroxidase (HRP) was obtained from Bangalore Genei (Bangalore, India). The prednisolone, prednisolone-21-hemisuccinate (steroids) and other analogous steroids used for cross-reactivity were procured from steraloids, Inc. (Newport, RI, USA). The 96-wells microtitre plates were obtained from Costar, Corning Life Sciences (Tweksbury, MA 01876, USA).
2.2. Instruments
A Tecan Spectra micro-plate reader was purchased from Tecan Austria GmbH (5082, Grödig, Austria). Double beam evolution 220 UV–Visible Spectrophotometer (Thermo Electron Scientific Instruments LLC, Madison, WI, USA). Haryson lyophilizer was purchased from Haryson (New Delhi, India).
2.3. Methods
2.3.1. Buffers
Buffer “A:” Coating buffer pH 7.2 (10 mM PBS) was prepared using sodium phosphate dibasic (Na2HPO4·2H2O) 1.1 g/L, sodium phosphate monobasic dihydrate (NaH2PO4·2H2O) 0.52 g/L, sodium chloride (NaCl) 9 g/L and sodium azide (NaN3) 0.1%.
Buffer “B:” Enzyme conjugate dilution buffer was prepared by using Tris 2.42 g, NaCl 17.9 g, BSA 1.0 g, tween-80 1 mL, dextran 0.3 g/L, striped serum 5%, thimerosal 0.05% and glycerol 0.5%, adjust pH to 8.0 with 1M HCL.
Buffer “C:” Antibody dilution buffer was prepared by using Na2HPO4·2H2O 1.1 g/L, NaH2PO4·2H2O 0.52 g/L, sucrose (C12,H22,O11) 90 g/L, ammonium sulphate (NH4.2SO4) 100 g/L, BSA 2 g/L and sodium azide (NaN3) 0.5 g/L.
Buffer “D:” Blocking buffer was prepared using Tween Tris buffered saline (TTBS; 0.1% Tween 20, 50 mM Tris, 150 mM NaCl, 1 mM EDTA, pH 7.4) sucrose 92.0 g/L, bovine serum albumin (BSA) 2 g/L, glycerol 0.5% and thimerosal 0.1%.
Buffer “E:” Wash buffer for ELISA (10 mM PBS containing 0.05% Tween-20). Prepared by adding, 500 µL Tween-20 to 1 litre of PBS.
Buffer “F:” Citrate-phosphate buffer, pH 5.0 Citric acid 22.2 mM and Na2HPO4 51.3 mM.
HRP Substrate: Mix 200 µL of 41 mM TMB solution in dimethyl sulfoxide (DMSO) having 25 mM sodium tartrate as stabilizer, 3 µL 30% H2O2 with 200 ppm of acetanilide as stabilizer to 9.8 ml citrate-phosphate buffer, pH 5.0.
Stop solution: 1N HCL.
2.3.2. Preparation of PSL-21-HS-BSA immunogen
PSL-21-HS was coupled to BSA by using N-hydroxysuccinimide mediated carbodiimide reaction, previously reported with minor modification (Shrivastav, Chaube, Kariya, & Kumar, Citation2012; Wang et al., Citation2016). Five milligrams of PSL-21-HS was taken, and each of 200 μL of dimethyl formamide, dioxan and 100 μL of distilled water containing 10 mg of N-hydroxysuccinimide and 20 mg of 1-ethyl-3-(3-dimethyl-amino-propyl) carbodiimide-HCL were added into it. The reaction mixture was mixed and kept at 4°C for overnight. Following the overnight incubation, aqueous BSA solution (1 mg/0.5 mL) was added to the activated steroid reaction mixture and vortex-mixed and kept at 4°C for overnight. The resultant conjugate was dialyzed against distilled water for 3–4 changes at 4°C. Subsequently, the dialyzed PSL-21-HS-BSA was frozen at −20°C, lyophilized and stored at 4°C.
2.3.2.1. Characterization of PSL-21-HS-BSA immunogen
2.3.2.1.1. Ultraviolet (UV) Spectroscopy
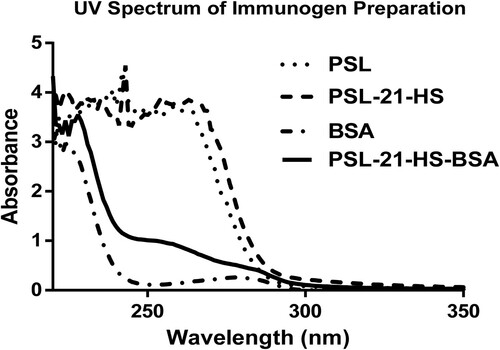
Characterization of immunogen by ultraviolet spectroscopy was performed with some modification as per the procedure of Chen et al. and Kong et al. (Chen et al., Citation2017; Kong, Xie, Liu, Song, & Kuang, Citation2017). One milligram each of BSA and PSL-21-HS-BSA were dissolved in Milli-Q water (1.0 mg/mL), similarly each one milligram of PSL and PSL-21-HS were dissolved in ethanol (1.0 mg/5 mL). Each sample was scanned from 220 nm to 350 nm. The changes in absorption spectra were determined by Evolution 220 UV–Visible Spectrophotometer.
2.3.3. Immunization of rabbits and collection of antiserum
The polyclonal antibody was raised in New Zealand white rabbits against PSL-21-HS-BSA as per previously described procedure (Shrivastav, Chaube, Kariya, & Kumar, Citation2012; Wei, Zhao, Wang, & Wang, Citation2014). Briefly, PSL-21-HS-BSA (1 mg) was dissolved in 0.9% saline (0.5 mL) and emulsified with (0.5 mL) Freund’s (complete) adjuvant. The prepared emulsion (250 μL) was injected intramuscularly in each limb of the rabbits. Primarily, the first five booster injections were prepared with Freund’s complete adjuvant and given per week that was further followed by monthly booster injection prepared using Freund’s incomplete adjuvant. Further, the rabbit was bled after 9–14 days after the booster dose injections. The blood was collected from rabbit and after incubation at room temperature, antiserum was collected by centrifugation at 1000 g for 15 min. The separated antiserum was stored at −30°C.
2.3.4. Collection of normal rabbit serum (NRS) and generation of anti-rabbit gamma globulin (ARGG)
For the collection of normal rabbit serum, blood was withdrawn from the non-immunized New Zealand white rabbits. The collected blood was centrifuged and clear serum separated and stored as NRS at −30°C until use.
The immunoglobulins (IgG) of non-immunized rabbits were used for generating anti-rabbit gamma globulin (ARGG) by immunizing the goat (Shrivastav, Chaube, Kariya, & Kumar, Citation2012; Shrivastav, Chaube, Kariya, Kumari, et al., Citation2011; Shrivastav, Chaube, Kariya, Singh, et al., Citation2011; Shrivastav, Chaube, Kariya, Singh, et al., Citation2012). Following, the booster injection, blood was collected from the goat and centrifuged. The obtained antiserum was stored at −30°C for further use.
2.3.5. Preparation of PSL-21-HS-HRP-enzyme conjugate
PSL-21-HS was conjugated to HRP using an active ester method with some modification (Shrivastav, Citation2003; Tao et al., Citation2014). Two hundred microlitres of each of Dioxan, DMF and water were added to 5 mg of PSL-21-HS, 10 mg NHS and 20 mg EDAC. The above mixture was vortex-mixed and kept for activation at 4°C for 24 h. Further, HRP solution (1 mg/mL) was added to activated PSL-21-HS solution and kept at 4°C for 24 h. The PSL-21-HS-HRP-enzyme conjugate was purified by passing through the pre-equilibrated (10 mM PBS containing 0.1% thimerosal) G-25 column. The purified brown-coloured fraction of the HRP-PSL-21-HS conjugate with enzyme activity was collected and then an equal volume of ethylene glycol was added along with 1% of ammonium sulphate, BSA and sucrose. The PSL-21-HS-HRP-enzyme conjugate was aliquoted and stored at −30°C for further use.
2.3.6. Checkerboard assay
A checkerboard assay was performed to obtain the optimal dilutions of antibody and enzyme conjugate for the development of assay.
2.3.6.1. Coating of antibody to microtitre plates
The 96 well microtitre plates were coated using the immunobridge technique for primary antibody immobilization according to the method of Shrivastav et al. (Shrivastav, Chaube, Kariya, Kumari, et al., Citation2011; Shrivastav, Chaube, Kariya, Singh, et al., Citation2011; Shrivastav, Chaube, Kariya, Singh, et al., Citation2012; Shrivastav, Kariya, Prasad, Chaube, & Kumar, Citation2014). Briefly, 250 µL of normal rabbit serum (NRS) diluted (1:250) in water was dispensed into each well and incubated at 37°C overnight. Following incubation, the plate was washed two to three times using washing solution (buffer E). To the NRS coated wells, 250 µL of 1:1000 diluted goats anti-rabbit gamma globulin (ARGG) was added and incubated for 5 h at 37°C or overnight at 4°C. Thereafter the plate was washed thoroughly using washing solution (buffer E). The raised antiserum against immunogen (PSL-21-HS-BSA) was serially diluted in antibody dilution buffer to 1:500, 1:1000, 1:2000, 1:4000 and 150 μL was added per ARGG coated wells (single dilution per 8 well strip); for nonspecific binding (NSB), 150 μL of antibody dilution buffer was added in a separate ARGG coated 8-well strip and incubated for 2 h at 37°C. Unabsorbed antibody was then washed with buffer E and 250 μL of buffer “D” was added to block the unoccupied sites of the plate. The plate was kept at room temperature (RT) for 1 h. The contents were decanted and the plate was dried at RT; packed and stored at 4°C in zip-lock bags for further use.
2.3.6.2. Determination of optimal loading of primary antibody using prednisolone-21-HS-HRP conjugate
In order to determine the optimum dilution of antibody and enzyme conjugates required for assay development, 100 μL of serially diluted (1:500, 1:1000, 1:2000 and 1:4000) enzyme conjugate (PSL-21-HS-HRP) was added in the above coated wells (one dilution per two wells vertically) and kept at 37°C for 1 h. Thereafter, the plate was decanted and washed two to three times using washing solution (buffer E). Further, to measure the bound enzyme activity (which is a direct function of the antibody), 100 μL of substrate solution TMB/H2O2 was added to each well and incubated for 15 min in dark at RT. 100 μL of 1 N HCL was used to stop the reaction and the developed colour was measured at 450 nm using Tecan Spectra micro-plate reader (TECAN, Austria). The dilutions of antiserum and enzyme conjugate combination showing maximum zero binding and least nonspecific binding were selected for assay development.
2.3.7. Preparation of prednisolone standard
Eight prednisolone working standards (0.0, 0.62, 1.25, 2.5, 5.0, 10.0, 20.0 and 40.0 ng/mL) were prepared in stripped pooled serum. Steroid stripped serum was prepared by charcoal treatment 100 mL of pooled human serum was taken and to it 5% charcoal was added and stirred for 2 h at RT. This solution was centrifuged at 10,000×g for 15 min to remove the charcoal and then filtered using 0.45 μM membrane filter. Add one pinch of thimerosal as a preservative.
2.3.8. Assay procedure for measurement of PSL in serum or PSL-21-HS-BSA conjugate
One hundred microlitres of PSL standard of different concentrations were added followed by serum samples or diluted PSL-21-HS-BSA conjugate to the wells coated with optimal dilution of PSL-21-HS-BSA-antibody. To each well, 100 µL of optimal dilution of enzyme conjugate (PSL-21-HS-HRP) was added and incubated for 1 h at 37°C. Thereafter the plate was decanted, and washed with buffer E. Further, 100 µL of TMB/H2O2 substrate was added to each wells and incubated for 15 min at RT, to estimate the bound enzyme activity. Finally, 100 µL of 1 N HCL was added to each well. The colour intensity was measured at 450 nm using a micro-plate reader. The concentration of PSL in serum sample or attached to BSA was obtained by interpolation of their respective OD from standard curve obtained by plotting OD at Y-axis and standard on X-axis.
2.3.9. Preparation of recovery pools
Six recovery pools were prepared in serum by spiking the serum with different known concentrations of prednisolone, viz., 0.0, 0.62, 2.5, 5.0, 15.0 and 25.0 ng/mL.
3. Data analysis
3.1. Preparation of standard curve and determination of affinity constant and sensitivity
Standard curve was plotted by software Graph Pad Prism 6.0 and Microsoft Excel programme. The concentration and the percent ratio of mean absorbance of standard and mean absorbance at zero dose (A/A0 × 100) were plotted on X-axis (log-scale) and Y-axis, respectively. A QBASIC language-based program developed in house has been used to compute the values of unknown samples that uses the logit-log regression method (Shrivastav, Chaube, Kariya, Singh, et al., Citation2011). The affinity constant of PSL antibody and sensitivity of the developed assay were calculated by the Feldman and Rodbard method (Shrivastav, Chaube, Kariya, Singh, et al., Citation2012).
3.2. Recovery
Recovery is often confused with accuracy although these two concepts are quite separate. Recovery is an indirect assessment of accuracy. Recovery assesses more than just whether or not an assay is correctly calibrated. It is the ability of a test to recover, or measure, a known incremental amount of an analyte from a sample matrix. The experimental protocol for a study includes adding a known amount of analyte (A) to a base (B) and measuring the concentration (C) (Shrivastav et al., Citation2014). The percentage of recovery can be calculated by using the formula: (C − B)/A * 100.
3.3. Precision
Precision is probably the most important technical aspect of an immunoassay performance. Precision, also sometimes referred to as reproducibility, is a statistical measure of the variation between repeated determinations on the same sample, either within the same run or from day to day, i.e. between runs. Imprecision is the opposite of precision.
Serum pools containing various prednisolone concentrations (i.e. high, high-medium, medium, low-medium and low) were used for measuring the level of imprecision in the standardized assay by quantitating prednisolone concentrations, in each serum pool by repeating the assay eight times and in eight different assays. The statistics conventionally used to express the precision profile of an assay are the mean (X), standard deviation (SD) and the coefficient of variation, CV. The % coefficient of variation is calculated as % CV = (SD/X) * 100 at a particular analyte level (Shrivastav et al., Citation2014).
3.4. Method comparison (correlation)
A newly developed immunoassay method should produce results similar to those of other reliable, clinically validated immunoassays or methods of assay. A graph of the two methods plotted against one another with identical axes, along with the bisecting line of perfect agreement, allows a good visual assessment of the degree of agreement between the two methods. Simple linear regression analysis is widely used to estimate the Pearson correlation coefficient (R²) (Chen et al., Citation2017).
3.5. Statistical analysis
Statistical analysis, including average, standard deviations (SD), logit-log transformation and correlation coefficient, was calculated using Microsoft Excel and graph was plotted by OriginPro 2016 (64) Bit software.
4. Results
4.1. Characterizations of immunogen by UV and visible spectroscopic methods
The UV spectrum of BSA gave single peak at 280 nm, which is a characteristic peak of proteins due to amino acid tryptophan and tyrosine. However, PSL, PSL-21-HS and PSL-21-HS-BSA (after conjugation) showed three peaks at 242, 243 and 254 nm respectively as given in , which represents the characteristic peak of hapten (PSL, PSL-21-HS) and hapten-protein (PSL-21-HS-BSA) conjugate.
4.2. Analysis of the hapten density in immunogen by ELISA
The amount of PSL-21-HS-BSA conjugated in immunogen was found to be 16 μg of prednisolone per mg of immunogen by ELISA.
4.3. Standard curve
The composite dose–response curve of prednisolone ELISA using PSL-21-HS-BSA antiserum and PSL-21-HS-HRP-enzyme conjugate has been shown in . The standard curve obtained after repetition of several assays remained precise and stable. The CV % of the developed ELISA, for the A/A0 ratio of each standard ranged from 2.56% to 9.93%. The logit-log transformation of standard curve in which y = −1.845x + 0.759 ng/mL and R² = 1. The affinity constant (Ka) of the PSL-21-HS-BSA antibody for prednisolone antigen was found to be 6.29 × 10−8 (L/mol) calculated by a Scatchard plot. The slope and intercept of the curves were calculated by logit-log transformation of standard curve data, as shown in .
Figure 2. Composite dose–response curve of homologous ELISA of prednisolone using PSL-21-HS-BSA-antibody with PSL-21-HS-HRP-enzyme conjugate. Each value is a mean ± SD of eight assays (In duplicate). The coefficient of variation at each concentration is shown in parentheses.
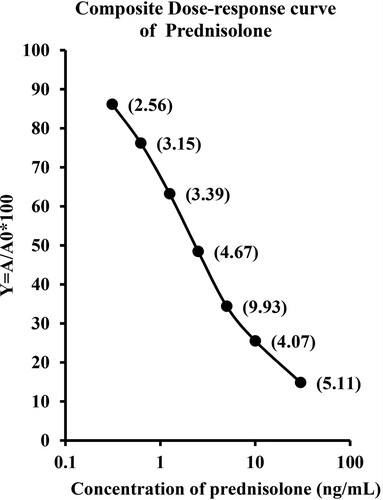
Table 1. Slope (m), Intercept (c), Sensitivity, Affinity, ED50 and R² of prednisolone assay, using PSL-21-HS-BSA-antibody with PSL-21-HS-HRP-enzyme conjugate.
4.4. Sensitivity
Sensitivity is the capability to measure the minimum quantity of target analyte under defined standard condition (Shrivastav et al., Citation2014). The sensitivity of the assay is expressed in terms of its lowest detection dose (LDD) and the effective dose at 50% (ED50). The LDD is the lowest concentration of analyte (A) that responds to statistically different from that detected in the lack of analyte (A0). It is calculated as A0 − 2× SD after 32-times determination of A0. The ED50 is the effective concentration at which 50% of inhibition in the binding of enzyme conjugates occurs in an assay in the presence of analyte. It is calculated as ED50 ± SD, after eight times determination of ED50. The values obtained were interpolated from the standard curve, and the concentration found in the low detection dose and the ED50 of the developed assay were 0.078 and 2.64 ng/mL, respectively.
4.5. Specificity
The specificity of the PSL-21-HS-BSA-antibody was estimated as the percentage of cross-reaction with commercially available fifty one C27, C21, C19 and C18 steroids with analogous structure. Out of 51 analogous steroids, only 4 steroids (i.e. progesterone 1.76%, 17α-OH progesterone 5.89%, cortisol 17.69% and prednisone – 21.13%) showed cross-reaction as given in the . Cross-reactivity of analogous steroid compounds with PSL-21-HS-BSA-antibody using PSL-21-HS-HRP-enzyme conjugate assay was calculated. The % cross-reaction was calculated using the following formula (Shrivastav, Chaube, Kariya, Kumari, et al., Citation2011; Shrivastav, Chaube, Kariya, Singh, et al., Citation2011; Shrivastav, Chaube, Kariya, Singh, et al., Citation2012; Tao et al., Citation2014):
Table 2. Cross-reactivity of steroid compounds with Prednisolone in homologous and bridge heterologous assays of Prednisolone using PSL-21-HS-BSA-antibody with PSL-21-HS-HRP-enzyme conjugates by ELISA.
4.6. Recovery
The capability of the assay to accurately measure PSL in serum samples was verified. Six allocates of pooled serum were prepared by externally adding different concentrations of PSL. After spiking, the concentration of PSL was measured and recovery was calculated for each pool. The recovery ranged from 94.84% to 100.17%, as shown in .
Table 3. Recovery of prednisolone from exogenously spiked serum pools.
4.7. Precision
The serum samples having roughly the similar concentrations of PSL were pooled and five different pools were prepared containing different concentrations of PSL. Each pool was examined eight times within the assay (intra-assay) and between separate assays (inter-assay) (). The intra-assay and inter-assay CV% (n = 8, replicate of each pool) was <7.22%. The intra-assay coefficients of variation range from 1.39% to 6.15%. The inter-assay coefficients of variation range from 1.58% to 7.22%.
Table 4. Inter- and intra-assay CV % for the measurement of prednisolone in serum pool.
4.8. Correlation coefficient
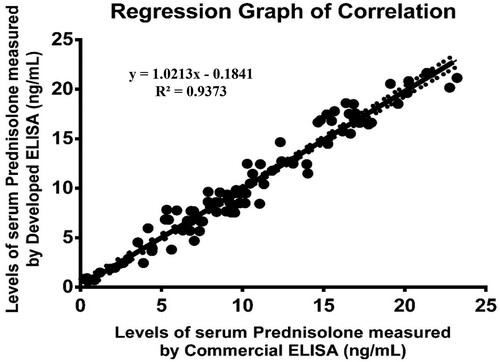
The newly developed technique must yield results analogous to those of the already available conventional method. The correlation coefficient for values of prednisolone in serum samples (n = 64) measured by developed ELISA and commercial prednisolone ELISA kit catalogue no. E13651458 (purchased from Sincere Biotech Co. Ltd., Beijing, China) was found to be r2 = 0.93. The regression analysis has been performed in which both X and Y was subject for measurement error. showed that method fit a straight line to a two-dimensional data where both the variables, X and Y, are measured with errors that can accommodate differences in measurement error between the test and the reference method. The linear regression curve of the correlated data was plotted by Graph Pad Prism version 6.0 for MS windows.
4.9. Estimation of PSL in patient treated for different diseases
We determined the PSL in serum of patient taking treatment through oral, local or intramuscular routes in different diseases such as chronic asthma (17 male and 16 female), rheumatoid arthritis (18 male and 14 female), allergic (22 male and 13 female) and in healthy (38 male, 22 female) age group between 10 and 72 years ().
Table 5. Determination of PSL in patient receiving PSL medication for different patient by using developed ELISA.
5. Discussion
In a homologous immunoassay, haptens used to generate the antibody and those used to prepare enzyme conjugate are the same. In the present study, the PSL-21-HS was used to couple to BSA and HRP so as to prepare immunogen and enzyme conjugate, respectively.
Various analytical methods have been developed, including RIA, HPLC, LC-MS, GC-MS, LC-MS/MS and RP-HPLC-UV, for prednisolone measurement. Syedanaglbashl et al. (Citation1980) developed RIA for PSL estimation using the antiserum generated in rabbits to prednisolone-21-hemisuccinate-bovine serum albumin and [3H]-prednisolone as tracer, where the detection limits were 2 ng/mL (Syedanaglbashl et al., Citation1980). Although, RIA has been the standard method for the measurement of steroids and other hormones in biological fluid and tissues but it has a number of serious disadvantages that restrict its applicability. Apart from this, the availability of radiolabeled steroids from commercial sources is very limited and severely restricts the range of steroids that can be measured.
Nawab Sher et al. (Citation2016) utilized the HPLC method using a C18 analytical column as stationary phase. The mobile phase was 30:70 methanol: pH 2.5 phosphate buffer at a flow rate of 1.0 mL/min with absorbance detection at 235 nm. The method was linear for concentrations ranged from 40 to 10,000 ng/mL. However, the retention time of prednisolone was 10 min. The LOD, LOQ and coefficient of correlation (r2) were 2.11, 6.39 ng/mL and 0.9995, respectively, for PSL in human serum (Sher et al., Citation2016). Jusko et al. (Citation1994) quantified prednisolone in human plasma by high-performance liquid chromatography (HPLC) with ultraviolet (UV) at 254 nm, where the detection limit was 10 ng/mL (Jusko et al., Citation1994). Similarly Doppenschmitt et al. (Citation1995) determined prednisolone levels using HPLC-UV in blood (serum) and the LOD was 2 ng/mL, where samples were extracted from 1.0 mL serum with 3 mL (1:1 v/v) ethyl acetate/tert-methyl butyl ether and 0.1 mL phosphoric acid (Doppenschmitt et al., Citation1995).
Cho et al. (Citation2003) used reversed-phase high-performance liquid chromatography (RP-HPLC-UV) for estimation of prednisolone in blood (plasma) samples. The intra and inter-assay variabilities ranged from 1.8% to 10.5% and from 0.7% to 9.5%, respectively, for PSL. The LOD, LOQ and coefficient of correlation (r2) were 0.5, 2 ng/mL and 0.998, respectively, for PSL (Cho et al., Citation2003). In addition, Kurakula et al. (Citation2011) also developed RP-HPLC method for detection of prednisolone in proliposomal formulation. However, the retention time of prednisolone was 6.4 min. The LOD and LOQ were 35 and 62.5 ng/mL, respectively. The linearity of the drug was in the range of 1–5 μg/mL with coefficient of correlation r2 = 0.999. The % recovery of the PSL was 100.64% (Kurakula et al., Citation2011).
Brett et al. (2010) describe an ultra-high-performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS) method suitable for a laboratory to determine endogenous and exogenous glucocorticoids (PSL) in plasma, plasma ultra-filtrate, urine and saliva in a single analytical run. The limit of quantitation and CV% for PSL were 5.0 nmol/L and 7.6%, respectively (McWhinney et al., Citation2010). Shobha et al. (Citation2012) estimated prednisolone using HPLC (+) Electrospray ionization (ESI) -MS/MS method. The concentrations of calibration standard were 15–120 ng/mL. The inter CV was within ±15% of the actual value. The coefficient of correlation was r2 = 0.99 and LOD was 15 ng/mL (Shobha et al., Citation2012). Leporati et al. (Citation2013) and Vincenti et al. (Citation2012) expanded and revalidated existing quantitative LC-MS/MS method for the detection of PSL in beef cattle(Leporati et al., Citation2013) and cow urine (Vincenti et al., Citation2012). The LOD of PSL in beef cattle and in cow urine was 0.42 and 0.5 ng/mL, respectively. Shibasakia et al. (Citation2008) estimated plasma concentrations of prednisolone, prednisone, cortisol and cortisone simultaneously using gas chromatography-mass spectrometry (GC–MS). The LOD was 114.0 ng/mL for prednisolone (Shibasakia et al., Citation2008). Amendola et al. (Citation2003) also determined prednisolone in urine by (GC-MS) with electron impact ionization, where limit of detection was 4 ng/mL, but it also requires additional preparation such as extraction, outgassing and derivatization, etc. and therefore it is time-consuming (Amendola et al., Citation2003).
The present study showed that PSL-21-HS-based homologous assay was more sensitive and very simple than others available system for PSL detection counterpart. The sensitivity and the ED50 of the developed homologous assay was 0.078 and 2.64 ng/mL, respectively. This developed assay is specific for PSL estimation and it’s showed less cross-reaction with commercially available fifty five steroids such as C27, C22, C21, C19 and C18 with analogous structure. This assay showed cross-reaction with only four steroids; i.e. progesterone 1.76%, 17α-OH progesterone 5.89%, cortisol 7.69% and prednisone 1.13% out of 55 analogous C18, C19, C21 and C27 steroids. The percent recovery of prednisolone from the exogenously spiked human serum pools was in the range of 94.84–100.17%. The intra-assay and inter-assay CV% was <7.22%. The correlation coefficient for values of prednisolone in serum samples (n = 64) measured by developed ELISA and commercially available assay was found to be r2 = 0.93. Also we determined the PSL in patient receiving PSL medication under different diseases by using developed ELISA. Serum samples from healthy volunteers (38 male, 22 female) and in patient taking treatment with prednisolone drug through oral, locally or injection in diseases such as chronic asthma (17 male and 16 female), acute rheumatic (18 male and 14 female) and allergic (22 male and 13 female) having age group between 10 and 72 years. The developed assay is sensitive, specific, precise and requires an assay time of 1 h and 15 min only to complete; so it is rapid when compared to others methods.
6. Conclusion
A sensitive, specific, reproducible direct homologous assay for prednisolone quantitation has been developed, which require low volume of serum sample as well as less time to perform the assay. The analytical tool developed for detection of prednisolone may be extended for its measurement as drug of abuse in an athlete sports person, horse and meat of veal calves, bulls and old cows.
Acknowledgements
We acknowledge the volunteers for providing their blood for prednisolone estimation. The National Institute of Health and Family Welfare (NIHFW), New Delhi, India, supported this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Amendola, L., Garribbam, F., & Botre, F. (2003). Determination of endogenous and synthetic glucocorticoids in human urine by gas chromatography-mass spectrometry following microwave assisted derivatization. Analytica Chimica Acta, 489, 233–243. doi: https://doi.org/10.1016/S0003-2670(03)00703-7
- Beotra, A., Gupta, Y. K., Ahi, S., Dubey, S., Upadhyay, A., Priyadarshi, R. … Jain, S. (2009). Preliminary studies on detection of corticosteroids in adulterated herbal drugs. Recent Advances in Doping Analysis 18, 208–211.
- Chen, X., Li, Z., Guo, J., Li, D., Gao, H., Wang, Y., & Chuanlai, X. (2017). Simultaneous screening for marbofloxacin and ofloxacin residues in animal-derived foods using an indirect competitive immunoassay. Food and Agricultural Immunology, 28(3), 489–499. doi: https://doi.org/10.1080/09540105.2017.1297780
- Chen, C.-M., Xia, Y.-C., Zhang, X.-G., Peng, C.-H., Liu, F.-Y., Peng, Y.-M., & Sun, L. (2014). HPLC determination and clinical significance of serum prednisone in patients with nephrotic syndrome. International Journal of Clinical and Experimental Medicine, 7(12), 5517–5522.
- Cho, C. Y., Shin, B. S., & Yoo, S. D. (2003). Sensitive analysis of prednisolone and prednisone in human plasma by RP-HPLC with UV detection. Analytical Letters, 36(8), 1573–1585. doi: https://doi.org/10.1081/AL-120021540
- Delgado, J. N., & Remers, W. A. (1998). Wilson and Gisvold’s textbook of organic medicinal and pharmaceutical chemistry (10th ed., pp. 772–774). Philadelphia, NY: Lippincott Raven.
- Deventer, K., Thuyne, W. V., Mikulcikova, P., Van Eenoo, P., & Delbeke, F. T. (2007). Detection of selected stimulants as contaminants in solid nutritional supplements by liquid chromatography–mass spectrometry. Food Chemistry, 103(4), 1508–1513. doi: https://doi.org/10.1016/j.foodchem.2006.09.015
- Doppenschmitt, S. A., Scheidel, B., Harrison, F., & Surmann, J. P. (1995). Simultaneous determination of prednisolone, prednisolone acetate and hydrocortisone in human serum by high-performance liquid chromatography. Journal of Chromatography B: Biomedical Sciences and Applications, 674(2), 237–246. doi: https://doi.org/10.1016/0378-4347(95)00317-7
- Fidani, E. M., Pompa, G., Mungiguerra, F., Alessio, C., Fracchiolla, M. L., & Francesco, A. (2012). Investigation of the presence of endogenous prednisolone in equine urine by high-performance liquid chromatography mass spectrometry and high-resolution mass spectrometry. Rapid Communications in Mass Spectrometry, 26, 879–886. doi: https://doi.org/10.1002/rcm.6169
- Fiel, S. B., & Vincken, W. (2006, June–July). Systemic corticosteroid therapy for acute asthma exacerbations. Review. Journal of Asthma, 43(5), 321–331. doi: https://doi.org/10.1080/02770900600567163
- Frerichs, V. A., & Tornatore, K. M. (2004). Determination of the glucocorticoids prednisone, prednisolone, dexamethasone, and cortisol in human serum using liquid chromatography coupled to tandem mass spectrometry. Journal of Chromatography B, 802(2), 329–338. doi: https://doi.org/10.1016/j.jchromb.2003.12.015
- Jusko, W. J., Pyszczynski, N. A., Bushway, M. S., D’Ambrosio, R., Mis, S. M. (1994, August 5). Fifteen years of operation of a high-performance liquid chromatographic assay for prednisolone, cortisol and prednisone in plasma. Journal of Chromatography B: Biomedical Sciences and Applications, 658(1), 47–54. doi: https://doi.org/10.1016/0378-4347(94)00218-5
- Kong, D., Xie, Z., Liu, L., Song, S., & Kuang, H. (2017). Development of ic-ELISA and lateral-flow immunochromatographic assay strip for the detection of citrinin in cereals. Food and Agricultural Immunology, 28(5), 754–766. doi: https://doi.org/10.1080/09540105.2017.1312293
- Kurakula, M., Mohd, A. B., Samhuidrom, P. R., & Diwan, P. V. (2011, October–December). Estimation of prednisolone in proliposomal formulation using RP HPLC method. International Journal of Research in Pharmaceutical and Biomedical Sciences, 2(4), 1663–1669.
- Leporati, M., Capra, P., Cannizzo, F. T., Biolatti, B., Nebbia, C., & Vincenti, M. (2013). Determination of prednisolone metabolites in beef cattle. Food Additives & Contaminants Part A, 30(6), 1044–1054. doi: https://doi.org/10.1080/19440049.2013.777975
- McWhinney, B. C., Briscoe, S. E., Ungerer, J. P. J., & Pretorius, C. J. (2010). Measurement of cortisol, cortisone, prednisolone, dexamethasone and 11-deoxycortisol with ultra-high performance liquid chromatography–tandem mass spectrometry: Application for plasma, plasma ultrafiltrate, urine and saliva in a routine laboratory. Journal of Chromatography B, 878, 2863–2869. doi: https://doi.org/10.1016/j.jchromb.2010.08.044
- Nebbia, C., Capra, P., Leporati, M., Girolami, F., Barbarino, G., Gatto, S., & Vincenti, M. (2014). Profile of the urinary excretion of prednisolone and its metabolites in finishing bulls and cows treated with a therapeutic schedule. BMC Veterinary Research, 10, 622. doi: https://doi.org/10.1186/s12917-014-0237-0
- Ramsay, H. M., Goddard, W., Gill, S., & Moss, C. (2003). Herbal creams used for atopic eczema in Birmingham, UK illegally contains potent corticosteroids. Archives of Disease in Childhood, 88(12), 1056–1057. doi: https://doi.org/10.1136/adc.88.12.1056
- Rao, L. V., Petersen, J. R., Bissell, M. G., Okorodudu, A. O., & Mohammad, A. A. (1999). Development of urinary free cortisol assay using solid-phase extraction-capillary electrophoresis. Journal of Chromatography B: Biomedical Sciences and Applications, 730, 123–128. doi: https://doi.org/10.1016/S0378-4347(99)00151-6
- Schimmer, B. P., & Parker, K. L. (2001). Adrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs; inhibitors of the synthesis and actions of adrenocortical hormones. In A.G. Gilman (Ed.), Goodman and Gilman’s. The pharmacological basis of therapeutics (10th ed., pp. 1649–1677). New York, NY: McGraw-Hill.
- Sher, N., Fatima, N., Perveen, S., & Siddiqui, F. A. (2016). Determination of benzimidazoles in pharmaceuticals and human serum by high-performance liquid chromatography. Instrumentation Science And Technology, 44(6), 672–682. doi: https://doi.org/10.1080/10739149.2016.1184162
- Shibasakia, H., Nakayama, H., Furuta, T., Kasuya, Y., Tsuchiya, M., Soejima, A., … Nagasawa, T. (2008). Simultaneous determination of prednisolone, prednisone, cortisol, and cortisone in plasma by GC–MS: Estimating unbound prednisolone concentration in patients with nephrotic syndrome during oral prednisolone therapy. Journal of Chromatography B, 870(2), 164–169. doi: https://doi.org/10.1016/j.jchromb.2008.03.003
- Shobha, A., Sachin, D., Awanish, U., Yadav, S., Rakesh, S., Rahul, P., & Alka, B. (2012). Identification of prednisolone, methylprednisolone and their metabolites in human urine using HPLC (+) ESI-MS/MS and detection of possible adulteration in Indian herbal drug preparations. Ibnosina Journal of Medicine and Biomedical Sciences, 4(2), 44–52. doi: https://doi.org/10.4103/1947-489X.210755
- Shrivastav, T. G. (2003). Horseradish peroxidase hydrazide and its use in immunoassay. Journal of Immunoassay and Immunochemistry, 24(3), 301–309. doi: https://doi.org/10.1081/IAS-120022939
- Shrivastav, T. G., Chaube, S. K., Kariya, K. P., & Kumar, D. (2012). Heterologous enzyme linked immunosorbent assay for measurement of testosterone in serum. Journal of Immunoassay and Immunochemistry, 33, 253–269.
- Shrivastav, T. G., Chaube, S. K., Kariya, K. P., Kumari, P., Singh, R., & Kumar, D. (2011). Influence of different homologous and heterologous combinations of antibodies and horseradish peroxidase-labeled dehydroepiandrostosterone derivatives on sensitivity and specificity of DHEA ELISA. Journal of Immunoassay and Immunochemistry, 32, 114–127. doi: https://doi.org/10.1080/15321819.2010.543221
- Shrivastav, T. G., Chaube, S. K., Kariya, K. P., Singh, R., Kumar, D., Jain, P., … Deshmukh, B. (2012). Influence of different length linker containing DHEA-7-CMO-enzyme conjugates on sensitivity and specificity of DHEA-3-HS-antibody. Journal of Immunoassay and Immunochemistry, 33, 1–17. doi: https://doi.org/10.1080/15321819.2011.591475
- Shrivastav, T. G., Chaube, S. K., Kariya, K. P., Singh, R., Kumar, D., Pandit, D., … Pandey, B. (2011). Influence of different length linker containing DHEA-7-CMO-enzyme conjugates on sensitivity and specificity of DHEA-17-CMO-antibody. Journal of Immunoassay and Immunochemistry, 32, 269–283. doi: https://doi.org/10.1080/15321819.2011.560325
- Shrivastav, T. G., Kariya, K. P., Prasad, P. K., Chaube, S. K., & Kumar, D. (2014). Development of enzyme-linked immunosorbent assay for estimation of urinary albumin. Journal of Immunoassay and Immunochemistry, 35(3), 300–313. doi: https://doi.org/10.1080/15321819.2013.849729
- Syedanaglbashl, K. Y., Mizuchl, A., Yotsumoto, H., & Mlyachl, Y. (1980). Separation of serum prednisolone and prednisolone-21-hemisuccinate by extraction and their concurrent determination by radioimmunoassay. Clinical Chemistry, 28/9, 1301–1303.
- Tao, X., Jiang, H., Zhu, J., Wang, X., Wang, Z., Niu, L., … Shen, J. (2014). An ultrasensitive chemiluminescent ELISA for determination of chloramphenicol in milk, milk powder, honey, eggs and chicken muscle. Food and Agricultural Immunology, 25(1), 137–148. doi: https://doi.org/10.1080/09540105.2012.753513
- Thrower, B. W. (2009, January). Relapse management in multiple sclerosis. The Neurologist, 15(1), 1–5 Review. doi: https://doi.org/10.1097/NRL.0b013e31817acf1a
- Tripathi, K. D. (2008). Essentials of medical pharmacology (6th ed., pp. 217–218). New Delhi: Jaypee Brothers Medical.
- Uchiyama, N., Hanajiri, R. K., Kawahara, N., & Goda, Y. (2009). Identification of a cannabimimetic indole as a designer drug in a herbal product. Forensic Toxicology, 27(2), 61–66. doi: https://doi.org/10.1007/s11419-009-0069-y
- van der Hoeven, R. A. M., Hofte, A. J. P., Frenay, M., Irth, H., Tjaden, U. R., van der Greef, J., … Edholm, L. E. (1997). Liquid chromatography –mass spectrometry with on-line solid-phase extraction by a restrictedaccess C18 precolumn for direct plasma and urine injection. Journal of Chromatography A, 762, 193–200. doi: https://doi.org/10.1016/S0021-9673(96)01004-7
- Vincenti, M., Leporati, M., Capra, P., Gatto, S., Attucci, A., Barbarino, G., & Nebbia, C. (2012). A field survey on the presence of prednisolone and prednisone in urine samples from untreated cows. Food Additives & Contaminants Part A, 29(12), 1893–1900. doi: https://doi.org/10.1080/19440049.2012.719645
- Wang, Z., Zou, S., Xing, C., Song, S., Liu, L., & Xu, C. (2016). Preparation of a monoclonal antibody against testosterone and its use in development of an immunochromatographic assay. Food and Agricultural Immunology, 27(4), 547–558. doi: https://doi.org/10.1080/09540105.2015.1137276
- Wei, X., Zhao, Y., Wang, B., & Wang, Y. (2014). Enzyme-linked immunosorbent assay-based two different polyclonal antibodies for the detection of cypermethrin with phenoxybenzene multiresidue. Food and Agricultural Immunology, 25(3), 364–374. doi: https://doi.org/10.1080/09540105.2013.805732