ABSTRACT
In this study, we examined whether Lactobacillus plantarum C29 could restore 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced T helper 17 (Th17)/regulatory T cells (Tregs) imbalance in mice. Treatment with C29 inhibited the differentiation of splenic T cells into Th17 cells and the expression of retinoic acid receptor-related orphan receptor gamma t (RORγt) and IL-17 in vitro, whereas promoting the differentiation into Tregs. Oral administration of Lactobacillus plantarum C29 in mice attenuated TNBS-induced colon shortening, myeloperoxidase (MPO) activity, inducible Nitric oxide (NO) synthase, and cyclooxygenase-2 expression, and activation of NF-κB in the colon of mice. C29 treatment downregulated TNF-α, IL-17, and IL-1β expression, while increasing IL-10 expression. C29 treatment suppressed TNBS-induced Th17 cell differentiation and reduced IL-17 and RORγt expression, while promoting the TNBS-suppressed Tregs differentiation and IL-10 and forkhead box P3 expression. These findings suggest that C29 can alleviate colitis by modulating NF-κB activation as well as Th17/Treg balance.
Introduction
Inflammatory bowel disease (IBD), including ulcerative colitis and Crohn’s disease, is a chronically relapsing inflammatory disease of the gastrointestinal (GI) tract (Maloy & Powrie, Citation2011). The pathogenesis of IBD involves genetic susceptibility and host innate and adaptive immunity (Du et al., Citation2015; Niess, Leithäuser, Adler, & Reimann, Citation2008). The stimulation of pathogens in the GI tracts is continuously defended by the gut immune system, which consists of neutrophils, macrophages, dendritic cells (DCs), and T cells involved in innate and adaptive immunity (Du et al., Citation2015; Niess et al., Citation2008; Nourshargh & Alon, Citation2014). Activation of innate immune cells, including DCs and macrophages, by these antigens stimulates the adaptive immune cells such as T cells: the secretion of TNF-α, IL-10, and IL-12 in the immune cells stimulate the differentiation of naïve CD4+ T cells into effector T cells, such as Th1, Th17, and regulatory T cells (Tregs) (Atreya, Atreya, & Neurath, Citation2008; Owen & Mohamadzadeh, Citation2013; Rutella & Locatelli, Citation2011). TNF-α, IL-12, and IL-17 are highly expressed in the inflamed colons of mice and humans with IBD; however, IL-10 expression is down-regulated, leading to colitis (Kaistha & Levine, Citation2014; Leppkes et al., Citation2009). Therefore, the down-regulation of IL-12 and TNF-α expression and up-regulation of IL-10 expression may be important for the prevention and treatment of colitis.
Probiotics including lactobacilli and bifidobacteria are frequently found in fermented foods and in the microbiota of humans and animals (Liong, Citation2008; Xu, Shen, Wu, & Li, Citation2017). Many lactobacilli exhibit a variety of beneficial effects such as inhibiting the growth of pathogens, preventing inflammation and memory impairment, and the modulation of the host immune system (Jung, Jung, Kim, Han, & Kim, Citation2012; Nagpal et al., Citation2016). Of these, lactobacilli including Lactobacillus sakei, Lactobacillus fermentum, and Lactobacillus reuteri are known to mitigate colitis in rodents with 2,4,6-trinitrobenzenesulfonic acid (TNBS)- or dextran sulfate sodium (DSS)-induced colitis through the inhibition of innate immune responses such as macrophage activation (Lee et al., Citation2008; Lim et al., Citation2017 ; Eun, Lim, Jang, Han, & Kim, Citation2016; Petrella, Citation2016). Lactobacillus plantarum CLP-0611, which was isolated from kimchi, also suppressed the lipopolysaccharide (LPS)-induced NF-κB activation in macrophages (Jang, Han, Kim, & Kim, Citation2014; Lee et al., Citation2008). Lactobacillus plantarum C29, a kimchi probiotic, also suppressed NF-κB activation in LPS-stimulated macrophages (Jang et al., Citation2014; Lee et al., Citation2008), as well as in the colon of aged rats (Jeong et al., Citation2015; Jung et al., Citation2012). Furthermore, C29 suppressed the scopolamine-induced memory impairment in mice (Jeong et al., Citation2015; Jung et al., Citation2012). Additionally, Lactobacillus casei attenuated DSS-induced colitis in mice by regulating the imbalance of T helper 17 (Th17) cells/regulatory T cells (Wang et al., Citation2017). Lactobacillus plantarum A7 with Bifidobacterijm animalis PTCC1631 alleivated neuroinflammation in mice with experimental multiple sclerosis by increasing Th2 and Treg cell differentiation (Salehipour et al., Citation2017). Nevertheless, the effects of lactobacilli including C29 on the T cell differentiation have been studied thoroughly.
Therefore, in the present study, to understand the effect of C29 on the T cell differentiation, we investigated its effect on the differentiation of splenocytes into Th17/Treg cells and TNBS-induced Th17/Treg imbalance in mice.
Materials and methods
Materials
Rosewell Park Memorial Institute (RPMI) 1640 medium, TNBS, radioimmuno-precipitation assay (RIPA) buffer, and 3,3′,5,5′-tetramethyl benzidine (TMB) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies were obtained from Cell Signalling Technology (Beverly, MA, USA). Enzyme-linked immunosorbent assay (ELISA) kits were supplied from R&D Systems (Minneapolis, MN, USA). Pan T Cell Isolation Kit II was provided by MiltenyiBiotec (Bergisch Gladbach, Germany). Anti-CD28, anti-CD3, recombinant IL-6 (rIL-6), and recombinant Transforming growth factor (rTGF)-β were purchased from BioGems International Inc. (Westlake Village, CA, USA). The mRNA isolation kit was purchased from Qiagen (Hilden, Germany). The other chemicals used were of the highest grade available.
Culture of Lactobacillus plantarum C29
Lactobacillus plantarum C29 (KCCM10885, Korea Culture Center of Microorganisms, Seoul, Korea) was cultured in MRS broth as previously reported (Jang et al., Citation2013). Briefly, C29 was cultured at 37°C for 24 h in MRS broth (10 mL), successively inoculated in MRS broth (2 L) and cultured at 37°C to an optical density between 1.5 and 2 at a wavelength of 600 nm. Cells were harvested by centrifugation (10,000 g for 20 min), washed with saline, and freeze-dried. Freeze-dried cells were suspended in 1% glucose for the in vivo study (oral gavage in mice), or in 20 mM phosphate buffer (pH7.0) for the in vitro study (experiments in Th cells isolated from spleens).
Animals
Male C57BL/6 mice (20–22 g, 6-week old) were purchased from RaonBio Inc. (Seoul, Korea). Animals were acclimatized for 7 days before experiments. All mice were housed in wire cages at 20–22°C and 50 ± 10% humidity and fed with standard laboratory chow and water ad libitum. All animal experiments were approved by the Committee for the Care and Use of Laboratory Animals in the Kyung Hee University (IRB No., KHUASP(SE)-16-027) and performed in accordance with the Kyung Hee University Guidelines for Laboratory Animal Care and Usage.
Preparation and differenitation of Th cells from spleens
Spleens were aseptically removed from twelve mice (male, 6 weeks-old), crushed, and treated with Tris-buffered ammonium chloride (Lim et al., Citation2016). The cell suspension was prepared in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum. T cells were isolated from the cell suspension using the Pan T Cell Isolation Kit II. The differentiation of T cells (1 × 105 cells/well) into Th17 was induced through their stimulation with anti-CD28 (1 μg/mL), anti-CD3 (1 μg/mL), rTGF-β (1 ng/mL), and rIL-6 (20 ng/mL) in the absence or presence of C29 (1 × 103 and 1 × 105 colony forming unit [CFU]/mL) for 4 days. In addition, T cells (1 × 105 cells) were subjected to stimulation with anti-CD28 (1 μg/mL) and anti-CD3 (1 μg/mL) in the absence or presence of C29 (1 × 105 CFU/mL) for 4 days to induce differentiation into Tregs. Cells were fixed, stained with anti-FoxP3 (phycoerythrin [PE]) or anti-IL-17A (fluorescein isothiocyanate [FITC]) antibodies, and measured using a flow cytometer (C6 Flow Cytometer® System, San Jose, CA, USA).
Preparation of mice with TNBS-induced colitis and memory impairment
Mice were divided into four groups to measure the effective dose of C29 against TNBS-induced colitis and memory impairment. Each group consisted of 6 mice. Animals were anesthetized with ether and 2.5% (w/v) TNBS solution (100 μL, dissolved in 50% ethanol) was intrarectally administered into the colon 3.5–4 cm proximal to the anus of mice, apart from mice in the first and the second groups (Jang et al., Citation2014). Mice were held in a vertical position for 30 s after TNBS administration. Mice in the first and second groups were treated with saline instead of TNBS. After 24 h from the administration of the TNBS treatment, test agents (1 × 109 CFU/mouse of C29 for the third and 50 mg/kg of sulfasalazine for the fourth group) were orally administered once a day for 3 days. Mice in the first and third groups were vehicle treated (1% dextrose). Their colons removed, longitudinally opened, and macroscopically scored (grade 0, no ulcer and inflammation; grade 1, no ulceration and local hyperaemia; grade 2, ulceration with hyperaemia; grade 3, ulceration and inflammation at one site only; grade 4, two or more sites of ulceration and inflammation; and grade 5, ulceration extending more than 2 cm) (Lim et al., Citation2016). Colon tissues were washed with ice-cold phosphate-buffered saline, divided into eight segments, and used for other experiments. Colon tissues were stored at −80°C for immunoblotting and ELISA analysis. Histological examination of colon tissues (first and fifth segments) was performed according to the method of Jang et al. (Citation2014).
Myeloperoxidase (MPO) activity assay
Colon tissues (second and sixth segments) were homogenized in 10 mM potassium phosphate buffer (pH 7.0) containing 0.5% hexadecyl trimethyl ammonium bromide, and centrifuged for 10 min (20,000 × g at 4°C) (Lim et al., Citation2016). The supernatant (50 μL) was incubated with 0.75 mL of the pre-incubated reaction mixture containing 0.1 mM hydrogen peroxide and 1.6 mM TMB at 37°C for 5 min and periodically monitored for its absorbance at a wavelength of 650 nm.
Immunoblotting and ELISA
Colon (third and seventh segments) stored at −80°C were homogenized on ice in 1 mL of RIPA lysis buffer containing 1% each of phosphatase and protease inhibitor cocktail and centrifuged at 15,000 × g for 15 min at 4°C (Jang et al., Citation2014). Immunoblotting analysis and cytokines assays were performed according to the method of Kim, Gu, Lee, Joh, and Kim (Citation2012).
Flow cytometric analysis of Th17 and Tregs in the lamina propria of the colon
Colon tissues (fourth and eighth segments) were cut into small pieces and agitated in 2.5 mM ethylenediaminetetraacetic acid (EDTA) at 37°C for 20 min. Samples treated with EDTA were minced, digested at 37°C for 20 min in RPMI containing 1 mg/mL type VIII collagenase, and lamina propria cells were separated (Lim et al., Citation2016; Roy & Kumar, Citation2015). T cells were purified using the Pan T cell Isolation Kit II. Cells were fixed and stained with anti-Foxp3 or anti-IL-17A antibodies, and the number of Th17 and Treg cells was estimated using a flow cytometer.
Quantitative real time – polymerase chain reaction (qPCR)
RNA (2 μg) was isolated from the colon tissue and subjected to qPCR for the analysis of IL-10, IL-17, Foxp3, retinoic acid receptor-related orphan receptor gamma t (RORγt), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) using a Takara thermal cycler and SYBR premix reagents (Lim et al., Citation2016). Thermal cycling conditions were as follows: denaturation at 95°C for 5 min, followed by 35 cycles of amplification at 95°C for 10 s and 60°C for 30 s. The normalized expression of the assayed genes, with respect to GAPDH, was computed for all samples using the Microsoft Excel data spreadsheet. And Primers used were as follows: forward, 5′-TGGCCCAGAAATCAAGGAGC-3′ and reverse, 5′-CAGCAGACTCAATACACACT-3′ for IL-10; forward, 5′-TCAGCGTGTCCAAACACTGAG-3′ and reverse, 5′-CGCCAAGGGAGTTAAAGACTT-3′ for IL-17; forward, 5′-TTGGCCAGCGCCATCTT-3′ and reverse, 5′-TGCCTCCTCCAGAGA GAAGTG-3′ for Foxp3; forward, 5′-TGAGGCCATTCAGTATGTGG-3′ and reverse 5′-CTTCCATTGCTCCTGCTTTC-3′ for RORγt; and forward, 5′-TGCAGTGGCAAAGTGGAGAT-3′ and reverse 5′-TTTGCCGTGAGTGGAGTCATA-3′ for GAPDH.
Statistical analysis
All experimental data are indicated as the mean ± standard deviation (SD) and statistical significance was analyzed using one-way ANOVA followed by a Student–Newman–Keuls test (P < .05).
Results
C29 suppressed the differentiation of splenic Th cells into Th17 cells and induced the differentiation into Tregs
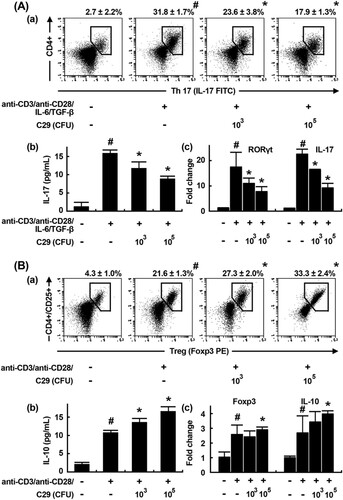
To investigate whether C29 could modulate the T cell differentiation involved in the adaptive immunity, we purified Th cells from mouse splenocytes, stimulated them with Th17-differentiating cytokines (anti-CD3, anti-CD28, rIL-6, and rTGF-β) in the presence or absence of C29, and measured subsets of Th17 cells as well as the expression of the Th17-specific transcription factor RORγt and cytokine IL-17 using a flow cytometer and qPCR (). The cytokine treatment significantly induced the differentiation of Th17 cells and increased the expression of RORγt and IL-17. In contrast, C29 treatment (1 × 105 CFU/mL) significantly inhibited the differentiation of Th cells into Th17 cells and the expression of RORγt and IL-17 by 52.3%, 60.1%, and 62.0%, respectively. However, C29 treatment (1 × 105 CFU/mL) showed a 1.68-fold increase in the differentiation of Th cells into Tregs as well as a 1.21- and a 1.76-fold increase in the expression of Foxp3 and IL-10, respectively.
Figure 1. Effect of C29 on the differentiation of splenic Th cells into Th17 and Tregs. (A) Effect on Th17 cell differentiation. (a) Effect on Th17 differentiation measured by a flow cytometer (fluorescence-activated cell sorting, FACS). (b) Effect on IL-17 expression measured by ELISA. (c) Effect on RORγt and IL-17 mRNA expression using qPCR. (B) Effect on Treg differentiation. (a) Effect on Treg differentiation measured by FACS. (b) Effect on IL-10 expression measured by ELISA. (c) Effect on Foxp3 and IL-10 expression using qPCR. qPCR values (fold changes), which was compared to that of normal control group, were indicated. All data indicate mean ± SD (n = 3); #P < .05 vs. normal control group; *P < .05 vs. group stimulated with anti-CD3/anti-CD28 and IL-6/TGF-β.
C29 alleviated TNBS-induced colitis in mice
C29 potently inhibited the Th17 cell differentiation and induced Treg differentiation in vitro. Therefore, to confirm whether C29 could regulate the T cell differentiation, we orally treated C29 at a dose of 1 × 109 CFU/mouse in mice with TNBS-induced colitis and investigated its anti-inflammatory effects involved in the innate and adaptive immunities. TNBS treatment significantly caused colon shortening and increased MPO activity in the colon. Histological examination revealed the disruption of epithelial cells in the colon by TNBS treament, attributed to inflammation and ulceration (). However, treatment with C29 significantly inhibited TNBS-induced colon shortening and MPO activity as well as the infiltration of neutrophils in the colon. Treatment with C29 or sulfasalazine increased TNBS-suppressed expression of colonic tight junction proteins, such as claudin-1, occludin, and ZO-1. We also addressed the effect of C29 on the expression of inflammatory markers. TNBS treatment induced the activation of NF-κB and increased the expression of iNOS, and COX-2 (). Conversely, treatment with C29 had the opposite effect, leading to the inhibition in expression for TNBS-induced markers such as COX-2 and iNOS and to the activation of NF-κB. Furthermore, C29 treatment suppressed TNBS-induced expression of TNF-α, IL-1β, IL-6, and IL-17 and increased IL-10 expression ().
Figure 2. Effects of C29 and sulfasalazine on body weight (A), macroscopic disease (B), colon length (C), colonic MPO activity (D), histological examination (E), and tight junction proteins (F) in mice with TNBS-induced colitis. All data are mean ± SD (n = 6). #P < .05 vs. normal control group. *P < .05 vs. group treated with TNBS alone.
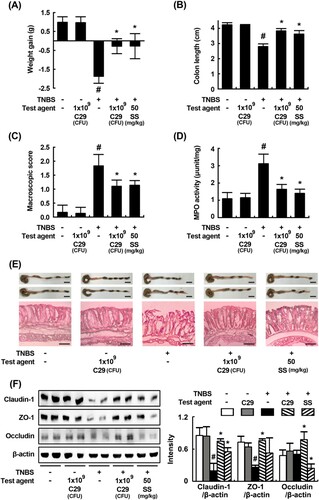
Figure 3. Effects of C29 and sulfasalazine on the expression of iNOS and COX-2 (A), activation of NF-κB (B) in mice with TNBS-induced colitis.
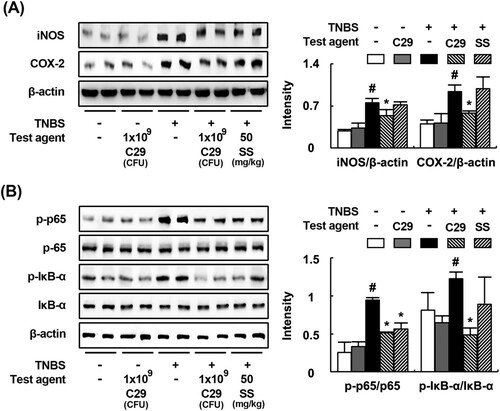
Figure 4. Effects of C29 and sulfasalazine on the expression of TNF-α (A), IL-1β (B), IL-10 (C), IL-6 (D), an IL-17 (E) in mice with TNBS-induced colitis. All values are mean ± SD (n = 6). #P < .05 vs. normal control group. *P < .05 vs. group treated with TNBS alone.
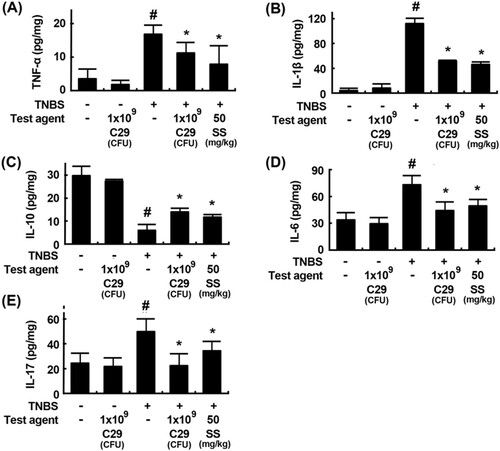
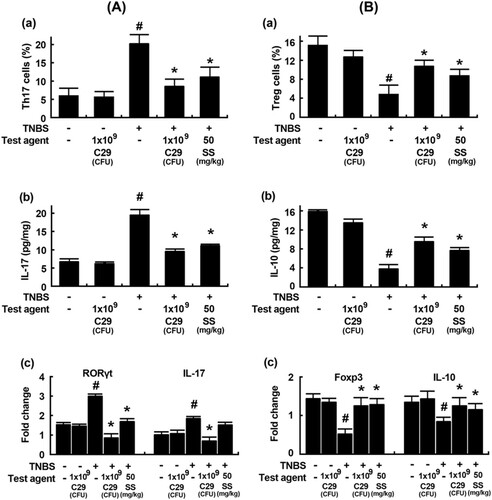
Next, we examined the effect of C29 on the Th17 and Treg cell differentiation in mice with TNBS-induced colitis. Treatment with TNBS increased the differentiation of Th cells into Th17 cells and suppressed their differentiation into Tregs in the colon (). On the contrary, treatment with C29 inhibited TNBS-induced Th17 differentiation and increased TNBS-suppressed Treg differentiation. Furthermore, we confirmed the differentiation of Th17 and Tregs by evaluating the expression levels of Th17 and Treg markers such as IL-10, IL-17, RORγt, and Foxp3 using qPCR. Treatment with C29 significantly inhibited TNBS-induced expression of IL-17 and RORγt in the colon and increased TNBS-suppressed expression of Foxp3 and IL-10. The effect of C29 (1 × 109 CFU/mouse) was similar to that exerted by the treatment with sulfasalazine (50 mg/kg).
Figure 5. Effects of C29 and sulfasalazine on the differentiation of Th17 and Tregs and expression of their transcription factors and cytokines in mice with TNBS-induced colitis. (A) Effects on Th17 differentiation (a) and RORγt (b) and IL-17 expression (c). (B) Effects on Treg differentiation (a) and Foxp3 (b) and IL-10 expression. RORγt, Foxp3, IL-10, and IL-17 were assessed by qPCR. qPCR values (fold changes), which was compared to that of normal control group, were indicated. All values are mean ± SD (n = 6). #P < .05 vs. normal control group. *P < .05 vs. group treated with TNBS alone.
Discussion
The inflammatory cytokines TNF-α, IL-1β, IL-6, IL-10, and IL-23 are secreted by the innate immune system and regulate the proliferation and differentiation of T cells of the adaptive immune system into Th1, Th2, Th17, and Tregs (Geremia, Biancheri, Allan, Corazza, & Di Sabatino, Citation2014; Lord, Citation2015). Furthermore, the overexpression of TNF-α and IL-17 accelerates the occurrence of colitis, while the anti-inflammatory cytokine IL-10 attenuates colitis (Dharmani & Chadee, Citation2008). Therefore, inhibitors of TNF-α and IL-17 (e.g. aminosalicylates and infliximab) and inducers of IL-10 (e.g. methotrexate) are the frequently used therapeutics for colitis (Egan & Sandborn, Citation1998; Lopez & Peyrin-Biroulet, Citation2013; Robinson, Citation1998).
The present study showed that oral administration of C29 improved the symptoms of TNBS-induced colitis in mice, suppressed colon shortening, and decreased MPO activity and iNOS and COX-2 expression. C29 also restored TNBS-suppressed tight junction protein ZO-1, occludin, and claudin-1. Moreover, C29 suppressed TNBS-induced differentiation of Th17 cells and t downregulated the expression of IL-17 and that of RORγt, a Th17-specific transcription factor. However, C29 treatment increased TNBS-suppressed Treg cell differentiation and enhanced the expression of IL-10 and of the Treg transcription factor Foxp3. These results suggest that C29 could modulate the innate immune response, as well as the adaptive immune responses including helper T cell differentiation. It was supported by the present study that C29 increased the differentiation of splenic Th cells into Tregs and enhanced the expression of IL-10 and Foxp3 in vitro, while it inhibited the differentiation of Th cells into Th17 cells and the expression of IL-17, TNF-α, and RORγt and the previous study that C29 inhibited LPS-induced NF-κB activation in macrophage cells.
Lactobacillus rhamnosus RC007 and Lactobacillus plantarum 21 improved TNBS-induced colitis in mice and rats, respectively, through the inhibition of TNF-α expression (Dogi, García, De Moreno de LeBlanc, Greco, & Cavaglieri, Citation2016; Satish Kumar et al., Citation2015). Studies have reported that Lactobacillus brevis K65 and Lactobacillus paracasei ameliorated DSS-induced ulcerative colitis in mice through the suppression of TNF-α expression (Liu et al., Citation2016; Pan et al., Citation2014). Lactobacillus brevis G-101 attenuated colitis by inhibiting TNF-α and IL-1β expression, and by increasing IL-10 expression (Jang et al., Citation2013). These results suggest that these probiotic organisms may mitigate colitis through the inhibition of innate immune responses such as macrophage activation that stimulates TNF-α and inhibits IL-10 expression. However, Lactobacillus plantarum CLP-0611 attenuated colitis in mice through M1 to M2 macrophage polarization induced via an increased IL-10 expression (Jang et al., Citation2014). Miyauchi et al. reported that Bifidobacterium longum alleviates inflammatory diseases by suppressing IL-17 expression (Miyauchi et al., Citation2013). In the previous study, we also reported that C29 inhibited LPS-induced NF-κB activation in macrophages, resulting in the attenuation of colitis in aged mice (Jeong et al., Citation2015). These results suggest that some lactobacilli can regulate the expression of TNF-α, IL-10, and IL-17, as well as the differentiation of T cells, leading to the attenuation of chronic inflammatory diseases such as colitis. Conclusively, C29 may ameliorate colitis by inhibiting NF-κB activation and restoring the imbalanced Th17/Treg cells.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Atreya, I., Atreya, R., & Neurath, M. F. (2008). NF-kappaB in inflammatory bowel disease. Journal of Internal Medicine, 263, 591–596. doi: https://doi.org/10.1111/j.1365-2796.2008.01953.x
- Dharmani, P., & Chadee, K. (2008). Biologic therapies against inflammatory bowel disease: A dysregulated immune system and the cross talk with gastrointestinal mucosa hold the key. Current Molecular Pharmacology, 1, 195–212. doi: https://doi.org/10.2174/1874467210801030195
- Dogi, C., García, G., De Moreno de LeBlanc, A., Greco, C., & Cavaglieri, L. (2016). Lactobacillus rhamnosus RC007 intended for feed additive: Immune-stimulatory properties and ameliorating effects on TNBS-induced colitis. Benefical Microbes, 7, 1–2. doi: https://doi.org/10.3920/BM2016.x001
- Du, Z., Hudcovic, T., Mrazek, J., Kozakova, H., Srutkova, D., Schwarzer, M., … Kverka, M. (2015). Development of gut inflammation in mice colonized with mucosa-associated bacteria from patients with ulcerative colitis. Gut Pathogen, 7, 32. doi: https://doi.org/10.1186/s13099-015-0080-2
- Egan, L. J., & Sandborn, W. J. (1998). Drug therapy of inflammatory bowel disease. Drugs of Today, 34, 431–446. doi: https://doi.org/10.1358/dot.1998.34.5.485242
- Eun, S. H., Lim, S. M., Jang, S. E., Han, M. J., & Kim, D. H. (2016). Lactobacillus sakei K17, an inducer of IL-10 expression in antigen-presenting cells, attenuates TNBS-induced colitis in mice. Immunopharmacology and Immunotoxicology, 38, 447–454. doi: https://doi.org/10.1080/08923973.2016.1233981
- Geremia, A., Biancheri, P., Allan, P., Corazza, G. R., & Di Sabatino, A. (2014). Innate and adaptive immunity in inflammatory bowel disease. Autoimmunity Reviews, 13, 3–10. doi: https://doi.org/10.1016/j.autrev.2013.06.004
- Jang, S. E., Han, M. J., Kim, S. Y., & Kim, D. H. (2014). Lactobacillus plantarum CLP-0611 ameliorates colitis in mice by polarizing M1 to M2-like macrophages. International Immunopharmacology, 21, 186–192. doi: https://doi.org/10.1016/j.intimp.2014.04.021
- Jang, S. E., Hyam, S. R., Han, M. J., Kim, S. Y., Lee, B. G., & Kim, D. H. (2013). Lactobacillus brevis G-101 ameliorates colitis in mice by inhibiting NF-κB, MAPK and AKT pathways and by polarizing M1 macrophages to M2-like macrophages. Journal of Applied Microbiology, 115, 888–896. doi: https://doi.org/10.1111/jam.12273
- Jeong, J. J., Kim, K. A., Jang, S. E., Woo, J. Y., Han, M. J., & Kim, D. H. (2015). Orally administrated Lactobacillus pentosus var. plantarum C29 ameliorates age-dependent colitis by inhibiting the nuclear factor-kappa B signaling pathway via the regulation of lipopolysaccharide production by gut microbiota. PLoS One, 10, e0116533. doi: https://doi.org/10.1371/journal.pone.0116533
- Jung, I. H., Jung, M. A., Kim, E. J., Han, M. J., & Kim, D. H. (2012). Lactobacillus pentosus var. plantarum C29 protects scopolamine-induced memory deficit in mice. Journal of Applied Microbiology, 113, 1498–1506. doi: https://doi.org/10.1111/j.1365-2672.2012.05437.x
- Kaistha, A., & Levine, J. (2014). Inflammatory bowel disease: The classic gastrointestinal autoimmune disease. Current Problems in Pediatric and Adolescent Health Care, 44, 328–334. doi: https://doi.org/10.1016/j.cppeds.2014.10.003
- Kim, K. A., Gu, W., Lee, I. A., Joh, E. H., & Kim, D. H. (2012). High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One, 7, e47713. doi: https://doi.org/10.1371/journal.pone.0047713
- Lee, H. S., Han, S. Y., Bae, E. A., Huh, C. S., Ahn, Y. T., Lee, J. H., & Kim, D. H. (2008). Lactic acid bacteria inhibit proinflammatory cytokine expression and bacterial glycosaminoglycan degradation activity in dextran sulfate sodium-induced colitic mice. International Immunopharmacology, 8, 574–580. doi: https://doi.org/10.1016/j.intimp.2008.01.009
- Leppkes, M., Becker, C., Ivanov, I. I., Hirth, S., Wirtz, S., Neufert, C., … Neurath, M. F. (2009). RORgamma-expressing TH17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology, 136, 257–267. doi: https://doi.org/10.1053/j.gastro.2008.10.018
- Lim, S. M., Choi, H. S., & Kim, D. H. (2017). The mixture of Anemarrhena asphodeloides and Coptis chinensis attenuates high-fat diet-induced colitis in mice. American Journal of Chinese Medicine, 45, 1033–1046. doi: https://doi.org/10.1142/S0192415X17500550
- Lim, S. M., Kang, G. D., Jeong, J. J., Choi, H. S., Kim, D. H., & Lim, S. M. (2016). Neomangiferin modulates the Th17/Treg balance and ameliorates colitis in mice. Phytomedicine, 23, 131–140. doi: https://doi.org/10.1016/j.phymed.2016.01.002
- Liong, M. T. (2008). Safety of probiotics: Translocation and infection. Nutrition Reviews, 66, 192–202. doi: https://doi.org/10.1111/j.1753-4887.2008.00024.x
- Liu, Y. W., Ong, W. R., Su, Y. W., Hsu, C. C., Cheng, T. H., & Tsai, Y. C. (2016). Anti-inflammatory effects of Lactobacillus brevis K65 on RAW 264.7 cells and in mice with dextran sulphate sodium-induced ulcerative colitis. Beneficial Microbes, 7, 387–396. doi: https://doi.org/10.3920/BM2015.0109
- Lopez, A., & Peyrin-Biroulet, L. (2013). 5-Aminosalicylic acid and chemoprevention: Does it work? Digestive Diseases, 31, 248–253. doi: https://doi.org/10.1159/000353806
- Lord, J. D. (2015). Promises and paradoxes of regulatory T cells in inflammatory bowel disease. World Journal of Gastroenterology, 21, 11236–11245. doi: https://doi.org/10.3748/wjg.v21.i40.11236
- Maloy, K. J., & Powrie, F. (2011). Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature, 474, 298–306. doi: https://doi.org/10.1038/nature10208
- Miyauchi, E., Ogita, T., Miyamoto, J., Kawamoto, S., Morita, H., Ohno, H., … Tanabe, S. (2013). Bifidobacterium longum alleviates dextran sulfate sodium-induced colitis by suppressing IL-17A response: Involvement of intestinal epithelial costimulatory molecules. PLoS One, 8, e79735. doi: https://doi.org/10.1371/journal.pone.0079735
- Nagpal, R., Kumar, M., Yadav, A. K., Hemalatha, R., Yadav, H., Marotta, F., & Yamashiro, Y. (2016). Gut microbiota in health and disease: An overview focused on metabolic inflammation. Beneficial Microbes, 7, 181–194. doi: https://doi.org/10.3920/bm2015.0062
- Niess, J. H., Leithäuser, F., Adler, G., & Reimann, J. (2008). Commensal Gut flora drives the expansion of proinflammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. Journal of Immunology, 180, 559–568. doi: https://doi.org/10.4049/jimmunol.180.1.559
- Nourshargh, S., & Alon, R. (2014). Leukocyte migration into inflamed tissues. Immunity, 41, 694–707. doi: https://doi.org/10.1016/j.immuni.2014.10.008
- Owen, J. L., & Mohamadzadeh, M. (2013). Microbial activation of gut dendritic cells and the control of mucosal immunity. Journal of Interferon & Cytokine Research, 33, 619–631. doi: https://doi.org/10.1089/jir.2013.0046
- Pan, T., Guo, H. Y., Zhang, H., Liu, A. P., Wang, X. X., & Ren, F. Z. (2014). Oral administration of Lactobacillus paracasei alleviates clinical symptoms of colitis induced by dextran sulphate sodium salt in BALB/c mice. Beneficial Microbes, 5, 315–322. doi: https://doi.org/10.3920/BM2013.0041
- Petrella, C. (2016). Lactobacillus reuteri treatment and DSS colitis: New insight into the mechanism of protection. Acta Physiologica, 217, 274–275. doi: https://doi.org/10.1111/apha.12719
- Robinson, M. (1998). Medical therapy of inflammatory bowel disease for the 21st century. The European Journal of Surgery, Supplement 582, 90–98.
- Roy, S., & Kumar, T. (2015). Chaudhuriassessment of Th1 and Th2 cytokine modulatory activity of an edible fern, Diplazium esculentum. Food and Agricultural Immunology, 26, 690–702. doi: https://doi.org/10.1080/09540105.2015.1007449
- Rutella, S., & Locatelli, F. (2011). Intestinal dendritic cells in the pathogenesis of inflammatory bowel disease. World Journal of Gastroenterology, 17, 3761–3775. doi: https://doi.org/10.3748/wjg.v17.i33.3761
- Salehipour, Z., Haghmorad, D., Sankian, M., Rastin, M., Nosratabadi, R., Soltan Dallal, M. M., … Mahmoudi, M. (2017). Bifidobacterium animalis in combination with human origin of Lactobacillus plantarum ameliorate neuroinflammation in experimental model of multiple sclerosis by altering CD4+ T cell subset balance. Biomedicine & Pharmacotherapy, 95, 1535–1548. doi: https://doi.org/10.1016/j.biopha.2017.08.117
- Satish Kumar, C. S., Kondal Reddy, K., Reddy, A. G., Vinoth, A., Ch, S. R., Boobalan, G., & Rao, G. S. (2015). Protective effect of Lactobacillus plantarum 21, a probiotic on trinitrobenzenesulfonic acid-induced ulcerative colitis in rats. International Immunopharmacology, 25, 504–510. doi: https://doi.org/10.1016/j.intimp.2015.02.026
- Wang, K., Dong, H., Qi, Y., Pei, Z., Yi, S., Yang, X., … Hu, G. (2017). Lactobacillus casei regulates differentiation of Th17/Treg cells to reduce intestinal inflammation in mice. Canadian Journal of Veterinary Research, 81, 122–128.
- Xu, R., Shen, Q., Wu, R., & Li, P. (2017). Structural analysis and mucosal immune regulation of exopolysaccharide fraction from Bifidobacterium animalis RH. Food and Agricultural Immunology, 28, 1226–1241.
