 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The present study aimed to examine the immunomodulatory properties of the methanolic (MeOH) extract from Pouteria. campechiana leaves in peritoneal macrophages of Balb/c mice. Peritoneal macrophages isolated from mice and Vero cells were treated with the MeOH extract from leaves. Cell viability of the macrophages and Vero cells were evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide method. The phagocytic activity, as nitric oxide (NO), hydrogen peroxide (H2O2), interleukin 6 (IL-6) and tumour necrosis factor α (TNF-α) production were evaluated on peritoneal macrophages. Results showed that the MeOH extract from leaves was able to stimulate the phagocytic activity and increase NO, H2O2 and cytokines production. The viability assays do not show cytotoxic effect on cell viability and cause a significative proliferative effect in the macrophages of a concentration-dependent manner. These results conclude that the MeOH extract from P. campechiana leaves possessed a stronger immunostimulatory effect in a concentration-dependent manner without affect the cell viability.
1. Introduction
Macrophages play an important role in early innate immune responses as part of the host’s defense mechanism (Muralidharan & Mandrekar, Citation2013). The first lines of defense against pathogens are the phagocytes cells, in which macrophages are included, which play a significant role in cellular and humoral immunity responses (Navegantes et al., Citation2017). Macrophages are activated by a range of stimuli that includes lipopolysaccharides (LPS), interferon γ and phorbol esters (Kelly & O’Neill, Citation2015). Those stimulus enhances proliferation, induces phagocytosis of pathogens, increases their oxygen consumption through the NADPH (nicotinamide adenine dinucleotide phosphate) oxidase and generates different reactive oxygen species (ROS), in a process called respiratory burst (Rahal et al., Citation2014). The hydrogen peroxide (H2O2) and nitric oxide (NO) are identified as the major effector molecules in the destruction of microorganisms by macrophages (Flannagan, Heit, & Heinrichs, Citation2015; Liu, Li, Xu, & Li, Citation2017). Since macrophages play important roles in maintaining tissue homeostasis and fighting disease, immunostimulation of macrophages that specifically contribute to the amelioration of various diseases present themselves as attractive targets for therapeutic intervention (Labonte, Tosello-Trampont, & Hahn, Citation2014; Wynn, Chawla, & Pollard, Citation2013). Plant extracts have been widely investigated in different parts of the world for their possible immunomodulatory properties and can provide an alternative to conventional therapy, which can potentialise the immune function for a variety of diseases (Ghonime et al., Citation2015; Pan et al., Citation2013).
Pouteria campechiana (Sapotaceae) is commonly known as canistel, yellow sapote or egg fruit, and is a native plant from Mexico, Belize, Guatemala and El Salvador. P. campechiana is an evergreen tree and 6–12-m tall with slender and glossy leaves (Cook, Citation2016). In traditional medicine, the bark has been used to treat fevers and skin eruptions, while the seeds have been used to treat ulcers (Elsayed, El-Tanbouly, Moustafa, Abdou, & El Awdan, Citation2016).
Although scientific reports on P. campechiana remain limited, previous studies have shown that it possesses antioxidant activities. Kong, Khoo, Prasad, Chew, and Amin (Citation2013) found that ethanolic extracts of pulp and peel isolated from P. campechiana fruit have significant antioxidant activities by the DPPH (α-diphenyl-β-picrylhydrazyl) and ABTS (2,2'-azino-bis[3-ethylbenzothiazoline-6-sulphonic acid]) radical scavenging assays. Also, Murakami et al. (Citation2005) demonstrated that the chloroform extract from P. campechiana fruits possess antioxidant activity on a test of TPA (12-O-tetradecanoylphorbol-13-acetate)-induced superoxide anion (O2−) generation in differentiated HL-60 (Human promyelocytic leukemia) cells, like antinitrosative activity on a test of LPS-induced NO generation in RAW 264.7 macrophages. Hernandez, Villaseñor, Joseph, and Tolliday (Citation2008) evaluated the ethyl acetate fraction of P. campechiana leaves, showing a positive result in a cell-based assay for anti-mitotic activity using HeLa cells. Even, they isolated stilbenoids and flavonoids from the leaves extract, concluding that ampelopsin B (stilbenoid) could be the responsible molecule for the anti-mitotic activity. Aseervatham et al. (Citation2014) demonstrated that the polyphenolic-rich aqueous extract of P. campechiana fruit has significant hepatoprotective effect against acetaminophen-induced hepatotoxicity in rats, due to a reduction in elevated liver marker enzymes such as aspartate transaminase, alanine transaminase and alkaline phosphatase. Finally, Elsayed et al. (Citation2016) analysed the ethanol, de-fatted ethanol extracts and n-hexane fractions from the leaves and seeds of P. campechiana, and they concluded that this plant have anti-inflammatory, analgesic and gastroprotective activity. In addition, the authors suggested that the biological effects found for P. campechiana may be attributed to its phenolic and flavonoid content.
However, there was paucity of data available on the immune effects of the extract of P. campechiana over the macrophage functions. Therefore, the present study was undertaken to evaluate the immunomodulatory effects of the MeOH extract from P. campechiana leaves and elucidating its cytotoxic effects on normal cell line, to support their safety on further clinic studies.
2. Materials and methods
2.1. Reagents
Dulbecco’s modified eagle medium (DMEM), fetal bovine serum (FBS) and penicillin–streptomycin were purchased from Gibco-BRL® (Grand Island, NY, USA). Bovine serum albumin (BSA), ethylenediaminetetraacetic acid (EDTA), Trypan-blue dye, dimethyl sulfoxide (DMSO), Cisplatin (CDDP), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), LPS (Escherichia coli 0111:B4), propidium iodide (PI), H2O2, sodium nitrite (NaNO2), phenol red and type I HRP, Dextrose and Griess Reagent were purchased from Sigma-Aldrich® (St Louis, MO, USA). Murine interleukin 6 (IL-6) enzyme-linked immunosorbent assay (ELISA) development kit (900-K50) and murine tumour necrosis factor α (TNF-α) ELISA development kit (900-K54) were obtained from Peprotech® (London, UK).
2.2. Plant material
Samples from P. campechiana leaves were collected on October 2013 in Mérida, Yucatán, México and were identified by a specialist (Salvador Flores Guido, UADY). The leaves were reserved at the herbarium of Universidad Autónoma de Yucatán (UADY) for future reference (voucher number 12867).
2.3. Preparation of extract
Dried leaves of P. campechiana (80 g) were extracted (3 times) with methanol (MeOH) at room temperature during 72 h. The extract was filtered, and the solvent was then evaporated under reduced pressure to give the crude MeOH extract.
2.4. Animals
Balb/c male mice (6–8 week of age, 20 ± 5 g of weight) were obtained from Centro de Investigaciones Regionales (CIR) “Dr. Hideyo Noguchi” from UADY. The animals were maintained in accordance with the principles and guidelines of NIH Guide for Treatment and Care for Laboratory Animals and by Mexican Official Norm for Animal Care and Handing (NOM-062-ZOO-1999). The animals were housed in standard polypropylene cages and were kept under standard laboratory conditions: access to special food and purified water ad libitum, free environment of pathogen and stress, temperature of 22 ± 2°C and light controlled room with a 12-h light/dark cycle.
2.5. Cell line
Green monkey kidney cell line (Vero) was employed in this study, which was obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). The cells were grown in supplemented DMEM media with 10% FBS and 1% penicillin–streptomycin. Vero cells were maintained in a humidified incubator at 37°C and 5% CO2 atmosphere with 95% humidity.
2.6. Isolation and treatment of peritoneal murine macrophages
The peritoneal murine macrophages were isolated according to a standard protocol described previously by Arana-Argáez et al. (Citation2016). The mice were sacrificed by the cervical dislocation method and peritoneal macrophages were isolated by lavages of the peritoneal cavity with cold phosphate buffered saline (PBS). In a biological safety cabinet, the peritoneum of each animal was exposed, cleaned with 70% ethanol and injected with 10 mL of cold PBS. The resident macrophages were collected by aspiration of the lavage fluid into a 10-mL plastic syringe and harvested by centrifugation for 15 min at 150× g, 4°C. The cells were resuspended in supplemented DMEM media with 10% FBS and 1% penicillin–streptomycin. The viability of macrophages was determined in a hemacytometer by trypan-blue dye exclusion and was typically found to be ≥95%. Between 1 × 104 cells/mL were seeded into each well of a 96-well plate for the cell viability assay, H2O2, NO, IL-6 and TNF-α production, while 1 × 105 cells/mL were placed in each well of a 24-well plate for the phagocytic activity assay. The plates were placed in a humidified incubator for 48 h (37°C, 5% CO2). Thereafter, macrophages were treated with the MeOH extract from P. campechiana leaves at 1, 10, 100 and 200 µg/mL dissolved in supplemented DMEM media with 0.1% DMSO (200 µL for 96-well plates or 500 µL for 24-well plates). The plates were incubated for 24 h (37°C, 5% CO2). Finally, macrophages were activated with LPS (1 µg/mL) and incubated during 48 h (37°C, 5% CO2). Macrophages without treatment or stimuli were used as negative control and activated macrophages with LPS (1 µg/mL) without treatment were employed as positive control in the H2O2, NO, IL-6 and TNF-α assays.
2.7. Measurement of NO production
The supernatants of a 96-well plate with treated and activated macrophages were collected and analysed for nitrites’ production as indicator of NO synthesis through the Griess reaction described by Zamani Taghizadeh Rabe, Mahmoudi, Ahmadsimab, Zamani Taghizadeh Rabe, and Emami (Citation2014). Briefly, 50 µL of the Griess reagent (0.1% N-(1-naphthyl) ethylenediamine dihydrochloride in H2O and 1% sulphanilamide in 5% H3PO4) were added to 50 µL of each supernatant in 96-well plates. The absorbance was measured at 490 nm on the iMark Microplate Reader (Bio-Rad®, USA) and nitrites concentration was determined by comparison with a NaNO2 standard curve (0–50 µM).
2.8. Measurement of H2O2 release
The supernatants of a 96-well plate with treated and activated macrophages were collected and analysed for H2O2 release according to Toffoli-Kadri et al.’s (Citation2014) protocol. Cell culture supernatants (100 µL) were mixed with an equal volume of fresh phenol red solution (5.5 mM dextrose, 0.056 g phenol red and 8.5 U/mL type I HRP in DPBS) in a 96-well plate and incubated for 3 h. The reaction was stopped adding 10 µL of NaOH 1 N solution. The absorbance was measured at 620 nm on the iMark Microplate Reader and the H2O2 concentration was determined by standard curve of H2O2 (0–50 µM).
2.9. IL-6 and TNF-α production
Cytokines (IL-6 and TNF-α) were analysed from cell culture medium of treated and activated macrophages through commercial ELISA kits according to the manufacturer’s instructions (Peprotech®, London, UK). A capture antibody was used at 2 µg/mL for IL-6 and 1 µg/mL for TNF-α. Serial dilutions of recombinant IL-6 (0–4000 pg/mL) or TNF-α (0–2000 pg/mL) in diluent were used as standard curve. The cytokines presented in the supernatants were detected with a biotinylated avidin-HRP conjugated second antibody (0.5 µg/mL for IL-6 and 0.25 µg/mL for TNF-α). The absorbance was measured at 490 nm on the iMark Microplate Reader, and the cytokines concentration (pg/mL) was determined by extrapolation of the absorbance in the standard curve.
2.10. Phagocytic activity
The phagocytic activity assay was carried out as described previously by Alonso-Castro et al. (Citation2012). Treated and activated macrophages were co-cultured with yeasts of Saccharomyces cerevisiae (5 × 106 yeasts/well) labelled with PI (100 µg/mL) and incubated 90 min (37°C, 5% CO2). Later, non-ingested labelling yeasts were removed, and 500 µL of separation buffer (0.5% BSA, 6.29 mM EDTA) were added. The plate was incubated for 10 min (37°C, 5% CO2). The obtained cell suspension was transferred to 1.5-mL tube and centrifuged for 10 min at 150× g, 4°C. Supernatants were removed by aspiration, and the pellets were resuspended in 1% formaldehyde. The amount of S. cerevisiae yeasts phagocytosed by macrophages was determined measuring the cellular fluorescent intensity using a Cell Lab Quanta SC flow cytometer (Beckman Coulter®). Macrophages with S. cerevisiae yeasts labelled with PI (1 µg/mL) were used as negative control, while activated macrophages with LPS (1 µg/mL) and S. cerevisiae yeasts labelled with PI (1 µg/mL) were employed as positive control.
2.11. Cell viability
Cell viability was examined by the MTT assay as described previously (Abdullah, Abdulghani, Ismail, and Abidin Citation2017). Macrophages of 96-well plates (1 × 104 cells/well) were treated with the MeOH extract from P. campechiana leaves at 1, 10, 100 and 200 µg/mL in supplemented DMEM media or DMSO (100%) as positive control. Also, pre-cultured Vero cells of 96-well plates (2.5 × 104 cells/well) were treated with the MeOH extract from P. campechiana leaves at 75, 150 and 300 µg/mL in supplemented DMEM media or CDDP (1 µg/mL) as positive control. Macrophages and Vero cells without treatment were used as negative control. After 24 h, the supernatants were removed and subsequently, 20 µL of the MTT solution (5 mg/mL in PBS) were added with 180 µL of supplemented DMEM media per well. Cells were incubated for 4 h (37°C, 5% CO2), the supernatants were removed and 100 µL of DMSO (100%) were added to each well, to dissolve produced-formazan crystals. The reduction of MTT was quantitated by measurement of the absorbance at 490 nm on the iMark Microplate Reader. The percentage (%) of cell viability was calculated by the following formula:
2.12. Statistical analysis
Experimental values were expressed as the mean ± standard deviation of three experiments in triplicate. Data were analysed using one-way analysis of variance, followed by post hoc Dunnett’s tests. Levels of p < .05 were considered as indicative of significance. Letter “a” indicated significant differences in comparison to negative control or C(−) and letter “b” indicated significant differences in comparison to positive control or C(+). All calculations were carried out using the GraphPad Prism® V5.03 software (GraphPad Software Inc.®, CA, USA).
3. Results
3.1. Phagocytic activity
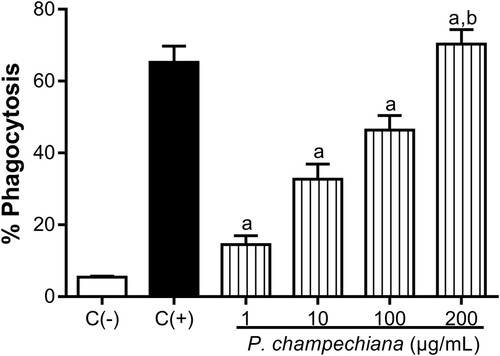
The results from flow cytometry show that the MeOH extract from P. campechiana leaves increased the phagocytic activity of macrophages over S. cerevisiae yeasts in a concentration-dependent manner up to 14.53, 32.68, 46.40 and 70.31% at 1, 10, 100 and 200 µg/mL, respectively (). When LPS (1 µg/mL) was added to macrophages, the phagocytic activity was increased up to 65.20%, a value that was considered as the higher percentage of phagocytosis (positive control), while the basal phagocytic activity (5.43%) corresponded to the negative control.
Figure 1. Effect of the MeOH extract of P. campechiana leaves on the macrophages’ phagocytic activity. The results represent the mean ± SD of three independent experiments (n = 3) and were analysed using the ANOVA test followed by Dunnett’s post hoc test. Letter “a” indicates significant differences in comparison to negative control or C(−), with p < .05. Letter “b” indicates significant differences in comparison to positive control or C(+), with p < .05. C(−): macrophages and S. cerevisiae yeasts labelled with PI (1 µg/mL), C(+): activated macrophages with LPS (1 µg/mL) and S. cerevisiae yeasts labelled with PI (1 µg/mL).
3.2. NO production of macrophages
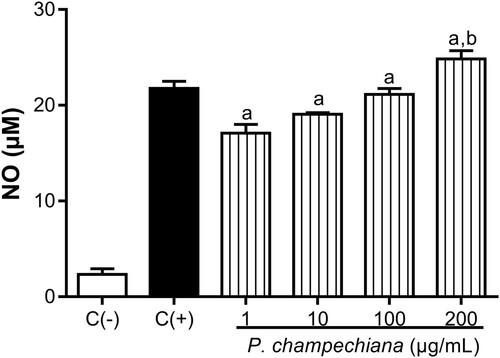
Treatment with the MeOH extract from leaves on the macrophages increased the nitrites’ production by these cells in a concentration-dependent manner to 17.08, 19.06, 21.15 and 24.83 µM at the evaluated concentrations (). NO production in positive control was increased up to 21.77 µM and the obtained value of the negative control was 2.32 µM.
Figure 2. Effect of the MeOH extract of P. campechiana leaves on the macrophages’ NO production. The results represent the mean ± SD of three independent experiments (n = 3) and were analysed using the ANOVA test followed by Dunnett’s post hoc test. Letter “a” indicates significant differences in comparison to negative control or C(−), with p < .05. Letter “b” indicates significant differences in comparison to positive control or C(+), with p < .05. C(−): macrophages without treatment or stimulus, C(+): macrophages activated with LPS (1 µg/mL).
3.3. H2o2 production of macrophages
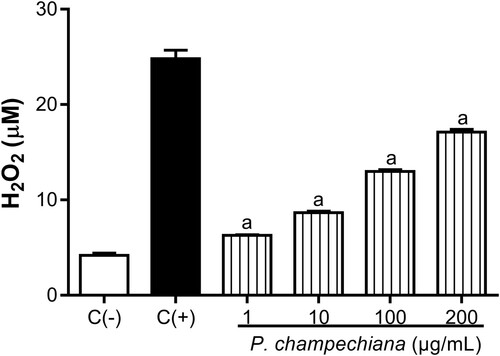
The H2O2 released by macrophages incubated with the MeOH extract from P. campechiana increased in a concentration-dependent manner up to 6.27, 8.67, 12.98 and 17.12 µM at the evaluated concentrations (). LPS (1 µg/mL) were added to the cells and the level of H2O2 was increased up to 24.80 µM (positive control). In contrast, the H2O2 concentration of the negative control was 4.17 µM.
Figure 3. Effect of the MeOH extract of P. campechiana leaves on the macrophages H2O2 production. The results represent the mean ± SD of three independent experiments (n = 3) and were analysed using the ANOVA test followed by Dunnett’s post hoc test. Letter “a” indicates significant differences in comparison to negative control or C(−), with p < .05. C(−): macrophages without treatment or stimulus, C(+): macrophages activated with LPS (1 µg/mL).
3.4. IL-6 and TNF-α production by macrophages
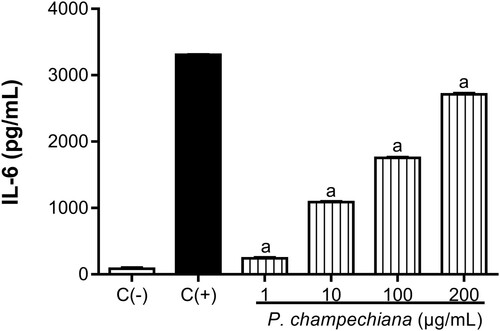
In our study, macrophages were treated with various concentrations of the MeOH extract from leaves, and the IL-6 and TNF-α levels were measured by ELISA kits. Results show that P. campechiana leaves significantly increased the cytokines’ production in contrast to their respective positive control. The concentrations of IL-6 were 242, 1089, 1755 and 2712 pg/mL at 1, 10, 100 and 200 µg/mL, respectively (). The IL-6 positive control value was 3309 pg/mL, while the concentration of the negative control was 85.33 pg/mL.
Figure 4. Effect of the MeOH extract of P. campechiana leaves on the macrophages IL-6 production. The results represent the mean ± SD of three independent experiments (n = 3) and were analysed using the ANOVA test followed by Dunnett’s post hoc test. Letter “a” indicates significant differences in comparison to negative control or C(−), with p < .05. C(−): macrophages without treatment or stimulus, C(+): macrophages activated with LPS (1 µg/mL).
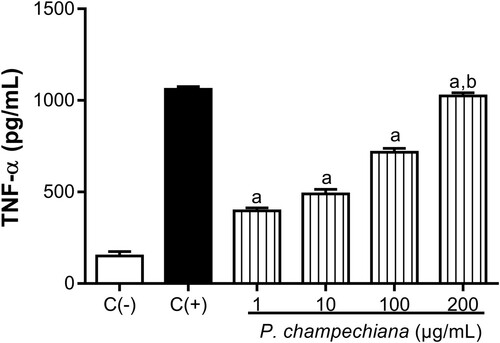
Also, the concentrations of TNF-α were 397.30, 489, 717.30 and 1024 pg/mL at 1, 10, 100 and 200 µg/mL, respectively (). TNF-α value in positive control was 1061 pg/mL, while the level of TNF-α corresponding to the negative control was 150.70 pg/mL.
Figure 5. Effect of the MeOH extract of P. campechiana leaves on the macrophages TNF-α production. The results represent the mean ± SD of three independent experiments (n = 3) and were analysed using the ANOVA test followed by Dunnett’s post hoc test. Letter “a” indicates significant differences in comparison to negative control or C(−), with p < .05. Letter “b” indicates significant differences in comparison to positive control or C(+), with p < .05. C(−): macrophages without treatment or stimulus, C(+): macrophages activated with LPS (1 µg/mL).
3.5. Cell viability
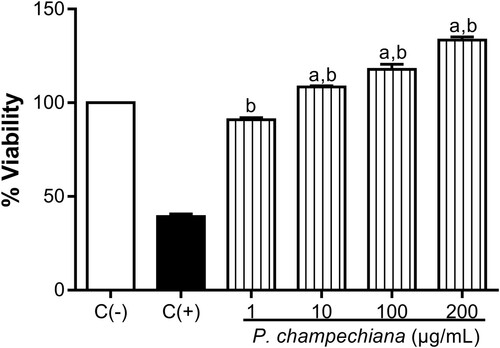
Cytotoxic effects of the MeOH extract from P. campechiana on peritoneal murine macrophages and Vero cells were evaluated using the MTT assay, demonstrating that the MeOH extract does not affect cell viability of these cells at different concentrations. Even, the results showed an increase in the proliferation of the macrophages in a concentration-dependent manner and the percentages obtained were 90.98%, 108.50%, 117.80% and 133.40% at 1, 10, 100 and 200 µg/mL, respectively (). Macrophages without treatment or stimuli were employed as negative control (100%), while macrophages incubated with DMSO (100%) were used as positive control (39.29%).
Figure 6. Effect of the MeOH extract of P. campechiana leaves on the macrophages cell viability. The results represent the mean ± SD of three independent experiments (n = 3) and were analysed using the ANOVA test followed by Dunnett’s post hoc test. Letter “a” indicates significant differences in comparison to negative control or C(−), with p < .05. Letter “b” indicates significant differences in comparison to positive control or C(+), with p < .05. C(−): macrophages without treatment or stimulus, C(+): macrophages with DMSO (100%).
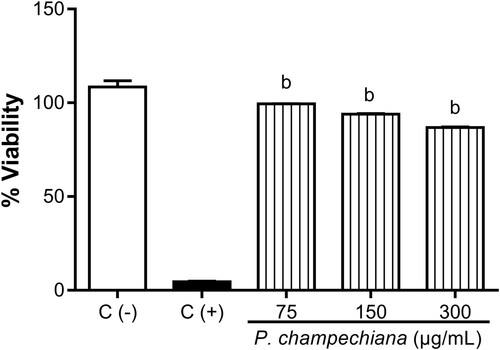
In Vero cells, the percentages showed that the MeOH extract did not exert a significant cytotoxic effect (99.40%, 93.97% and 86.76% at 75, 150 and 300 µg/mL, respectively), with median cytotoxic concentrations (CC50) values >300 µg/mL (). Vero cells without treatment or stimuli were employed as positive control (100%), while Vero cells with CDDP (1 µg/mL) were used as negative control (4.59%).
Figure 7. Effect of the MeOH extract of P. campechiana leaves on Vero cells viability. The results represent the mean ± SD of three independent experiments (n = 3) and were analysed using the ANOVA test followed by Dunnett’s post hoc test. Letter “b” indicates significant differences in comparison to positive control or C(+), with p < .05. C(−): Vero cells without treatment or stimulus, C(+): Vero cells with CDDP (1 µg/mL).
4. Discussion
In the traditional medicine, P. campechiana have been employed to treat fevers, ulcers and skin eruptions (Elsayed et al., Citation2016). Previously, some studies have reported the antioxidant, anti-mitotic, hepatoprotective, anti-inflammatory, analgesic, gastroprotective and tyrosinase inhibitory activities of this plant, but have not been evaluated its effect over the immune system (Aseervatham et al., Citation2014; Baky, Kamal, Elgindi, & Haggag, Citation2016; Elsayed et al., Citation2016; Kong et al., Citation2013; Souza et al., Citation2012). Then, the aim of this work was evaluate the immunomodulatory effect of the MeOH extract from P. campechiana leaves over peritoneal murine macrophage functions.
Macrophages have an important role in recognition of foreign antigens, homeostasis, tissue repair and immunity. Phagocytosis process is clearly central to macrophage function because they destroy pathogens in part through ROS as H2O2 or reactive nitrogen species (RNS) as NO (Flannagan et al., Citation2015; Rahal et al., Citation2014; Zhu, Pan, Yang, & Zhou, Citation2015). In this work, the MeOH extract of P. campechiana enhanced the phagocytic activity of the macrophages stimulated by LPS in a significative concentration-dependent manner. The NO and H2O2 production followed the same trend. Nevertheless, in the scientific literature no studies have evaluated the extract of leaves or some metabolites isolated from P. campechiana. Murakami et al. (Citation2005) demonstrated that the chloroform extract from P. campechiana fruits showed suppressive activity on NO generation in RAW 264.7 macrophages, in contrast with the results obtained in our study. Even, some reports have shown the immunostimulatory effect of plants belonging to the Sapotaceae family. For example, Manosroi, Saraphanchotiwitthaya, and Manosroi (Citation2005) showed that the methanolic stem bark extract from Pouteria cambodiana (Sapotaceae) presents a good dose–response effect in a phagocytosis assay on macrophages isolated from BALB/c mice. Another study carried out by Manosroi, Saraphanchotiwitthaya, and Manosroi (Citation2006) examined the acetone extracts of stem bark from P. cambodiana on murine macrophage phagocytosis and concluded that the extract upgrade the phagocytic response in a lysosomal enzyme activity test. These latter data coincide with the results of our work, in part due at the genus and chemical composition of P. campechiana, which contains similar metabolites (Sapotaceae is a family with a wide range of chemical constituents such as flavonoids and polyphenolic compounds) that might be responsible for the resulting immunostimulatory effect (Baky et al., Citation2016).
During an infection or inflammation, innate activation of macrophages results in secretion of pro-inflammatory cytokines, such as IL-6 and TNF-α (Wynn et al., Citation2013). These cytokines help in acute phase response by acting on a variety of cells. Also, the TNF-α induce the expression of adhesion molecules, causing chemotaxis of leucocytes, inhibiting cellular proliferation and inducing cell apoptosis (Duque & Descoteaux, Citation2014). In our study, the MeOH extract of P. campechiana leaves increased the levels of IL-6 and TNF-α in a concentration-dependent manner. No previous study has shown the effect of P. campechiana on cytokines’ production by macrophages. Likewise, few studies have reported the effect of plants from Sapotaceae family on IL-6 and TNF-α levels. For example, Merini et al. (Citation2014) demonstrated the anti-inflammatory effect of phonophoresis (application of topical drugs to the external layer of the skin with the aid of pulsed ultrasound) with Elaeoluma nuda (Sapotaceae) gel on interleukin 1α (IL-1α) and TNF-α. Meira et al. (Citation2014) showed that chloroform fraction obtained from Chrysophyllum cainito (Sapotaceae) leaves reduce the interleukin 1β (IL-1β) and TNF-α levels in mouse paw. However, these studies contrast with the results obtained in our study, due to the different genus and other metabolites present in the plants, which could be causing a synergism and inducing the immunostimulatory effect.
Some research has reported that monocytes are prominently produced in the bone marrow. In response to stimuli, the monocytes are mobilised from bone marrow, migrate into tissues and differentiate into macrophages. Also, the macrophages have no proliferative capacity, as evidenced in some reports (Italiani & Boraschi, Citation2015). In our study, the MeOH extract from P. campechiana leaves did not cause a significant cytotoxic effect in macrophages and Vero cells. Also, the MeOH extract showed a low but significative increase in the proliferation of the macrophages in a concentration-dependent manner.
In conclussion, in this work we found that the MeOH extract from P. campechiana leaves exhibit an immunostimulatory effect on phagocytic activity, through the overproduction of NO, H2O2, IL-6 and TNF-α in peritoneal murine macrophages. Various metabolites are present in P. campechiana leaves and it is possible that these metabolites (or others) are implicated in the immunostimulatory effects. Despite these results, further phytochemical and pharmacological investigations of this plant are necessary.
5. Conclusion
This is the first study about the evaluation of the immunomodulatory effect of the MeOH extract from P. campechiana leaves, demonstrating an important immunostimulatory effect in the proliferation of murine macrophages, as the phagocytic activity, NO, H2O2 and cytokines (IL-6 and TNF-α) production of those cells, in a concentration-dependent manner.
Acknowledgements
The authors are grateful to Salvador Flores Guido, PhD from Herbarium of the Universidad Autónoma de Yucatán, for the identification of the vegetal material.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Ivan Chan-Zapata http://orcid.org/0000-0001-8589-0134
Additional information
Funding
References
- Abdullah, N., Abdulghani, R., Ismail, S. M., & Abidin, M. H. Z. (2017). Immune-stimulatory potential of hot water extracts of selected edible mushrooms. Food and Agricultural Immunology, 28(3), 374–387. doi: https://doi.org/10.1080/09540105.2017.1293011
- Alonso-Castro, A. J., Ortiz-Sánchez, E., Domínguez, F., Arana-Argáez, V., Juárez-Vázquez, M. d. C., Chávez, M., … García-Carrancá, A. (2012). Antitumor and immunomodulatory effects of Justicia spicigera Schltdl (Acanthaceae). Journal of Ethnopharmacology, 141, 888–894. doi: https://doi.org/10.1016/j.jep.2012.03.036
- Arana-Argáez, V. E., Chan-Zapata, I., Canul-Canche, J., Fernández-Martín, K., Martín-Quintal, Z., Torres-Romero, J. C., … Ramírez-Camacho, M. A. (2016). Immunosuppresive effects of the methanolic extract of Chrysophyllum cainito leaves on macrophage functions. African Journal of Traditional, Complementary and Alternative Medicines, 14(1), 179–186. doi: https://doi.org/10.21010/ajtcam.v14i1.20
- Aseervatham, G. S. B., Sivasudha, T., Sasikumar, J. M., Christabel, P. H., Jeyadevi, R., & Ananth, D. A. (2014). Antioxidant and hepatoprotective potential of Pouteria campechiana on Acetaminophen-induced hepatic toxicity in rats. Journal of Physiology and Biochemistry, 70(1), 1–14. doi: https://doi.org/10.1007/s13105-013-0274-3
- Baky, M. H., Kamal, A. M., Elgindi, M. R., & Haggag, E. G. (2016). A review on phenolic compounds from family Sapotaceae. Journal of Pharmacognosy and Phytochemistry, 5(2), 280–287. Retrieved from http://www.phytojournal.com/archives/?year=2016&vol=5&issue=2&part=D&ArticleId=844
- Cook, S. (2016). The forest of the Lacandon Maya: An ethnobotanical guide. New York, NY: Springer.
- Duque, G. A., & Descoteaux, A. (2014). Macrophage cytokines: Involvement in immunity and infectious diseases. Frontiers in Immunology, 5(491), 897–812. doi: https://doi.org/10.3389/fimmu.2014.00491
- Elsayed, A. M., El-Tanbouly, N. D., Moustafa, S. F., Abdou, R. M., & El Awdan, S. A. W. (2016). Chemical composition and biological activities of Pouteria campechiana (Kunth) Baehni. Journal of Medicinal Plants Research, 10(16), 209–215. doi: https://doi.org/10.5897/JMPR2015.6031
- Flannagan, R. S., Heit, B., & Heinrichs, D. E. (2015). Antimicrobial mechanisms of macrophages and the immune evasion strategies of Staphylococcus aureus. Pathogens (basel, Switzerland), 4(4), 826–868. doi: https://doi.org/10.3390/pathogens4040826
- Ghonime, M., Emara, M., Shawky, R., Soliman, H., El-Domany, R., & Abdelaziz, A. (2015). Immunomodulation of RAW 264.7 murine macrophage functions and antioxidant activities of 11 plant extracts. Immunological Investigations, 44(3), 237–252. doi: https://doi.org/10.3109/08820139.2014.988720
- Hernandez, C. L. C., Villaseñor, I. M., Joseph, E., & Tolliday, N. (2008). Isolation and evaluation of antimitotic activity of phenolic compounds from Pouteria campechiana Baehni. Philippine Journal of Science, 137(1), 1–10. Retrieved from http://agris.fao.org/agris-search/search.do?recordID=PH2009000796
- Italiani, P., & Boraschi, D. (2015). New insights into tissue macrophages: From their origin to the development of memory. Immune Network, 15(4), 167–176. doi: https://doi.org/10.4110/in.2015.15.4.167
- Kelly, B., & O’Neill, L. A. J. (2015). Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Research, 25, 771–784. doi: https://doi.org/10.1038/cr.2015.68
- Kong, K. W., Khoo, H. E., Prasad, N. K., Chew, L. Y., & Amin, I. (2013). Total phenolics and antioxidant activities of Pouteria campechiana fruit parts. Sains Malaysiana, 42(2), 123–127. Retrieved from http://www.ukm.my/jsm/pdf_files/SM-PDF-42-2-2013/0120K.W.20Kong.pdf
- Labonte, A. C., Tosello-Trampont, A. C., & Hahn, Y. S. (2014). The role of macrophage polarization in infectious and inflammatory diseases. Molecules and Cells, 37(4), 275–285. doi: https://doi.org/10.14348/molcells.2014.2374
- Liu, L., Li, H., Xu, R. H., & Li, P. L. (2017). Expolysaccharides from Bifidobacterium animalis RH activates RAW 264.7 macrophages through toll-like receptor 4. Food and Agricultural Immunology, 28(1), 149–161. doi: https://doi.org/10.1080/09540105.2016.1230599
- Manosroi, A., Saraphanchotiwitthaya, A., & Manosroi, J. (2005). In vitro immunomodulatory effect of Pouteria cambodiana (Pierre ex Dubard) Baehni extract. Journal of Ethnopharmacology, 101, 90–94. doi: https://doi.org/10.1016/j.jep.2005.03.031
- Manosroi, A., Saraphanchotiwitthaya, A., & Manosroi, J. (2006). Effects of Pouteria cambodiana extracts on in vitro immunomodulatory activity of mouse immune system. Fitoterapia, 77, 189–193. doi: https://doi.org/10.1016/j.fitote.2006.01.003
- Meira, N. A., Klein Jr., L. C., Rocha, L. W., Martin Quintal, Z., Delle Monache, F., Cechinel Filho, V., & Meira Quintão, N. L. (2014). Anti-inflammatory and anti-hypersensitive effects of the crude extract, fractions and triterpenes obtained from Chrysophyllum cainito leaves in mice. Journal of Ethnopharmacology, 151, 975–983. doi: https://doi.org/10.1016/j.jep.2013.12.014
- Merini, L. R., Furtado, S. d. C., Brito de Oliveira, M., M., Basilo Carneiro, A. L., … Marques Barcellos J. F. (2014). Attenuation of adjuvant-induced arthritis in rats by phonophoresis with an aqueous gel of the Amazonian plant Elaeoluma nuda (Sapotaceae). Cytokine, 65, 231–235. doi: https://doi.org/10.1016/j.cyto.2013.10.007
- Murakami, A., Ishida, H., Kubo, K., Furukawa, I., Ikeda, Y., Yonaha, M., … Ohigashi, H. (2005). Suppressive effects of Okinawan food items on free radical generation from stimulated leukocytes and identification of some active constituents: Implications for the prevention of inflammation-associated carcinogenesis. Asian Pacific Journal of Cancer Prevention, 6, 437–448. Retrieved from http://journal.waocp.org/?sid=Entrez:PubMed&id=pmid:16435988&key=2005.6.4.437
- Muralidharan, S., & Mandrekar, P. (2013). Cellular stress response and innate immune signaling: Integrating pathways in host defense and inflammation. Journal of Leukocyte Biology, 94(6), 1167–1184. doi: https://doi.org/10.1189/jlb.0313153
- Navegantes, K. C., Gomes, R. S., Pereira, P. A. T., Czaikoski, P. G., Azevedo, C. H. M., & Monteiro, M. C. (2017). Immune modulation of some autoimmune diseases: The critical role of macrophages and neutrophils in the innate and adaptive immunity. Journal of Translational Medicine, 15(36), 328–321. doi: https://doi.org/10.1186/s12967-017-1141-8
- Pan, S. Y., Zhou, S. F., Gao, S. H., Yu, Z. L., Zhang, S. F., Tang, M. K., … Ko, K. M. (2013). New perspectives on how to discover drugs from herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evidence-Based Complementary and Alternative Medicine, 2013, 1–25. doi: https://doi.org/10.1155/2013/627375
- Rahal, A., Kumar, A., Singh, V., Yadav, B., Tiwari, R., Chakraborty, S., … Dhama, K. (2014). Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Research International, 2014, 1–19. doi: https://doi.org/10.1155/2014/761264
- Souza, P. M., Elias, S. T., Simeoni, L. A., de Paula, J. E., Gomes, S. M., Guerra, E. N. S., … Magalhaes, P. O. (2012). Plants from Brazilian Cerrado with potent tyrosinase inhibitory activity. PLoS One, 7(11), e48589–7. doi: https://doi.org/10.1371/journal.pone.0048589
- Toffoli-Kadri, M. C., Carollo, C. A., Días Lourenço, L., Felipe, J. L., Brandini Néspoli, J. H., Campos Wollf, L. G., … de Siqueira, J. M. (2014). In vivo and in vitro anti-inflammatory properties of Achyrocline alata (Kunth) DC. Journal of Ethnopharmacology, 153(2), 461–468. doi: https://doi.org/10.1016/j.jep.2014.03.008
- Wynn, T. A., Chawla, A., & Pollard, J. W. (2013). Macrophage biology in development, homeostasis and disease. Nature, 496, 445–455. doi: https://doi.org/10.1038/nature12034
- Zamani Taghizadeh Rabe, S., Mahmoudi, M., Ahmadsimab, H., Zamani Taghizadeh Rabe, S. S., & Emami, A. (2014). Investigation of the biological activity of methanol extract from Eremostachys labiosa Bunge. Food and Agricultural Immunology, 25(4), 578–585. doi: https://doi.org/10.1080/09540105.2013.858311
- Zhu, S. J., Pan, J., Yang, J. J., & Zhou, A. (2015). Immune activation and toxicity evaluation of fresh Cordyceps militaris extracts by high-pressure processing. Food and Agricultural Immunology, 26(5), 645–658. doi: https://doi.org/10.1080/09540105.2015.1007445
