ABSTRACT
Zebrafish (Danio rerio) emerged as a model of diet-induced obesity because of its genetic homology to humans. Peanut is a rich source of monounsaturated fatty acid and its consumption is associated with decreased inflammatory markers and obesity control. This study evaluated the effects of peanut addition to the cafeteria diet (CAF) by analysis of fatty acids into the head, adiposity and expression of TNF, IL6 and FASN genes using zebrafish as experimental model. The zebrafish were maintained in tanks for 60 days and treated with standard (ST) and CAF diets, respectively. The CAF diet increased the oleic acid content in zebrafish heads, however the body weight, body mass index, adipose tissue and expression of inflammatory and lipid metabolism genes did not differ between the groups. This study suggests that the addition of peanut in the CAF diet can control weight gain, the inflammatory markers and lipid metabolism in zebrafish model.
GRAPHICAL ABSTRACT
1. Introduction
Obesity is a significant human health concern (Liu et al., Citation2017). According to the World Health Organization (WHO, Citation2014), over the past 30 years, more than 700 million people can be characterized as obese. Factors such as a diet rich in carbohydrates and fats, sedentary lifestyle and stress, are associated with obesity-related disorders (Fénero, Flores, & Câmara, Citation2016). High-calorie and high-energy fat diets are known as cafeteria (CAF) diets and are responsible for increasing the mass of adipose tissue and inducing obesity. Additionally, the CAF diet is accompanied by functional and metabolic changes, as well as deposition of body fat and chronic inflammation (Oliveira et al., Citation2015; Zeeni, Dagher-Hamalian, Dimassi, & Faour, Citation2015).
It is well established that obesity is associated with a state of chronic low-grade inflammation, characterized by alterations in circulating immune-modulatory factors and adipose tissue-resident immune cells, which may provide a causal link between increased adiposity (Gil-Cardoso et al., Citation2017). Adipose tissue is an endocrine organ, secreting a variety of pro-inflammatory cytokines, like tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6), play roles in mediating chronic inflammation and are produced predominantly by activated macrophages and CD4+ T cells (Hartati, Widjanarko, Widyaningsih, & Rifa’i, Citation2017) that could comprise up to 40% of the total cells in obese adipose tissue (Daniele et al., Citation2014).
Moreover, increased expression and activity of lipogenic pathways in adipose tissue may contribute to the development of obesity. Fatty acid synthase (FASN) is a key enzyme in lipogenesis and has been identified as a candidate gene for body fat determination in gene expression analyzes (Berndt et al., Citation2007). FASN is highly expressed in lipogenic tissues, such as adipose tissue. There is a low level of FASN expression in normal cells, because these cells obtain the necessary fatty acids from the diet, instead of lipogenesis (Abdel-Magid, Citation2015).
Zebrafish (Danio rerio) is emerging as a good research model of obesity, due to its similar genome and physiology to humans (Leibold & Hammerschmidt, Citation2015). Its applicability in lipid metabolism research as a model for lipid-related diseases, induced by diet and obesity has been demonstrated in some studies (Hasumura et al., Citation2012; Oka et al., Citation2010). Zebrafish have the key organs for energy homeostasis and metabolism in mammals, as well as other associated functions, such as appetite regulation in the brain (Broeder, Kopylova, Kamminga, & Legler, Citation2015), and lipid storage in white adipocytes (Oka et al., Citation2010). All of these characteristics make the zebrafish also an excellent model for the study of inflammatory pathologies, due to resemblance with the human genome (Fénero et al., Citation2016).
The CAF model is a widely used form in mice to evaluate the effect of high-fat concentrations (Zeeni et al., Citation2015). High-fat meals can stimulate innate immune cells and lead to a transient postprandial inflammatory response, altering our immune system and, subsequently, our inflammatory status (Gil-Cardoso et al., Citation2017). Saturated fatty acids (SFAs) are more prone to storage than monounsaturated fatty acids (MUFAs), promoting increased inflammation in adipose tissue. Peanuts are a rich source of MUFAs, and their consumption was linked to improved postprandial profiles of inflammatory markers (Alves et al., Citation2014). Evidence suggests that peanut ingestion may favour body weight control, by reducing food intake and modulating energy metabolism (Alves et al., Citation2014; Ha, Kim, Kim, & Kang, Citation2015).
This study evaluated the effect of 60 days of a peanut addition to the CAF diet by analysis of the composition of fatty acids into the head, adiposity and expression of TNF, IL6 and FASN genes in zebrafish as an experimental model.
2. Materials and methods
2.1. Experimental diets
The granulated diets used are described in . The standard (ST) and cafeteria (CAF) diet were formulated according to Néia et al. (Citation2018). The design of the cafeteria diet was based on studies already established for mice (Akyol, Langley-Evans, & McMullen, Citation2009; Higa, Spinola, Fonseca-Alaniz, & Evangelista, Citation2014) and adapted to the nutritional needs of zebrafish (Siccardi et al., Citation2009). The CAF diet was formulated from the ST diet (37.5%) and added: peanuts (25%), chocolate (25%) and biscuit (12.5%).
Table 1. Feed ingredients of experimental diets.
2.2. Feeding trial and fish sampling
This work was undertaken at the Laboratory PeixeGen of the State University of Maringa (Maringá, Paraná), from August to October 2016. Forty fish were divided equally and randomly, into two groups, according to the CAF and ST diet, respectively, in tanks with a 40 L h−1 water flow capacity, constant oxygenation, and external activated carbon filtration. Before the experiment, the fish received the ST diet for fifteen days, for nutritional adaptation. Next, 10 fish were euthanized, and zero time (0 day) analyzes were performed. Then, the animals were divided in two groups: experimental that received CAF diet and standard that received ST diet. The fish were fed four times daily ad libitum (Fowler et al., Citation2017; Karami, Groman, Wilson, Ismail, & Neela, Citation2017). After 60 days of feeding, the zebrafish were euthanized, weighed and measured. Five whole fish per treatment were used for histological analysis. The livers of five animals per treatment were collected for gene expression analyzes, and the heads of fifteen animals per treatment were harvested for fatty acid composition determination. The heads were stored in polyethylene bags at −18°C, until analysis. At the beginning of each analysis, the samples were equilibrated to room temperature and then homogenized. The collected livers were maintained in RNAlater® (Sigma-Aldrich) solution at 4°C, until RNA isolation. The full bodies of five zebrafish per experimental diet were collected and kept in paraformaldehyde. All animal experiments were performed in strict accordance with the regulations approved by the Ethics Committee on the Use of Animals (CEUA) of the State University of Maringa, with CEUA n° 5133220616 at the meeting of 08/07/2016.
2.3. Fatty acid composition
The fatty acid methyl esters (FAMEs) of the heads were prepared by total lipid methylation, as described by Figueiredo et al. (Citation2016). The FAMEs were separated by gas chromatography (GC) in a Thermo Scientific™ TRACE™ Ultra Gas chromatograph (Thermo Scientific™, USA), fitted with a flame ionization detector (FID) and a fused-silica capillary column (100 m × 0.25 mm i.d., 0.25 μm cyanopropyl CP-7420 select FAME). The ultra-pure gas flows were 1.2 mL min−1 carrier gas (hydrogen), 30 mL min−1 make-up gas (nitrogen), 300 mL min−1 synthetic air, and 35 mL min−1 hydrogen flame gas. The injected sample volume was 1 μL with a split injection ratio of 1/40. FAMEs were identified by comparing their retention times with those of standard methyl esters (Sigma, St. Louis, USA).
Quantification of fatty acids (mg g−1 of sample) was made using 23:0me and theoretical FID correction factor values were used according to Visentainer (Citation2012).
2.4. Measurement of zebrafish body weight, length and body mass index (BMI)
The body weight and length of zebrafish were measured both, at the beginning and end of treatment. The BMI was calculated, by dividing the body weight (g) by the square of the body length (cm2) (Oka et al., Citation2010).
2.5. Morphometric analysis of zebrafish fat tissue and adipocytes
For histological analysis, the fish were individually anesthetized, by immersion in a vessel with ice flakes and water at approximately 4°C. The anesthesia time was individualized and the fish were considered anesthetized after not responding to external stimuli. Five whole animals were used per treatment, fixed in 4% paraformaldehyde, decalcified in 20% sodium citrate and 50% formic acid, and cut transversely at the height of the dorsal fin. The samples were dehydrated in a series of alcohols of increasing concentrations, diaphanized in xylol and embedded in paraffin. The samples were positioned on the microtome and cut until reaching the liver region of each fish. From the liver region, serial cross sections (5 μm thick) were obtained and stained with hematoxylin and eosin (HE). The histological slides were analyzed under a Nikon® optical microscope (Eclipse 80i, Shinjuku, Japan), equipped with a high-resolution Nikon® camera (DS-Fi1c, Shinjuku, Japan), using a 20X objective. Two histological sections were analyzed per animal, capturing 20 fields per slide, totalling 100 images per treatment. Morphometric analysis was performed, using Image-Pro® Plus software version 4.5. In each histological field, morphometric analysis was performed on all subcutaneous adipose tissue. The number and the mean area (μm2) of all the adipocytes present in each field were analyzed. The different mean areas of the adipocytes found were grouped into classes of measurements and plotted in a histogram for each treatment, according to the various sizes of fish adipocytes.
2.6. RNA extraction and quantitative real-time PCR
Total RNA of the zebrafish liver was extracted from a random sample of five males per treatment diet, using QIAampH DNA blood mini kit Qiagen (Hilden, Germany), according to the manufacturer’s specifications. The liver tissue was maintained in RNA at 4°C, until the extraction. Subsequently, the samples were pretreated with RQ1 RNase-free DNase (Promega, USA) and reverse transcribed into first-strand cDNA using a Nanogen kit, according to the manufacturer’s instructions. The sequences of the PCR primers used have been described by Yang et al. (Citation2014) and Hasumura et al. (Citation2012): β-actin (endogenous control), ATGGATGAGGAAATCGCTG, ATGCCAACCATCACTCCCTG; TNF, GCTGGATCTTCAAAGTCGGGTGTA, TGTGAGTCTCAGCACACTTCCATC; IL6, AGACCGCTGCCTGTCTAAAA, TTTGATGTCGTTCACCAGGA and FASN, ATCTGTTCCTGTTCGATGGC, AGCATATCTCGGCTGACGTT. For real-time PCR, 3 µL of first-strand cDNA was reacted with a 25-µL mixture, consisting of 12.5 µL SYBR® green RT–PCR reaction mix, 0.5 µL of each primer (100 mM) and 6.5 µL of free-RNA water. The PCR programme involved an initial denaturation at 95°C for 5 min; 40 amplification cycles of 95°C for 30 s, 95°C for 45 s, and 60°C for 30 s. All reactions were done in duplicate, on a StepOne Plus real-time PCR system. Amplification plots indicating fluorescence intensity at each cycle were obtained, from which Ct values were measured for each sample. PCRs were run in duplicate for each sample, and Ct averages were acquired, followed by normalization to the average of the β-actin (housekeeping) gene, following the 2−ΔΔCt method (Livak & Schmittgen, Citation2001).
2.7. Statistical analysis
The results (mean ± standard deviation, SD) were submitted to analysis of variance (ANOVA) at 5% significance level, using GraphPad Prism 5 software (San Diego, CA, USA). The mean values of the zebrafish body weight, length, BMI, fat tissue, adipocytes and quantitative real-time PCR data were compared by Student’s t-test. The fatty acid compositions of the zebrafish heads were compared by Tukey’s test.
3. Results
3.1. Fatty acid composition of the diets
The CAF and ST diets were well accepted by the fish, and there was no death due to dietary intake of the treatments. The composition of fatty acids in the ST and CAF diets was described in Néia et al. (Citation2018). A total of 12 fatty acids were found in the diets. The major SFA, MUFA, and polyunsaturated fatty acid (PUFA) in both diets were palmitic (16:0), oleic (18:1n-9), and linoleic (18:2n-6) acids, respectively. The CAF diet presented high concentrations of oleic (102.58 ± 2.85 mg g−1) and linoleic (44.47 ± 1.32 mg g−1) acids.
3.2. Fatty acid composition in zebrafish’s head
After the supplemented diet administration, 20 fatty acids were identified and quantified in zebrafish heads. The profile of supplemented fatty acids in zebrafish heads at the end of supplementation (60 days) is shown in . The same classes of the fatty acids of SFAs, MUFAs, and PUFAs were found in heads from fish submitted to both diets, ST and CAF, however, in different concentrations. Palmitic acid (16:0) was the SFA found in the highest concentration. For MUFAs and PUFAs, oleic acid (18:1n-9) and linoleic acid (18:2n-6) predominated, respectively. The CAF diet increased the oleic acid content in zebrafish heads by 1.4-fold, from 28.17 mg g−1 (0-day of supplementation) to 40.21 mg g−1 (60-day of supplementation), such behaviour was not observed in fish fed the ST diet, in which there was a 1.3-fold decrease in oleic acid, from 28.17 mg g−1 (0-day of supplementation) to 21.58 mg g−1 (60-day of supplementation).
Table 2. Fatty acid composition (mg g−1 of sample) of zebrafish head submitted to different treatments.
3.3. Body fat distribution in zebrafish
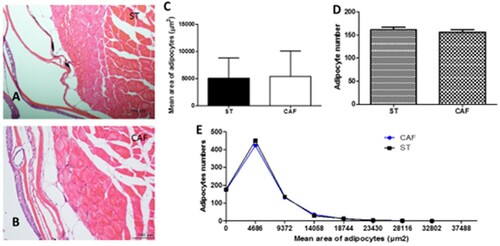
The histological and morphometric data demonstrated that there was no difference in the analysis of adipocyte sizes between the two experimental groups at the end of the 60 days, as illustrated in (a) and (b). The subcutaneous adipocyte average area ((c)) was 5103.3074 ± 3741.3847 µm2 for the ST diet and 5426.637 ± 4614.798 µm2 for the CAF diet. The number of adipocytes between the two experimental groups at the end of the 60 days ((d)) was 161.4 ± 5.55 and 156.6 ± 5.55 for the ST and CAF diet, respectively. The adipocyte area distribution histogram showed the adipocyte areas between the treatments were not significantly different ((e)).
Figure 1. Subcutaneous adipocytes. Transversal section of subcutaneous zebrafish adipocytes of ST (A) and CAF (B) diets. HE; bars = 100 μm. Comparison of the mean area of adipocytes (C), adipocyte numbers (D), and adipocyte mean area distribution histogram (E) between ST and CAF diets. The results of quantitative studies are expressed as mean ± standard error of the mean, according to the Student's t-test.
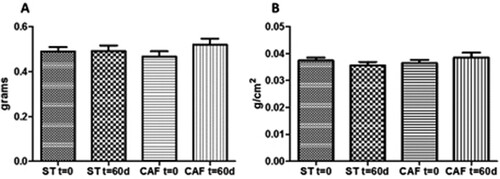
3.4. Body weight in zebrafish
The BMI is a useful measure of obesity in fish. In our study, BMI was evaluated by dividing body weight (g) by the square of body length (cm2), from the tip of the mouth to the end of the body (Oka et al., Citation2010). There was no significant difference between treatments in body weight (ST: 0.4915 ± 0.1081 g; CAF: 0.52 ± 0.1196 g), at the end of the 60 days ((a)). Also, the BMIs of the animals fed ST (0.0355 ± 0.00056 g/cm2) and CAF (0.0385 ± 0.00083 g/cm2) diets were not statistically different following 60 days of treatment ((b)).
3.5. Liver mRNA expression in zebrafish
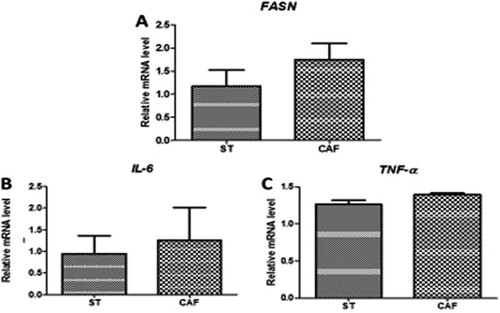
Analysis of the mRNA expression levels of the lipid metabolism gene (FASN) in the liver of DIO male zebrafish, revealed no significant differences of ((a)). Likewise, there were no significant differences in the mRNA expression levels of IL-6 ((b)) and TNF-α ((c)), in the liver of DIO male zebrafish. FASN plays a key role in lipogenesis, while both IL-6 and TNF-α play major role in the inflammatory process of obesity.
Figure 3. Effects of the CAF diet on the mRNA (A) expression of the fatty acid synthase (FASN) lipid metabolism gene in the liver, and the gene expression of the inflammatory cytokines interleukin-6 (IL-6) (B) and tumor necrosis factor-α(TNF-α) (C) in the liver of male zebrafish on day 60 of feeding. The expression of each mRNA was normalized against peptidylprolyl isomerase A (PPIA) mRNA expression and the corresponding expression in the diet-induced obese (DIO) group. Values are means ± standard error. Statistical analyses were performed using the Student's t test.
4. Discussion
4.1. Zebrafish as a useful animal model of diet-induced obesity (DIO)
Innumerable animal models have been used to study the etiology of obesity in order to gain a better understanding of molecular mechanisms and possible treatments (Broeder et al., Citation2015). In recent years, Zebrafish has emerged as an alternative vertebrate animal model for human physiology, including studies of energetic homeostasis and metabolic diseases (Leibold & Hammerschmidt, Citation2015) because of their similarity to the lipid metabolism of humans. Therefore Zebrafish is increasingly being used as a model for human diseases because of its ease of genetic manipulation and its low cost of reproduction (Fénero et al., Citation2016).
Some studies have used Artemia or Artemia cysts as a food source for DIO in zebrafish (Hasumura et al., Citation2012; Oka et al., Citation2010). However, we believe that our group is the first to report a CAF diet to induce obesity in zebrafish, similar to human diets (Néia et al., Citation2018) and evaluate the fatty acid intake in zebrafish fillets fed a CAF diet.
In humans, increasing availability of high-calorie, high-fat diets is considered a major contributor to obesity (Leibold & Hammerschmidt, Citation2015). In general, diets containing more than 30% of total energy from fat can lead to the development of obesity (Hariri & Thibault, Citation2010). In experimental rodents, a positive relationship was found between dietary fat content and body fat gain (Meguro, Hasumura, & Hase, Citation2015). The cafeteria diet (CAF) is widely used in animal models to induce obesity because of its high caloric and lipid density (Leibold & Hammerschmidt, Citation2015).
4.2. Effect of peanut addition on fatty acid composition of CAF diet
Peanuts were chosen because its consumption in the diet leads to an increase in the intake of MUFAs and PUFAs, in the presence of the reduction of SFAs, which presumably favours the lipid and glucose metabolism, reducing the risk of chronic non communicable diseases, such as obesity (Bes-Rastrollo et al., Citation2007). In this study, the addition of 25% peanut in the CAF diet, led to increased 4.5 times the MUFA content in CAF diet (Néia et al., Citation2018), from 27.47 mg g−1 (0-day of supplementation) to 106.12 mg g−1 (60-day of supplementation), as this oleaginous fraction is rich in oleic acid (18:1n-9), as described in the work of Ha et al. (Citation2015).
4.3. Effect of peanut addition on CAF diet on fatty acid composition into zebrafish’s head
The CAF diet used in this study presented higher concentrations of oleic acid (102.58 ± 2.85 mg g−1) than ST diet, due to the use of peanut in its preparation (Néia et al., Citation2018), which in our study increased the oleic acid (18: 1n-9) content in the zebrafish head from 28.17 mg g−1 (0 days of supplementation) to 40.21 mg g−1 (60 days of supplementation) in fish fed CAF diet. This increase may be related to increased satiety, generated by oleic acid in the head of the zebrafish once the central nervous system, primarily, the hypothalamus, is responsible for regulating energy balance and control of food consumption in vertebrates, from humans to zebrafish (Montalbano et al., Citation2016). Acoording with this fact, Cintra et al. (Citation2012) using fatty acid component of flax seed olive oil (rich in 18:1n-9) observed that unsaturated fatty acids could act either as nutrients or directly in the hypothalamus, reverting diet-induced inflammation, increase satiety and reducing body adiposity in a mouse DIO model.
Additionally, it has been described that ingestion of oilseeds at least twice a week may contribute to decreasing the risk of weight gain, even if there is an increase in caloric intake (Bes-Rastrollo et al., Citation2007). It is probable that body weight control is related to a reduction in the bioaccessibility of oil lipids given that part of these lipids is excreted in the feces and, therefore, not used as an energy source by the body (Hollis & Mattes, Citation2007). Furthermore, it is suggested that the satietogenic effect of these foods may contribute to reduced food intake (Alves et al., Citation2014).
4.4. Effect of peanut addition on CAF diet on zebrafish body composition
In our study, there was no significant difference in the number and area of subcutaneous adipocytes. These results were similar to those documented by Oliveira et al. (Citation2015), who evaluated the anti-inflammatory capacity of omega-3 and -9 fatty acids and adipose tissues in obese mice.
We observed that the increase in oleic acid, generated by the addition of 25% peanut in the CAF ration, may be related to the regulation of the increased number and area of the zebrafish subcutaneous adipocytes. Likewise, Alves et al. (Citation2014) observed that the regular consumption of peanuts, particularly the high oleic content type, in a hypocaloric diet, increased fat oxidation and decreased body fat in overweight and obese men. Barbour, Howe, Buckley, Bryan, and Coates (Citation2015), observed an inverse association between oleaginous food intake and obesity, inflammation, hyperlipidemia, and glucose intolerance. They investigated the effects of high oleic acid peanut intake versus a non-oleaginous diet on adiposity and cardiovascular risk markers of healthy subjects for 12 weeks.
We observed that the addition of peanut to the CAF ration and the increase in oleic acid might be related to the regulation of weight gain and the BMI of zebrafish. The study by Nouran, Kimiagar, Abadi, Mirzazadeh, and Harrison (Citation2010) supported the current findings. These authors added peanut oil to the diet of adult subjects for 8 weeks and observed a 43% lower than expected weight gain among overweight individuals, despite the distinct difference in the amount of energy consumed by the individuals.
In our study, 134.83 kcal of peanuts were added to the CAF diet, suggesting that it was sufficient to control weight gain and increase the BMI in fish. These results were comparable to those of Alper and Mattes (Citation2002), who added 505 kcal of peanuts to the diet of 15 eutrophic individuals, which led to a lower than expected weight gain. It was expected that one animal would gain 3.6 kg, yet, the weight gain was only 1 kg. At another time, individuals replaced food calories with peanuts, and there was no increase in body weight.
We also observed that the variables weight, BMI, and subcutaneous adipocytes did not present significant differences between the groups studied. These results correlated with Ha et al. (Citation2015), who investigated the effect of peanut bud extracts and variables, such as abdominal circumference and body fat in overweight and obese women. They noted that supplementation of peanut bud extract improved abdominal obesity, suggesting that an adequate amount of peanut sprouts may be a plausible effective agent for obesity and obesity-related health problems in obese women.
4.5. Effect of peanut addition on CAF diet on liver mRNA expression in zebrafish
Clinical and epidemiological evidence also indicates that peanut consumption can improve inflammatory markers with daily doses of 30 g of peanut (Barbour et al., Citation2015). In our study, we used 25% of peanut in the CAF diet, and it was not observed a significant difference in the FASN expressions between the experimental and standard groups, even though this gene was expressed more in the CAF than ST diet. There are conflicting literature results, regarding the expression of the FASN gene in adipose tissue and visceral tissue. Park et al. (Citation2011) observed downregulation of the FASN gene in the visceral adipose tissue of obese mice. In contrast, Letexier, Pinteur, Large, Fréring, and Beylot (Citation2003) reported that the FASN expression in adipose tissue was not affected by a high-fat diet in a wild type rodents’ model and is reduced in obese humans.
Crew, Waddell, and Mark (Citation2016) evaluated the expression of TNF-α, IL-6, IL-1β, toll-like receptor-2 (TLR2), TLR4, IL-1β, IL-1β, IL-1β, cyclooxygenase-2 and macrophage-surface marker (Emr1) in the fetal liver from two mice groups feed a CAF and normal diet, respectively. In support of the our findings, the authors did not observe a variation in the expression of these genes, concluding that maternal obesity induced by a CAF diet before and during pregnancy, does not increase the inflammatory state of the mother, placenta or fetus in late gestation.
Contrastingly, Gil-Cardoso et al. (Citation2017) illustrated that the CAF diet increased the expression of inflammatory genes, such as TNF. These authors examined the impact of an obesogenic diet on the intestinal health status of DIO-fed rats compared to the Zucker rat (fa/fa) female leptin receptor obesity model and evaluated gene expression of TNF-α and inducible nitric oxide synthase (iNOS). The authors stated that TNF-α was overexpressed in the CAF diet ileal and fa/fa groups, and ileal inflammation was associated with the degree of obesity and metabolic changes. However, many pathways could influence the TNF expression, due to the wide interactions and functions of this gene.
5. Conclusions
If it is important to understand the capability and limitation of mammalian models, the need is even greater for zebrafish, which are phylogenetically further removed from humans. However, among model systems that are amenable to screening, zebrafish stand out for their highly conserved integrative physiology, MacRae and Peterson (Citation2015) selected 65 examples of published chemical screens in zebrafish of many metabolic systems, including dietary lipid absorption. To the best of our knowledge, this is the first publication that uses CAF diet in a zebrafish model DIO.
In our experimental diet, the addition of peanut to the CAF diet led to an increased oleic acid in the CAF ration, which increased the incorporation of oleic acid in the head of the zebrafish. This finding may be related to the increased satiety of the fish and, consequently, to the control of weight gain and expression of inflammatory genes and lipid metabolism in fish fed a CAF diet.
Acknowledgments
The authors would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação Araucária de Apoio ao Desenvolvimento Científico e Tecnológico do Estado do Paraná, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and to Laboratory of Immunogenetics (Proc. n° 00639/99-DEG-UEM) for their financial support and fellowships.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Vanessa Bueno Moreira Javera Castanheira Néia http://orcid.org/0000-0003-2573-3457
Additional information
Funding
References
- Abdel-Magid, A. F. (2015). Fatty acid synthase (FASN) inhibitors as potential treatment for cancer, obesity, and liver related disorders. ACS Medicinal Chemistry Letters, 6, 838–839. doi: https://doi.org/10.1021/acsmedchemlett.5b00275
- Akyol, A., Langley-Evans, S. C., & McMullen, S. (2009). Obesity induced by cafeteria feeding and pregnancy outcome in the rat. British Journal of Nutrition, 102, 1601–1610. doi: https://doi.org/10.1017/S0007114509990961
- Alper, C. M., & Mattes, R. D. (2002). Effects of chronic peanut consumption on energy balance and hedonics. International Journal of Obesity, 26, 1129–1137. doi: https://doi.org/10.1038/sj.ijo.0802050
- Alves, R. D. M., Moreira, A. P. B., Macedo, V. S., Costa, N. M. B., Alfenas, R. C. G., & Bressan, J. (2014). High-oleic peanuts increase diet-induced thermogenesis in overweight and obese men. Nutrición Hospitalaria, 29, 1024–1032.
- Barbour, J. A., Howe, P. R. C., Buckley, J. D., Bryan, J., & Coates, A. M. (2015). Effect of 12 weeks high oleic peanut consumption on cardio-metabolic risk factors and body composition. Nutrients, 7, 7381–7398. doi: https://doi.org/10.3390/nu7095343
- Berndt, J., Kovacs, P., Ruschke, K., Klöting, N., Fasshauer, M., Schön, M. R., … Blüher, M. (2007). Fatty acid synthase gene expression in human adipose tissue: Association with obesity and type 2 diabetes. Diabetologia, 50, 1472–1480. doi: https://doi.org/10.1007/s00125-007-0689-x
- Bes-Rastrollo, M., Sabaté, J., Gómez-Gracia, E., Alonso, A., Martínez, J. A., & Martínez-González, M. A. (2007). Nut consumption and weight gain in a Mediterranean cohort: The SUN study. Obesity, 15, 107–116. doi: https://doi.org/10.1038/oby.2007.507
- Broeder, M. J. D., Kopylova, V. A., Kamminga, L. M., & Legler, J. (2015). Zebrafish as a model to study the role of peroxisome proliferating-activated receptors in adipogenesis and obesity. PPAR Research, 2015, 358029.
- Cintra, D. E., Ropelle, E. R., Moraes, J. C., Pauli, J. R., Morari, J., Souza, C. T., … Velloso, L. A. (2012). Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PLoS One, 7, e30571. doi: https://doi.org/10.1371/journal.pone.0030571
- Crew, R. C., Waddell, B. J., & Mark, P. J. (2016). Maternal obesity induced by a “cafeteria” diet in the rat does not increase inflammation in maternal, placental or fetal tissues in late gestation. Placenta, 39, 33–40. doi: https://doi.org/10.1016/j.placenta.2016.01.002
- Daniele, G., Guardado, M. R., Winnier, D., Fiorentino, T. V., Pengou, Z., Cornell, J., … Folli, F. (2014). The inflammatory status score including IL-6, TNF-α, osteopontin, fractalkine, MCP-1 and adiponectin underlies whole-body insulin resistance and hyperglycemia in type 2 diabetes mellitus. Acta Diabetologica, 51, 123–131. doi: https://doi.org/10.1007/s00592-013-0543-1
- Fénero, C. I. M., Flores, A. A. C., & Câmara, N. O. S. (2016). Inflammatory diseases modelling in zebrafish. World Journal of Experimental Medicine, 6, 9–20. doi: https://doi.org/10.5493/wjem.v6.i1.9
- Figueiredo, I. L., Claus, T., Júnior, O. O. S., Almeida, V. C., Magon, T., & Visentainer, J. V. (2016). Fast derivatization of fatty acids in different meat samples for gas chromatography analysis. Journal of Chromatography A, 1456, 235–241. doi: https://doi.org/10.1016/j.chroma.2016.06.012
- Fowler, L. A., Lacey, D., Dawson, J. A., Barry, R. J., Davis, J. L., Powell, M., … Watts, S. (2017). Sex-specific differences in the development of diet-induced obesity in a zebrafish model. The FASEB Journal, 31, 19. Retrieved from http://www.fasebj.org/doi/abs/10.1096/fasebj.31.1_supplement.792.19
- Gil-Cardoso, K., Ginés, I., Pinent, M., Ardévol, A., Terra, X., & Blay, M. (2017). A cafeteria diet triggers intestinal inflammation and oxidative stress in obese rats. British Journal of Nutrition, 117, 218–229. doi: https://doi.org/10.1017/S0007114516004608
- Ha, A. W., Kim, W. K., Kim, J. H., & Kang, N. E. (2015). The supplementation effects of peanut sprout on reduction of abdominal fat and health indices in overweight and obese women. Nutrition Research and Practice, 9, 249–255. doi: https://doi.org/10.4162/nrp.2015.9.3.249
- Hariri, N., & Thibault, L. (2010). High-fat diet-induced obesity in animal models. Nutrition Research Reviews, 23, 270–299. doi: https://doi.org/10.1017/S0954422410000168
- Hartati, F. K., Widjanarko, S. B., Widyaningsih, T. D., & Rifa’i, M. (2017). Anti-Inflammatory evaluation of black rice extract inhibits TNF-α, IFN-γ and IL-6 cytokines produced by immunocompetent cells. Food and Agricultural Immunology, 28(6), 1116–1125.
- Hasumura, T., Shimada, Y., Kuroyanagi, J., Nishimura, Y., Meguro, S., Takema, Y., & Tanaka, T. (2012). Green tea extract suppresses adiposity and affects the expression of lipid metabolism genes in diet-induced obese zebrafish. Nutrition & Metabolism, 9, 73. doi: https://doi.org/10.1186/1743-7075-9-73
- Higa, T. S., Spinola, A. V., Fonseca-Alaniz, M. H., & Evangelista, F. S. (2014). Comparison between cafeteria and high-fat diets in the induction of metabolic dysfunction in mice. International Journal of Physiology, Pathophysiology and Pharmacology, 6, 47–54.
- Hollis, J., & Mattes, R. (2007). Effect of chronic consumption of almonds on body weight in healthy humans. British Journal of Nutrition, 98, 651–656. doi: https://doi.org/10.1017/S0007114507734608
- Karami, A., Groman, D. B., Wilson, S. P., Ismail, P., & Neela, V. K. (2017). Biomarker responses in zebrafish (Danio rerio) larvae exposed to pristine low-density polyethylene fragments. Environmental Pollution, 223, 466–475. doi: https://doi.org/10.1016/j.envpol.2017.01.047
- Leibold, S., & Hammerschmidt, M. (2015). Long-term hyperphagia and caloric restriction caused by low- or high-density husbandry have differential effects on zebrafish postembryonic development, somatic growth, fat accumulation and reproduction. PLoS One, 10, e0120776. doi: https://doi.org/10.1371/journal.pone.0120776
- Letexier, D., Pinteur, C., Large, V., Fréring, V., & Beylot, M. (2003). Comparison of the expression and activity of the lipogenic pathway in human and rat adipose tissue. Journal of Lipid Research, 44, 2127–2134. doi: https://doi.org/10.1194/jlr.M300235-JLR200
- Liu, H., Pei, X., Shi, K., Wang, J., Han, F., & Li, A. (2017). Effects of replacing wheat flour with detoxified ginkgo nut powder on lipid metabolism of obese C57BL/6J male mice. Food and Agricultural Immunology, 1–17. doi:https://doi.org/10.1080/09540105.2017.1358255
- Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods, 25, 402–408. doi: https://doi.org/10.1006/meth.2001.1262
- MacRae, C. A., & Peterson, R. T. (2015). Zebrafish as tools for drug discovery. Nature Reviews Drug Discovery, 14, 721–731. doi: https://doi.org/10.1038/nrd4627
- Meguro, S., Hasumura, T., & Hase, T. (2015). Body fat accumulation in zebrafish is induced by a diet rich in fat and reduced by supplementation with green tea extract. Plos One, 10, e0120142. doi: https://doi.org/10.1371/journal.pone.0120142
- Montalbano, G., Mania, M., Guerrera, M. C., Abbate, F., Laurà, R., Navarra, M., … Germanà, A. (2016). Morphological differences in adipose tissue and changes in BDNF/trkb expression in brain and gut of a diet induced obese zebrafish model. Annals of Anatomy - Anatomischer Anzeiger, 204, 36–44. doi: https://doi.org/10.1016/j.aanat.2015.11.003
- Néia, V. B. M. J. C., Ambrosio-Albuquerque, E. P., Figueiredo, I. L., Boeing, J. S., Silva, T. C., Lewandowski, V., … Visentainer, J. V. (2018). Impact of cafeteria diet on the composition of fatty acids in zebrafish (Danio rerio) fillets. Journal of the Brazilian Chemical Society. doi: https://doi.org/10.21577/0103-5053.20170213
- Nouran, M. G., Kimiagar, M., Abadi, A., Mirzazadeh, M., & Harrison, G. (2010). Peanut consumption and cardiovascular risk. Public Health Nutrition, 13, 1581–1586. doi: https://doi.org/10.1017/S1368980009992837
- Oka, T., Nishimura, Y., Zang, L., Hirano, M., Shimada, Y., Wang, Z., … Tanaka, T. (2010). Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiology, 10, 21. doi: https://doi.org/10.1186/1472-6793-10-21
- Oliveira, V., Marinho, R., Vitorino, D., Santos, G. A., Moraes, J. C., Dragano, N., … Cintra, D. E. (2015). Diets containing α-linolenic (ω3) or oleic (ω9) fatty acids rescues obese mice from insulin resistance. Endocrinology, 156, 4033–4046. doi: https://doi.org/10.1210/en.2014-1880
- Park, H. J., DiNatale, D. A., Chung, M. Y., Park, Y. K., Lee, J. Y., Koo, S. I., … Bruno, R. S. (2011). Green tea extract attenuates hepatic steatosis by decreasing adipose lipogenesis and enhancing hepatic antioxidant defenses in ob/ob mice. The Journal of Nutritional Biochemistry, 22, 393–400. doi: https://doi.org/10.1016/j.jnutbio.2010.03.009
- Siccardi, A. J. I. I. I., Garris, H. W., Jones, W. T., Moseley, D. B., D’Abramo, L. R., & Watts, S. A. (2009). Growth and survival of zebrafish (Danio rerio) fed different commercial and laboratory diets. Zebrafish, 6, 275–280. doi: https://doi.org/10.1089/zeb.2008.0553
- Visentainer, J. V. (2012). Aspectos analíticos da resposta do detector de ionização em chama para ésteres de ácidos graxos em biodiesel e alimentos. Química Nova, 35, 274–279. doi: https://doi.org/10.1590/S0100-40422012000200008
- World Health Organization. (2014). Obesity and overweight. Retrieved from http://www.who.int/mediacentre/factsheets/fs311/en/
- Yang, L. L., Wang, G. Q., Yang, L. M., Huang, Z. B., Zhang, W. Q., & Yu, L. Z. (2014). Endotoxin molecule lipopolysaccharide-induced zebrafish inflammation model: A novel screening method for anti-inflammatory drugs. Molecules, 19, 2390–2409. doi: https://doi.org/10.3390/molecules19022390
- Zeeni, N., Dagher-Hamalian, C., Dimassi, H., & Faour, W. H. (2015). Cafeteria diet-fed mice is a pertinent model of obesity-induced organ damage: A potential role of inflammation. Inflammation Research, 64, 501–512. doi: https://doi.org/10.1007/s00011-015-0831-z