ABSTRACT
Pilose antler (PA) is used to treat many diseases, but its effect on breast cancer is still unclear. Here, we report the effects of PA on the growth of the mouse mammary tumour in vitro and in vivo, and its effects on immune system of the tumor-bearing mice. The 4T1 mouse mammary tumour cells were cultured with media supplemented with water extract of PA (WEPA), and the female BALB/c mice transplanted with 4T1 cells were treated by gavage with WEPA. The results showed that WEPA promoted the proliferation of 4T1 cells in vitro, while inhibited the growth of 4T1 tumour in vivo. In addition, WEPA increased the proportion of CD4+ T cells, CD8+ T cells and NK cells and reduced myeloid-derived suppressive cells in mouse peripheral blood. These results suggest that WEPA can inhibit the growth of mouse 4T1 tumours through modulating immune system of the mouse.
1. Introduction
Natural products from organisms are not only an important part of traditional Chinese medicine, but also an important source for modern medicine (Cragg & Newman, Citation2013). Therefore, it is necessary to identify the function and the bioactive components in these natural products (Hartati, Widjanarko, Widyaningsih, & Rifa’i, Citation2017; Imran et al., Citation2017). Pilose antler (PA), the not yet ossified young horn from the sika deer or red deer, is a traditional Chinese medicine containing hormones (Quan, Zhang, & Chang, Citation2014), polysaccharides, peptides (Zhang et al., Citation2012), cytokines (Lin, Deng, Wu, Chen, & Li, Citation2011), vitamins (Tseng et al., Citation2014) and other bioactive substances. PA has been widely used to treat kidney weakness, mental haziness, blood deficiency, body fatigue and child developmental delay (Sui, Zhang, Huo, & Zhang, Citation2014; Wu et al., Citation2013). In recent years PA has been used as a nutritional supplement to aid the recovery of cancer patients after surgery or chemotherapy in China. Some reports have shown that PA can stimulate some types of cells to proliferate (Wang, Luo, Zhang, Qu, & Tan, Citation2017; Yang et al., Citation2017; Zhang, Li, Qu, Luo, & Drummen, Citation2016). However, recent studies have shown that some peptides contained in PA have an inhibitory effect on tumour growth both in vitro and in vivo (Tang et al., Citation2015). This inhibitory effect in vivo may be associated with a regulatory effect of PA on the immune system (Zha et al., Citation2013; Zheng, Fan, Ji, & Ma, Citation2017). In practice, the total components of PA are used as medicine. Therefore, more detailed studies about the effects of the total components of PA on tumour growth and immune system of the tumor-bearing bodies is very necessary.
Breast cancer is a malignant tumour originating from mammary epithelial tissue, which can seriously endanger the patients’ health and even their lives. There is increasing evidence that the incidence of breast cancer is affected by the body’s immune state. Individuals in an immunocompromised state are more prone to developing breast cancer (Wang et al., Citation2015). In this study, the 4T1 mouse mammary tumour cells were cultured with media supplemented with water extract of PA (WEPA), and the female BALB/c mice transplanted with 4T1 cells were treated by gavage with WEPA to show the dynamic variation of immune cells. This study could reveal the effect of WEPA on growth of the mouse mammary tumour, and help the understanding of the relationship between tumour growth and immune changes caused by WEPA treatment.
2. Materials and methods
2.1. Materials
2.1.1. WEPA
Totally 0.4 g of the freeze-dried powder of PA (SiPing Limited Company of Deer Breeding Stock Ranch, China) was dissolved in 100 ml of distilled water, and shaked thoroughly overnight in a water bath at 60°C. Then the mixture was centrifuged at 4000 × g for 5 min and the supernatant of WEPA was collected.
2.1.2. Mice
Female BALB/c mice, 4–6 weeks of age, were purchased from the Liaoning Changsheng Biological Technology Company in China. Mice were kept under the SPF (Specific Pathogen Free) conditions and acclimatized for one week at room temperature with a 12-h light/dark cycle before treatment. The mice were fed with normal rodent chow and allowed free access to clean drinking water.
2.1.3. Cell line
The 4T1 mouse mammary tumour cells (Shanghai Institutes for Biological Sciences, CAS) was cultured in RPMI 1640 media supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (100 U/ml–0.1 mg/ml) at 37°C in a tissue culture incubator filled with 5% CO2.
2.1.4. Antibodies
CD4+ T cells were labelled with anti-CD4-FITC (Biolegend #100405), CD8+ T cells with anti-CD8a-PE (Biolegend #100707), NK cells with anti-CD49b-PE (Miltenyi #130-108-174), MDSCs with anti-CD11b-PE (Biolegend #101207), anti-Ly-6G/Ly-6C (Gr-1)-FITC (Biolegend #108405).
2.2. Methods
2.2.1. Effect of WEPA on cancer progression of mice transplanted with 4T1 cells
Forty mice were randomly and evenly divided into four groups: gavage with water (WC), gavage with water before and after 4T1 cell transplantation (WTC), gavage with WEPA before and after 4T1 cell transplantation (PTP), and gavage with water and WEPA before and after 4T1 cell transplantation, respectively (WTP). Up to 2 × 105 4T1 cells in the logarithmic phase of growth were transplanted into each mouse from groups of WTC, PTP and WTP after the one week of gavage. One week before 4T1 cell transplantation, each mouse from PTP group was gavaged with 200 µl of WEPA per day and each mouse from WC, WTC and WTP groups was gavaged with 200 µl of distilled water (DW) per day. For 4 weeks after 4T1 cell transplantation, every 3 days each mouse from PTP and WTP groups was gavaged with 200 µl of WEPA and each mouse from WC and WTC groups was gavaged with 200 µl of DW. After 4T1 cell transplantation, the long diameter (a) and the short diameter (b) of tumours were measured directly with a vernier calliper every 3 days. The tumour size was calculated according to the equation V = ab2/2 and tumour growth curves were drawn (Xue et al., Citation2012). At the 4th week after transplantation, mice were killed and dissected, and the tumours and spleens were observed and measured. The protocols used in this manuscript were approved by the Committee on the Ethics of Animal Experiments of Shenyang Agricultural University (Permit Number: 2015-007).
2.2.2. Effect of WEPA on immune system of mice transplanted with 4T1 cells
The peripheral blood samples were collected from facial veins of mice at four time points: 1, 2, 3 and 4 weeks after transplantation. Then lymphocytes were isolated from the blood samples using lymphocyte separation medium (MP Biomedicals, Califonia, US). For flow cytometric analysis, the lymphocytes from peripheral blood samples were washed twice with lymphocyte washing buffer (0.15 M PBS, 0.5% BSA, 0.1% NaN3), resuspended in 100 μl of the washing buffer. Then the lymphocytes were labelled with fluorochrome conjugated with different monoclonal antibodies at 4°C for 30 min in the dark. The labelled cells were then washed twice with washing buffer and used for flow cytometric analysis. Flow cytometry was conducted using a FACSCalibur flow cytometer (BD Biosciences), and the data were analyzed with the FlowJo software and the Graphpad Prism 8.0 software.
2.2.3. Effect of WEPA on the 4T1 cells in vitro
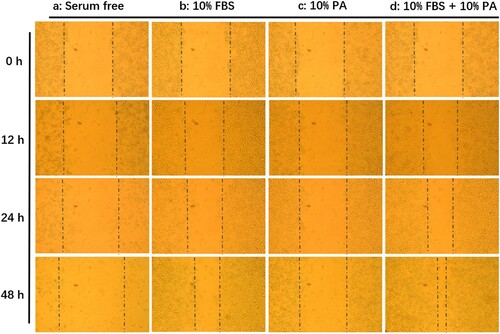
The 4T1 cells were seeded into 24-well assay plates and allowed to attach, spread, and form confluent monolayers, which were scratched and removed cells with needles (Hulkower & Herber, Citation2011). After scratch, the 4T1 cells in the plates were cultured in different media of RPMI-1640 (Group A), RPMI-1640 supplemented with 10% FBS (Group B), RPMI-1640 supplemented with 10% WEPA (Group C), RPMI-1640 supplemented with 10% FBS and 10% WEPA (Group D). The growth statuses of the 4T1 cells were observed at four time points: 0, 12, 24 and 48 h after culturing.
2.2.4. Statistical analysis
All data were presented as the mean ± S.D. Statistical significance between two groups was tested using one way ANOVA. P values <0.05 and <0.01 were statistically significant and extremely significant, respectively.
3. Results
3.1. Effect of WEPA on cancer progression of mice transplanted with 4T1 cells
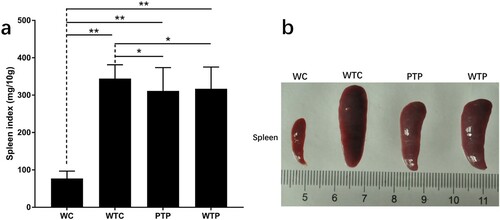
In this study, a set of experiments were carried out to show the effects of WEPA on breast tumour growth, which is illustrated in . As a result, the sizes of the 4T1 mouse mammary tumours in mice after different treatments increased over time, but their growth rates were obviously discrepant (a). Among the three treatments, the tumours from the mice transplanted with 4T1 cells but without WEPA gavage (WTC group) grew fastest, which were much larger than those from mice transplanted with 4T1 cells and gavaged with WEPA (i.e. WTP and PTP groups). Therefore, WEPA could significantly inhibit tumour growth in mice. Moreover, the inhibitory effect of PTP treatment (i.e. gavage with WEPA before and after 4T1 cell transplantation) on the tumour growth was remarkably larger than WTP treatment (i.e. gavage with WEPA only after 4T1 cell transplantation), indicating that treatment of cancer-free mice with WEPA could promote their capacity against cancer development. Four weeks after 4T1 cell transplantation the breast tumours were isolated from mice, which showed that the actual sizes of the tumours were in consistent with that measured in vivo (b). In addition, at the 4th week after 4T1 cell transplantation, spleen index were calculated to detect the immunity of mice after different treatments and spleens were measured to determine the effects of WEPA on spleens. Although the spleen index from PTP and WTP groups were higher than that from WC group, they are obviously lower than that from WTC group (a).The size of spleen from PTP and WTP groups were smaller than that from WTC group, even though all of the tumor-bearing mice groups showed larger size than WC group (b). This result suggested that gavage with WEPA could relieve the swelling of spleens and reduce the spleen index in mice transplanted with 4T1 cells.
Figure 1. A set of experiments designed to show the effects of WEPA on breast tumour growth. Day 1 to Day 7 were time points for gavage. Day 8 was the time point for 4T1 cell transplantation. Day 15 (Week 1) to Day 36 (Week 4) are time points for collection of peripheral blood samples after 4T1 cell transplantation.
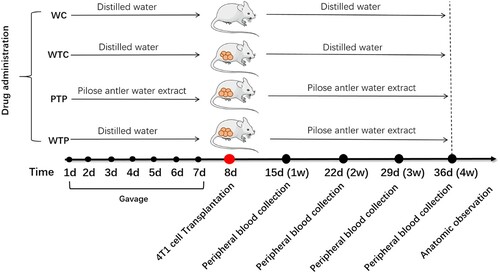
Figure 2. The states of tumours in tumor-bearing mice treated with or without WEPA. (a) shows the growth curves of tumours under different treatments. Error bars represent means ± standard deviations (n = 10). (b) the representative photograph shows the tumours under different treatments.
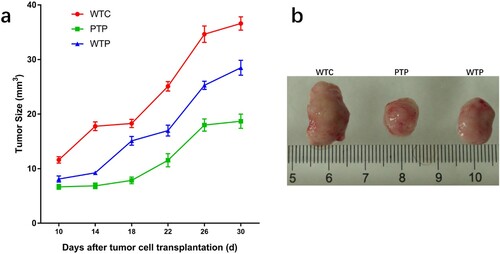
Figure 3. The states of spleens in tumor-bearing mice treated with or without WEPA. (a) shows the spleen index of different treatments. Spleen index (mg/10 g) = (Spleen weight/Mice weight) × 10. (b) the representative photograph shows the spleens under different treatments. Error bars represent means ± standard deviations (n = 10), *P < 0.05, **P < 0.01.
3.2. Effect of WEPA on immune system of mice transplanted with 4T1 cells
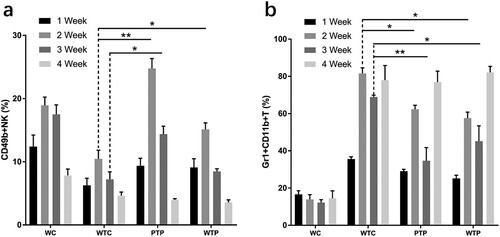
In this study, the lymphocytes from peripheral blood samples were labelled with different monoclonal antibodies and performed flow cytometric analysis to determine the effects of WEPA on the immune system of mice (a, and supplementary Figures 1 and 2).
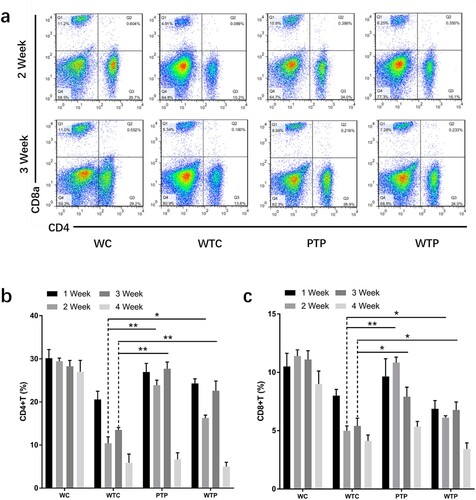
Figure 4. The levels of CD4+ T cells and CD8+ T cells from tumor-bearing mice treated with or without WEPA. (a) shows the representative flow cytometry plots of CD4+ T cells and CD8+ T cells; (b) and (c) show the levels of CD4+ T cells and CD8+ T cells under different treatments, respectively. Error bars represent means ± standard deviations (n = 10), *P < 0.05, **P < 0.01.
3.2.1. Effects of WEPA on CD4+ T cells and CD8+ T cells
The proportions of CD4+ and CD8+ T cells to all immune cells from WTC group were much lower than that from WC group, indicating that the levels of these T cells were obviously reduced by 4T1 cell transplantation. This effect became even more apparent with the development of tumour (b, c). At the end of week 4 after 4T1 cell transplantation, the proportion of CD4+ and CD8+ T cells in peripheral blood of the WTC group decreased to about 1/5 and 1/2 of those from WC group, respectively. However, gavage with WEPA could significantly prevent the reduction of the CD4+ and CD8+ T cell levels in tumor-bearing mice from PTP and WTP groups in the first three weeks after 4T1 cell transplantation. Unfortunately, at the end of fourth week after 4T1 cell transplantation, the levels of CD4+ and CD8+ T cells from the two groups decreased to the levels close to that from WTC group. The variations in the levels of CD4+ T cells in the first 3 weeks between the PTP and WTP treatments were obvious, and the levels of CD8+ T cells in the PTP group were clearly higher than in the WTP group in the first two weeks after 4T1 cell transplantation. These results indicated that gavage with WEPA before and after 4T1 cell transplantation was better than gavage only after the transplantation for maintaining the levels of CD4+ and CD8+ T cells.
3.2.2. Effects of WEPA on NK cells
The proportions of NK cells to all immune cells from TC group were much lower than that from WC group, indicating that the levels of NK cells were obviously reduced by 4T1 cell transplantation (a, and supplementary Figure 1). At the end of week 1 after 4T1 cell transplantation, the level of NK cells declined to approximately 50% of that in the healthy mice. However, gavage with WEPA could significantly prevent the decrease in the levels of NK cells in tumor-bearing mice (PTP and WTP groups) in the first three weeks after 4T1 cell transplantation, and the most obvious prevention was observed at the end of Week 2 in the PTP group. At the end of Week 4, both the PTP and WTP groups lost their preventive effects on the decrease of NK cell levels. The levels of NK cells in the PTP group were clearly higher than in the WTP group at Weeks 2 and 3. These results indicated that WEPA could prevent the decrease of NK cell levels in the early period after 4T1 cell transplantation.
3.2.3. Effects of WEPA on MDSCs
The proportions of MDSCs to all immune cells from WTC group were much higher than that from WC group, indicating that the levels of MDSCs were obviously improved by 4T1 cell transplantation (b, and supplementary ). Gavage with WEPA could generally reduce the levels of MDSCs in tumor-bearing mice (PTP and WTP groups) in the first three weeks after 4T1 cell transplantation, but this reduction effect disappeared at the end of Week4. The inhibitory effect of WEPA on MDSC levels in the PTP group was larger than those in the WTP group.
3.3. Effect of WEPA on the 4T1 cells in vitro
The 4T1 cells were treated with and without WEPA in vitro to determine whether WEPA could directly have effects on the proliferation and migration of 4T1 cells (). The gaps introduced by scratch would become narrower whenever the cells grow faster. The results showed that 4T1 cells could not grow in RPMI-1640 medium without FBS (a), while the cells proliferated and migrated well in complete medium containing 10% FBS (b). The cells in medium with 10% WEPA but without FBS could grow (c), but could not grow as well as these in medium containing 10% FBS. These results indicated that WEPA could stimulate the growth and migration of 4T1 cells in vitro, which was further supported by faster proliferation and migration of the 4T1 cells in the medium containing both 10% WEPA and 10% FBS (d).
4. Discussion
The results of this study showed that WEPA could obviously inhibit the growth of tumours, However, it was found that the addition of WEPA in complete medium could not inhibit, but strongly stimulate the growth and migration of tumour cells in vitro. Therefore, the real strategy of this strong anti-tumor function in vivo was worthy of further research. It was reported that crude extract or certain components of PA have immunomodulatory functions (Zha et al., Citation2013; Zheng et al., Citation2017). The ethanol-soluble components from PA could enhance macrophage phagocytosis and promote the level of immunoglobulins in mice (Suh, Eun, So, Seo, & Jhon, Citation1999; Wang & Zhou, Citation1991). The recombined polypeptides rVAP32 and nVAP32 from PA could significantly stimulate the proliferation of spleen cell and CD4+/CD8+ cell subsets, and strengthened the cytotoxicity of NK cells (Zha et al., Citation2016). In addition, rVAP32 could obviously upregulate the cytokines IL-2, IL-12, TNF-α and IFN-γ, but markedly decrease the levels of IL-4 and IL-10. It is known that IFN-γ, IL-2 and IL-12 can improve the cytotoxicity of NK cells and T cells against tumour cells, while cytokine IL-10 can help MDSCs to repress T cells. All these studies suggested that PA might exercise the anti-tumor function in vivo through modulating the immune system of mice. However, further and stronger evidences are still very necessary. In this study, the effects of WEPA on immune system of female BALB/c mice transplanted with 4T1 cells were studied. The results showed that the levels of cytotoxic T cells, helper T cells, and NK cells in peripheral blood of tumor-bearing mice were significantly increased and the level of MDSCs was significantly decreased by oral administration of WEPA. These results further suggested that WEPA could inhibit the growth of breast tumours possibly through modulating the immune system of tumor-bearing mice.
The spleen is an important immune organ containing a large number of B and T lymphocytes that are involved in humoral and cellular immunity. Its size can increase, known as splenomegaly, under the stimulation of certain factors such as immune inductive substances, virus, bacteria and cancers. The enlargement of the spleen within certain extent may be a result of immune enhancement induced by antigens (Ayeka, Bian, Githaiga, & Zhao, Citation2017; Chen et al., Citation2016). However, a prolonged splenomegaly and excessively large spleen are adverse to immunological competence (Bankoti & Stäger, Citation2012; Webb et al., Citation1980). Several disorders have been demonstrated to be associated with marked splenic enlargement in humans (Weinreb & Rosenbloom, Citation2013). In this study, the remarkable enlargement of the spleen was observed in mice transplanted with 4T1 cells and this enlargement was significantly reduced by WEPA treatments, but its mechanism is unknown. Some evidence suggests that B lymphocytes may be involved in the effect of WEPA. The patients with splenomegaly syndrome could overproduce immunoglobulins from B cells (Hoffman et al., Citation1984). A further clue comes from the experiment that rapamycin could inhibit B cells and reverse splenomegaly in Tg6/λ-MYC mice (Cen & Longnecker, Citation2011). The previous study by Zheng et al. (Citation2017) showed that WEPA gavage decreased the level of B cells in peripheral flood in mice. But, the pathway of inhibitory effect of WEPA on the spleen enlargement still needs more investigation.
Our results indicated that the gavage of WEPA had a preventive effect on the development of breast cancer in mice. The WEPA treatment both before and after the transplantation had a larger inhibitory effect on tumour growth than the treatment only after the transplantation (). This effect of WEPA may be involved in the production of effector memory T cells (TEMs) from the resting state central memory T cells (TCMs). TEMs, after stimulated by tumour antigens, immediately perform a cytotoxic action and secrete a large amount of IL-2, IL-4 and IFN-γ required for anti-tumor immunity (Farber, Yudanin, & Restifo, Citation2014). A study has shown that the patients with gastric cancer exhibited a lower level of TCMs and a higher level of TEMs in the peripheral blood before tumour resection, and TCMs were increased and TEMs reduced significantly after resection (Zhang, Li, Li, Yu, & Ren, Citation2014). A natural active substance glycoprotein from Neem leaf has been showed to maintain a long-term anti-tumor immune response and increase the levels of TCMs and TEMs in sarcoma-bearing mice (Ghosh et al., Citation2016).
Therefore, WEPA may be used as a valuable immunoprotectant for breast cancer treatment. However, WEPA might contain certain bioactive components that strongly stimulate the growth of tumour cells, which was a disadvantage of using WEPA for cancer treatment. Therefore, to identify and isolate immunomodulatory components from WEPA should be very valuable for application of PA in cancer therapy. In addition, further studies on the detailed mechanism of modulating the immune system of tumor-bearing mice with WEPA were necessary.
supplementary_data_R.docx
Download MS Word (2 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ayeka, P. A., Bian, Y. H., Githaiga, P. M., & Zhao, Y. (2017). The immunomodulatory activities of licorice polysaccharides (Glycyrrhiza uralensis Fisch.) in CT 26 tumor-bearing mice. BMC Complementary and Alternative Medicine, 17(1), 536. doi: https://doi.org/10.1186/s12906-017-2030-7
- Bankoti, R., & Stäger, S. (2012). Differential regulation of the immune response in the spleen and liver of mice infected with leishmania donovani. Journal of Tropical Medicine, 2012, 639304. doi: https://doi.org/10.1155/2012/639304
- Cen, O., & Longnecker, R. (2011). Rapamycin reverses splenomegaly and inhibits tumor development in a transgenic model of Epstein-Barr virus-related Burkitt’s lymphoma. Molecular Cancer Therapeutics, 10(4), 679–686. doi: https://doi.org/10.1158/1535-7163.MCT-10-0833
- Chen, J., Zhu, X. Q., Yang, L., Luo, Y., Wang, M. Y., Liu, X. T., … Gu, X. L. (2016). Effect of Glycyrrhiza uralensis Fisch polysaccharide on growth performance and immunologic function in mice in Ural City, Xinjiang. Asian Pacific Journal of Tropical Medicine, 9(11), 1078–1083. doi: https://doi.org/10.1016/j.apjtm.2016.08.004
- Cragg, G. M., & Newman, D. J. (2013). Natural products: A continuing source of novel drug leads. Biochimica et Biophysica Acta (BBA) - General Subjects, 1830(6), 3670–3695. doi: https://doi.org/10.1016/j.bbagen.2013.02.008
- Farber, D. L., Yudanin, N. A., & Restifo, N. P. (2014). Human memory T cells: Generation, compartmentalization and homeostasis. Nature Reviews Immunology, 14(1), 24–35. doi: https://doi.org/10.1038/nri3567
- Ghosh, S., Sarkar, M., Ghosh, T., Guha, I., Bhuniya, A., Saha, A., … Baral, R. (2016). Neem leaf glycoprotein promotes dual generation of central and effector memory CD8+ T cells against sarcoma antigen vaccine to induce protective anti-tumor immunity. Molecular Immunology, 71, 42–53. doi: https://doi.org/10.1016/j.molimm.2016.01.007
- Hartati, F. K., Widjanarko, S. B., Widyaningsih, T. D., & Rifa’i, M. (2017). Anti-Inflammatory evaluation of black rice extract inhibits TNF-α, IFN-γ and IL-6 cytokines produced by immunocompetent cells. Food and Agricultural Immunology, 28(6), 1116–1125. doi: https://doi.org/10.1080/09540105.2017.1332006
- Hoffman, S. L., Piessens, W. F., Ratiwayanto, S., Hussein, P. R., Kurniawan, L., Piessens, P. W., … Marwoto, H. A. (1984). Reduction of suppressor T lymphocytes in the tropical splenomegaly syndrome. New England Journal of Medicine, 310(6), 337–341. doi: https://doi.org/10.1056/NEJM198402093100601
- Hulkower, K. I., & Herber, R. L. (2011). Cell migration and invasion assays as tools for drug discovery. Pharmaceutics, 3(1), 107–124. doi: https://doi.org/10.3390/pharmaceutics3010107
- Imran, M., Nadeem, M., Saeed, F., Imran, A., Khan, M. R., Khan, M. A., … Rauf, A. (2017). Immunomodulatory perspectives of potential biological spices with special reference to cancer and diabetes. Food and Agricultural Immunology, 28(4), 543–572. doi: https://doi.org/10.1080/09540105.2016.1259293
- Lin, J. H., Deng, L. X., Wu, Z. Y., Chen, L., & Li, Z. (2011). Pilose antler polypeptides promote chondrocyte proliferation via the tyrosine kinase signaling pathway. Journal of Occupational Medicine and Toxicology, 6(1), 139–150. doi: https://doi.org/10.1186/1745-6673-6-27
- Quan, S., Zhang, X., & Chang, Z. (2014). Analysis of 3 kinds of sex hormone contents in antler velvet of Chinese sika deer. Special Wild Economic Animal & Plant Research. Retrieved from http://www.cnki.com.cn/
- Suh, J. S., Eun, J. S., So, J. N., Seo, J. T., & Jhon, G. J. (1999). Phagocytic activity of ethyl alcohol fraction of deer antler in murine peritoneal macrophage. Biological & Pharmaceutical Bulletin, 22(9), 932–935. doi: https://doi.org/10.1248/bpb.22.932
- Sui, Z., Zhang, L., Huo, Y., & Zhang, Y. (2014). Bioactive components of velvet antlers and their pharmacological properties. Journal of Pharmaceutical and Biomedical Analysis, 87, 229–240. doi: https://doi.org/10.1016/j.jpba.2013.07.044
- Tang, Y. J., Byong-Tae, J., Wang, Y. M., Eun-Ju, C., Yon-Suk, K., Jin-Woo, H., … Eun-Kyung, K. (2015). First evidence that sika deer (cervus nippon) velvet antler extract suppresses migration of human prostate cancer cells. Korean Journal for Food Science of Animal Resources, 35(4), 507–514. doi: https://doi.org/10.5851/kosfa.2015.35.4.507
- Tseng, S. H., Sung, C. H., Chen, L. G., Lai, Y. J., Chang, W. S., Sung, H. C., & Wang, C. C. (2014). Comparison of chemical compositions and osteoprotective effects of different sections of velvet antler. Journal of Ethnopharmacology, 151(1), 352–360. doi: https://doi.org/10.1016/j.jep.2013.10.060
- Wang, C., Chen, Y. G., Gao, J. L., Lyu, G. Y., Su, J., Zhang, Q. I., … Chen, S. H. (2015). Low local blood perfusion, high white blood cell and high platelet count are associated with primary tumor growth and lung metastasis in a 4T1 mouse breast cancer metastasis model. Oncology Letters, 10(2), 754–760. doi: https://doi.org/10.3892/ol.2015.3304
- Wang, Y., Luo, S., Zhang, D., Qu, X., & Tan, Y. (2017). Sika pilose antler type I collagen promotes BMSC differentiation via the ERK1/2 and p38-MAPK signal pathways. Pharmaceutical Biology, 55(1), 2196–2204. doi: https://doi.org/10.1080/13880209.2017.1397177
- Wang, B. X., & Zhou, Q. L. (1991). Advances in the chemical, pharmacological and clinical studies on pilose antler. Yao Xue Xue Bao: Acta Pharmaceutica Sinica, 26(9), 714–720. PMID: 1821093
- Webb, L. J., Ross, M., Markham, R. L., Webster, A. D., Thomas, H. C., & Sherlock, S. (1980). Immune function in patients with extrahepatic portal venous obstruction and the effect of splenectomy. Gastroenterology, 79(1), 99–103. PMID: 6966595
- Weinreb, N. J., & Rosenbloom, B. E. (2013). Splenomegaly, hypersplenism, and hereditary disorders with splenomegaly. Open Journal of Genetics, 3(1), 24–43. doi: https://doi.org/10.4236/ojgen.2013.31004
- Wu, F., Li, H., Jin, L., Li, X., Ma, Y., You, J., … Xu, Y. (2013). Deer antler base as a traditional Chinese medicine: A review of its traditional uses, chemistry and pharmacology. Journal of Ethnopharmacology, 145(2), 403–415. doi: https://doi.org/10.1016/j.jep.2012.12.008
- Xue, M., Ge, Y., Zhang, J., Wang, Q., Hou, L., Liu, Y., … Li, Q. (2012). Anticancer properties and mechanisms of Fucoidan on mouse breast cancer in vitro and in vivo. Plos One, 7(8), e43483. doi: https://doi.org/10.1371/journal.pone.0043483
- Yang, C., Cai, W., Wen, H., Sha, L., Zhao, C., Zhang, T., & Zhao, W. (2017). Pilose antler peptide protects osteoblasts from inflammatory and oxidative injury through EGF/EGFR signaling. International Journal of Biological Macromolecules, 99, 15–20. doi: https://doi.org/10.1016/j.ijbiomac.2017.02.056
- Zha, E., Dandan, L., Bai, X., Zhou, T., Li, Y., Shenyang, G., & Yue, X. (2016). A recombinant polypeptide from velvet antler of cervus nippon temminck exhibits similar immunomodulatory effects as its natural counterpart. Immunopharmacology and Immunotoxicology, 23, 1–5. doi: https://doi.org/10.1080/08923973.2016.1233978
- Zha, E., Li, X., Li, D., Guo, X., Gao, S., & Yue, X. (2013). Immunomodulatory effects of a 3.2 kDa polypeptide from velvet antler of cervus nippon temminck. International Immunopharmacology, 16(2), 210–213. doi: https://doi.org/10.1016/j.intimp.2013.02.027
- Zhang, Z. Y., Duan, L. X., Zhou, Q. L., Xin, J. L., Lin, Y., & Zhang, Y. (2012). Chemical composition and bioactivity of polypeptide from velvet antlers of cervus nippon temminck. Chemical Journal of Chinese Universities, 33(9), 2000–2004. doi: https://doi.org/10.3969/j.issn.0251-0790.2012.09.022
- Zhang, R., Li, F., Li, H., Yu, J., & Ren, X. (2014). The clinical significance of memory T cells and its subsets in gastric cancer. Clinical and Translational Oncology, 16(3), 257–265. doi: https://doi.org/10.1007/s12094-013-1066-5
- Zhang, M., Li, N., Qu, X. B., Luo, S., & Drummen, G. P. C. (2016). Total velvet-antler polypeptide extract from cervus nippon temminck induces cell proliferation and activation of the PI3K–Akt signalling pathway in human peripheral blood lymphocytes. Animal Production Science, 56(6), 1008–1015. doi: https://doi.org/10.1071/AN15103
- Zheng, K., Fan, Y., Ji, R., & Ma, S. (2017). Distinctive effects of pilose antler on mouse peripheral blood immune cell populations. Food and Agricultural Immunology, 28(6), 1181–1195. doi: https://doi.org/10.1080/09540105.2017.1332011