ABSTRACT
The consumption of oxidized cooking oils may exacerbate some allergic diseases. A contact hypersensitivity (CHS) mouse model was used to investigate the effects of naturally oxidized olive oil on the expression of inflammatory cytokines after a sensitization and elicitation phase. The mRNA expressions of IL-12, IL-18, and IFN-γ in the ear increased seven days after sensitization. IL-18 and IFN-γ mRNA expressions after elicitation were significantly enhanced by administration of oxidized olive oil, which exacerbated ear swelling and mononuclear cell infiltration from CHS after elicitation. IL-18 expression in the ear of oxidized oil-treated mice was highest before elicitation and decreased with an increase in IFN-γ expression after elicitation. The acceleration of an immune reaction by administration of naturally oxidized olive oil in the CHS mouse model was caused by increased expression of IL-18 in the ear auricles after sensitization, which was followed by a significant increase in IFN-γ expression after elicitation.
Introduction
The consumption of frying oil and processed fats is increasing because of ease and simplification of dietary habits and has increased in industrialized countries. Olive oil is a frequently used cooking oil and contains glycerol triesters of unsaturated fatty acids, such as oleic acid and linoleic acid, and saturated fatty acids such as palmitic acid and stearic acid (Yun & Surh, Citation2012). When unsaturated fatty acids are oxidized by heating, free radicals are generated at the carbon–carbon double bond-neighboring methylene groups (Denisov & Afanasev, Citation2005; Min & Boff, Citation2002). These radicals react easily with other lipid molecules to produce ketones, aldehydes, free organic acids, and hydroperoxides through a series of chain reactions (Bastida & Sanchez-Muniz, Citation2001; Guillen & Goicoechea, Citation2008), including autoxidation, polymerization, cyclization, and decomposition, during the oxidation process (Takeoka, Full, & Dao, Citation1997) . The elevation of peroxide value (POV) means an accumulation of these radicals and oxidation products (Totani, Burenjargal, Yawata, & Ojiri, Citation2008). The adverse effects of thermally oxidized cooking oils with high POVs have been shown to induce oxidative stress (Huang, Kang, Li, & Shaw, Citation2009), lipid peroxidation (Nwanguma, Achebe, Ezeanyika, & Eze, Citation1999), and damage to muscle proteins and erythrocyte membranes (Hayam, Cogan, & Mokady, Citation1997). Despite the adverse effects of oxidative stress on health, little information is available on the relationship between ingestion of oxidized frying oils or oxidation intermediates and the increased incidence of allergic diseases.
Because skin is the primary site of the most prevalent human allergic diseases, animal models with allergic dermatitis, including atopic dermatitis and contact hypersensitivity (CHS), are useful tools for investigating the adverse effects of oxidized cooking oils. We previously investigated immediate and delayed allergic reactions of naturally oxidized olive oil with a high POV using mouse models of three types of allergic reactions: CHS as a delayed-type allergic reaction, active cutaneous anaphylaxis as an immediate-type allergic reaction, and DNFB-induced hypersensitivity as a biphasic allergic reaction (Ogino, Sakazaki, Okuno, Arakawa, & Ueno, Citation2015). The results suggested that hydroperoxides were generated by the natural oxidation of olive oil and might accelerate skin allergic reactions including CHS. CHS is a T cell-mediated allergic inflammation induced by exposure of the skin to low molecular weight chemicals such as oxazolone (OXA), one of the haptens. The sensitization phase of CHS is initiated by epicutaneous exposure to a hapten, generates hapten-specific T cells in the lymph nodes, and causes their migration to the dermis. During antigen presentation, antigen-presenting cells secrete IL-12 to drive T cell development into type-1 helper T cells (Th1) (Mactonia et al., Citation1995; Cella et al., Citation1996). In the elicitation phase during CHS, interferon (IFN)-γ produced by Th1, B cells, and natural killer (NK) cells, activates inflammatory cells (Fong & Mosmann, Citation1989). Inflammatory cells, such as macrophages and dendritic cells, promote the production of inflammatory cytokines from T cells via cytokines such as IL-18 (Okamura et al., Citation1995).
We investigated the relevance of naturally oxidized dietary oil and the expression of inflammatory cytokines, which enhance inflammatory lymphocytes, using a CHS mouse model. We examined the effect of oxidized olive oil on the mRNA expression levels of the inflammatory cytokines IL-1β, IL-12 p35, IL-12 p40, IL-18, and IFN-γ 0–7 days after sensitization and 0–24 hr after elicitation by quantitative reverse transcription-polymerase chain reaction (RT–PCR). We also evaluated the levels of IL-18 and IFN-γ in inflamed skin by immunohistochemical examination.
Methods
Preparation of oxidized olive oil
Oxidized olive oil was obtained by the preservation of olive oil at room temperature for more than three years. The POV of oxidized olive oil was 89.4 ± 2.02–93.8 ± 0.82 mEq/kg. The AV and TBARS of oxidized olive oil were similar to those of fresh olive oil.
Chemical analyses
POV was determined by the American Oil Chemists’ Society official method Ja 8-87 (‘Official method and recommended practices of the AOCS,’ Citation2010) using 0.01 mol/L sodium thiosulfate after dissolving oils in acetic acid-chloroform (3:2) solution. POV (mEq/kg) was calculated with the added amount of sodium thiosulfate.
Animals
The protocol used here met the Animal Experiment Guidelines of Setsunan University that were established by revising the guidelines of the Japanese Society for Pharmacology. This study was approved by the Committee for the Ethical Use of Experimental Animals at Setsunan University. All efforts were made to minimize animal suffering, reduce the number of animals used, and use alternatives to in vivo techniques. Female BALB/c mice (5–6 weeks old) were purchased from Japan SLC, Inc., Shizuoka, Japan, and were acclimated in a specific pathogen-free room at 23 ± 1 °C and 47–67% humidity under a 12 hr light/dark cycle (light on at 7:00 a.m.) for at least one week before the start of experiments.
CHS
Seven-week-old mice were sensitized by the topical application of 50 μL 3% OXA (Sigma-Aldrich Inc., St. Louis, MO, USA) in a 3:1 (v/v) mixture of ethanol and acetone on the dorsal skin and were orally administered 100 μL of the test oil once every two days for seven days. Mice were challenged on the next day to elicit an allergic reaction by applying 7.5 μL of 0.1% OXA-ethanol solution to the right ear. The thicknesses of both ears were measured using a digital thickness gauge (Ozaki MFG Co. Ltd., Tokyo, Japan) while taking care to avoid compressing edematous skin. The degree of ear swelling was calculated by subtracting the thickness of the left ear from that of the right ear.
Histological analysis
Ear skins were removed from mice, fixed in phosphate-buffered 4% formaldehyde, embedded in paraffin wax, sectioned into 10-μm thick slices, and stained with hematoxylin–eosin (HE).
IL-18 and IFN-γ expressions were detected by immunostaining. Skin sections were stained with rabbit anti-mouse IL-18 IgG (Rockland Immunochemicals, Inc., Gilbertsville, PA, USA) and rat anti-IFN-γ IgG (BioLegend, Inc., San Diego, CA, USA). The antibody was visualized using the ABC Staining System (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) according to the manufacturer’s protocol. Sections were counterstained with hematoxylin, and slides were examined by microscopy. Densitometric analysis was performed by ImageJ software (version 1.48v, National Institutes of Health, USA) using a dermis area randomly selected in each mouse.
Reverse transcription-polymerase chain reaction (RT–PCR)
Skin tissues were excised and immersed in Sepasol RNA I Super G (Nacalai Tesque, Inc., Kyoto, Japan). RNA extraction was performed according to the manufacturer’s protocol. An aliquot (2 μg) of total RNA sample was reverse transcribed using a Reverse Transcription Kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA), and 1 μL of the cDNA solution was used for PCR using SYBR Green I Master (Roche Diagnostics, GmbH, Mannheim, Germany) and LightCycler 480 System II (Roche Diagnostics, GmbH). Primer sets for IL-1β, IL-12 p35, IL-12 p40, IL-18, IFN-γ, and Ribosomal protein S18 (Rps18, an internal control) were obtained (TaKaRa Bio, Inc., Shiga, Japan). The mRNA levels were calculated as ratios relative to corresponding Rps18 mRNA levels.
Statistical analysis
Results were statistically analysed using one-way analysis of variance (ANOVA) followed by Bonferroni’s correction. Probability values (P < .05) were considered significant. Data in the figures are presented as the mean ± SD.
Results
Cytokine expression and histological change in the ear auricles after sensitization
To investigate whether inflammatory cytokines enhance CHS after administration of oxidized dietary oils, we measured auricular cytokine mRNA expression in the sensitization phase. Mice were orally administered oxidized olive oil on alternate days one week before sensitization and sensitized by the application of 3% OXA to the dorsal skin. The mRNA expressions of IL-1β, IL-12 p35, IL-12 p40, IL-18, and IFN-γ before sensitization and one, four, and seven days after the sensitization in the ear were measured. The mRNA of IL-18 at ear auricles of fresh olive oil-administered mice increased seven days after sensitization. A significantly greater increase in IL-18 mRNA expression was observed in the oxidized olive oil-administered group ((D)). Although the mRNA expression levels of IL-1β, IL-12 p35, IL-12 p40, and IFN-γ gradually increased after sensitization, there were no significant differences in mRNA expression from oxidized olive oil administration ((A–C, E)).
Figure 1. Cytokine mRNA expression levels in the ear after CHS sensitization. Mice were orally administered fresh olive oil (POV = 7.77 ± 0.27 mEq/kg) and oxidized olive oil (POV = 89.41 ± 2.02 mEq/kg) seven days before sensitization of CHS. The mRNA expression levels of target genes were normalized by Rps18. The relative expression levels of the fresh olive oil group before sensitization were designated as 100%. Day 0 means just before sensitization. Fresh olive oil (
 ). The values are mean ± SD (n = 4–5). *P < .05 vs. the fresh olive oil group.
). The values are mean ± SD (n = 4–5). *P < .05 vs. the fresh olive oil group.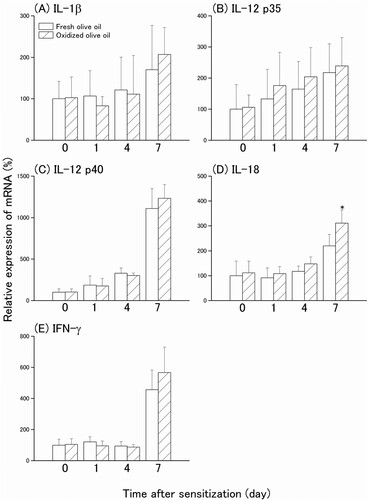
The ear auricles of sensitized mice were measured by HE staining and IL-18 immunostaining. Dermal infiltration of mononuclear cells was observed seven days after sensitization in both groups, and infiltrated cells increased by administration of oxidized olive oil ((A)). IL-18 immunostaining was observed in the dermis of ear auricles seven days after sensitization in fresh olive oil-administered mice and four and seven days after sensitization in oxidized olive oil-administered mice ((B)). The IL-18-positive area progressively increased one, four, and seven days after sensitization, and was significantly increased by oxidized olive oil administration after sensitization compared with that of fresh olive oil-administered mice ((C)).
Figure 2. Histological change and IL-18 expression after CHS sensitization. (A) HE staining. (B) IL-18 immunostaining. (C) IL-18-positive area. The arrowheads represent sites of IL-18. The letters e, d, and c represent epidermis, dermis, and cartilage, respectively. Scale bars, 100 μm. Day 0 means just before sensitization. Fresh olive oil (
 ). The values are mean ± SD (n = 4–5). **P < .01 vs. the fresh olive oil group.
). The values are mean ± SD (n = 4–5). **P < .01 vs. the fresh olive oil group.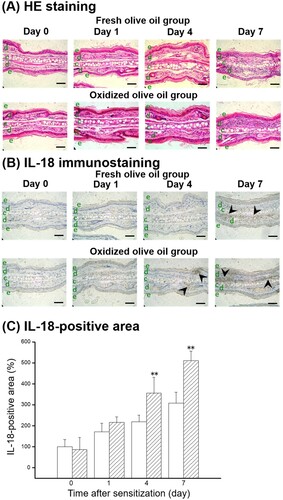
Cytokine expression and histological changes in the ear auricles after elicitation
In order to investigate the inflammatory cytokines that are implicated in the eliciting phase of the allergic reaction, CHS was elicited in oxidized olive oil-administered mice seven days after sensitization by the application of 0.1% OXA to the right ear. The mRNA expression levels of IL-1β, IL-12 p35, and IL-12 p40 peaked 12 hr after elicitation ((A–C)), but there were no significant differences in the expressions between fresh olive oil and oxidized olive oil groups. However, IL-18 mRNA expression level was highest just before elicitation and decreased over time ((D)). IL-18 mRNA expression in the oxidized olive oil-administered group was significantly higher than that in the fresh olive oil-administered group. IFN-γ mRNA expression reached a maximum 6 hr after elicitation in the oxidized olive oil-administered group, which was significantly greater than that of the fresh olive oil-administered group ((E)). Ear swelling was observed 3–24 hr after elicitation and the extent of ear swelling in the oxidized olive oil-administered mice was approximately twofold greater than that in fresh olive oil-administered mice ((A)). Inflamed ear auricles in elicited mice were examined histologically by HE staining ((B)). The dermis had edema 6–24 hr after elicitation. Infiltration of mononuclear cells was observed 12 hr after elicitation, which suggests an increase of infiltrating mononuclear cells in the oxidized olive oil-administered group compared with that in the fresh olive oil-administered group.
Figure 3. Time course of cytokine mRNA expression levels in the ear after CHS elicitation. Mice were orally administered fresh olive oil (POV = 1.47 ± 1.01 mEq/kg) and oxidized olive oil (POV = 67.8 ± 1.98 mEq/kg) seven days before elicitation of CHS. The mRNA expression levels of target genes were normalized by Rps18. The relative expression levels of the fresh olive oil group before sensitization were designated as 100%. 0 hr means just before elicitation. Fresh olive oil (
 ). The values are mean ± SD (n = 4–5). *P < .05, **P < .01 vs. the fresh olive oil group.
). The values are mean ± SD (n = 4–5). *P < .05, **P < .01 vs. the fresh olive oil group.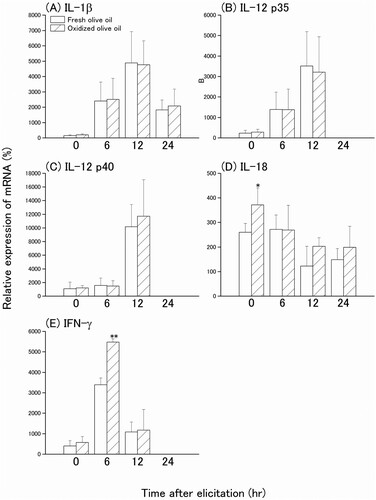
Figure 4. Time course and histological change of ear swelling from CHS. (A) Time-dependent effect of oxidized olive oil on ear thickness. (B) HE staining. The letters e, d, and c represent epidermis, dermis, and cartilage, respectively. Scale bars, 100 μm. 0 hr means just before elicitation. Fresh olive oil (
 ). The values are mean ± SD (n = 5 ). *P < .05, **P < .01 vs. the fresh olive oil group.
). The values are mean ± SD (n = 5 ). *P < .05, **P < .01 vs. the fresh olive oil group.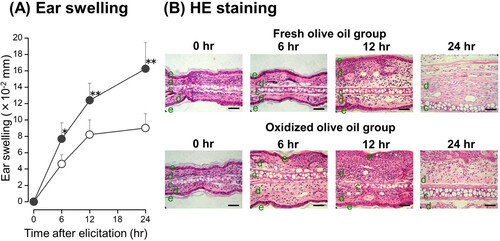
IL-18 and IFN-γ protein expressions in the auricles after elicitation were also estimated by immunostaining. IL-18 expression was observed in the dermis before elicitation and decreased over time ((A)). The IL-18-positive area peaked just before elicitation and was higher in the oxidized olive oil group than in the fresh olive oil group ((B)), which was similar to the pattern of IL-18 mRNA expression in (D). An IFN-γ-positive area was observed 6 hr after elicitation in the dermis ((A)). Administration of oxidized olive oil significantly enhanced IFN-γ expression in inflammation sites 0–6 hr after elicitation compared with that in fresh olive oil-administered mice ((B)).
Figure 5. Time course of IL-18 expression following CHS elicitation. (A) IL-18 immunostaining. (B) IL-18-positive area. The arrowheads represent sites of IL-18. The letters e, d, and c represent epidermis, dermis, and cartilage, respectively. Scale bars, 100 μm. 0 hr means just before elicitation. Fresh olive oil (
 ). The values are mean ± SD (n = 4–5). **P < .01 vs. the fresh olive oil group.
). The values are mean ± SD (n = 4–5). **P < .01 vs. the fresh olive oil group.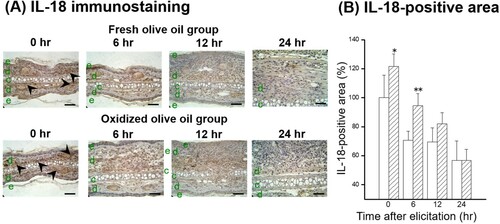
Figure 6. Time course of IFN-γ expression following CHS elicitation. (A) IFN-γ immunostaining. (B) IFN-γ-positive area. The arrowheads represent sites of IFN-γ. The letters e, d, and c represent epidermis, dermis, and cartilage, respectively. Scale bars, 100 μm. 0 hr means just before elicitation. Fresh olive oil (
 ). The values are mean ± SD (n = 4–5). **P < .01 vs. the fresh olive oil group.
). The values are mean ± SD (n = 4–5). **P < .01 vs. the fresh olive oil group.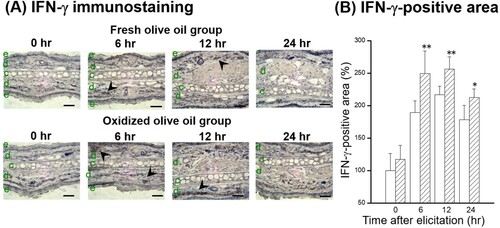
Discussion
Increased oxidative stress exacerbates allergic reactions. We previously demonstrated that oxidative dietary oils enhance allergic reactions such as CHS (Ogino et al., Citation2015). Oxidized dietary fat raises plasma oxidized unsaturated fatty acids, which are markers of oxidative stress in mice (Awada et al., Citation2012). Feeding animals oxidized fat increases the expression of antioxidants, such as glutathione peroxidase (GPx) and superoxide dismutase (SOD), via activation of Nrf2 or NF-κB, which indicates that ingestion of oxidized oil affects oxidative stress (Varady, Eder, & Ringseis, Citation2011; Liu et al., Citation2014). Sivaranjani, Rao, and Rajeev (Citation2013) demonstrated that increased lipid peroxidation increases atopic dermatitis, and suggested that accelerated oxidative stress by oxidized oil ingestion exacerbates allergic reactions.
We demonstrated that inflammatory cytokines enhance CHS after administration of oxidized olive oil. IL-12 p35 is constitutively expressed in small amounts in various cells, and the expression of IL-12 p40 is induced by antigen stimulation in macrophages and dendritic cells (Ma & Trinchieri, Citation2001). IL-12 p40 forms a heterodimer with p35 after antigen stimulation and is secreted as active IL-12 to induce Th1 cell development (O’Shea & Paul, Citation2002). IL-18 does not induce Th1 cell differentiation but enhances IL-12-mediated Th1 development as a strong costimulatory factor in the activation of Th1 cells stimulated by antigens (Robinson et al., Citation1997). The mRNA expression of IL-12, particularly p40, increased gradually after sensitization. IL-12 expression was not affected by administration of oxidized olive oil, but the mRNA and protein expression levels of IL-18 were significantly increased after four days of sensitization. The oxidized olive oil-induced increase of IL-18 protein-positive area in the dermis of the ear auricle is consistent with an increase of infiltrating cells four and seven days after sensitization. This indicates that enhancement of IL-18 production is caused not by epidermal keratinocytes or Langerhans cells but by macrophages or lymphocytes that have migrated to the dermis. These findings suggest that administration of oxidized olive oil promotes sensitization of CHS by increasing the expression of IL-18.
In the CHS-elicited ear auricle, ear swelling increased by administration of oxidized olive oil, which was accompanied by increased expressions of IL-18 and IFN-γ. IL-12 induces the production and release of IFN-γ to target cells, such as Th1, at sites of inflammation, and IL-18 enhances IL-12-induced IFN-γ production (Okamura et al., Citation1995). On the other hand, there was no detectable IL-4 mRNA expression in ear auricles after sensitization even under increased IL-18 expression by administration of oxidized olive oil (data not shown). This suggests that IL-18 may not promote IL-4 expression in Th2 cells in CHS, but induces IFN-γ expression by Th1 cells in cooperation with IL-12 expression. Oxidized olive oil did not enhance the expression levels of IL-1β and IL-12 in inflammatory ear auricles. IL-18 expression seven days after sensitization to the early phase of CHS elicitation was increased by oxidized olive oil administration, and thereafter IFN-γ expression increased. Wakabayashi et al. (Citation2005) have reported that IFN-γ plays important roles in the inflammation of CHS responses, which indicates that the increase of IFN-γ expression causes ear swelling. The absence of IFN-γ expression more than 12 hr after elicitation and an increase of IL-10 expression were observed in the fresh olive oil-administered group (data not shown). There was no increase in IL-10 expression 12 hr in the oxidized olive oil-administered group. These findings suggest that administration of oxidized olive oil decreases the expression of IL-10 produced from Th2 cells to suppress inflammation and prolongs the inflammatory response. Although ear thickness increased over time, IL-18 decreased after elicitation. There are no previous reports that examine the time course of IL-18 expression after CHS elicitation. Our results showed that elevation of IL-18 has an important role in the promotion of CHS sensitization, such as Th1 differentiation, but is not important for the elicitation phase. Enhancement of CHS may be related to other cytokines, such as thymic stromal lymphopoietin (TSLP), and inflammatory cytokines (Ashrin et al., Citation2014), and further investigation of other triggers of CHS is needed.
Lu (Citation2009) reported that NF-κB, a transcription factor that regulates gene expression of inflammatory cytokines including IL-18, is activated in a redox-dependent manner by oxidative stress, and inhibition of NF-κB activation prevents the induction of inflammatory cytokines. Production of IL-18 is also increased by the promoter activity of activator protein-1 (AP-1) via activation of c-Jun N-terminal kinase (JNK), which is a stress-responsive MAPK (Cho et al., Citation2002). Because dietary fats including olive oil contain a high amount of unsaturated fatty acids, hydroperoxides can be produced by oxidation (Denisov & Afanasev, Citation2005; Min & Boff, Citation2002 ). Oxidized olive oil with a high POV contains a high amount of hydroperoxides, but not fatty acids or TBARS, and enhances ear swelling by CHS (Ogino et al., Citation2015). Hepatic oxidized glutathione increases after long-term administration of oxidized olive oil (data not shown). These results suggest that an increase of CHS by administration of oxidized olive oil may be caused by accelerated cytokine expression from elevated promoter activity, such as NF-κB and AP-1, via an increase in oxidative stress. Further investigation of the relationship between oxidative stress from oxidized olive oil and cytokine expression is needed.
In summary, we found that oxidized olive oil administration did not affect the expression of IL-12, but enhanced the expression of IL-18 after sensitization of CHS. In the elicitation phase of CHS, elevated expression of IFN-γ was observed with an increase in inflammation. These results suggest that oxidized olive oil increased IL-18 expression, which resulted in increased production of IFN-γ and exacerbation of CHS.
Data availability statement
The data that support the findings of this study are available from the corresponding author, Ogino, H., upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ashrin, M. N. , Arakaki, R. , Yamada, A. , Kondo, T. , Kurosawa, M. , Kudo, Y. … Ishimaru, N. (2014). Acritical role for thymic stromal lymphopoietin in nickel-induced allergy in mice. Journal of Immunology , 192 (9), 4025–4031. doi: https://doi.org/10.4049/jimmunol.1300276
- Awada, M. , Soulage, C. O. , Meynier, A. , Debard, C. , Plaisancié, P. , Benoit, B. , … Michalski, M. C. (2012). Dietary oxidized n-3 PUFA induce oxidative stress and inflammation: Role of intestinal absorption of 4-HHE and reactivity in intestinal cells. Journal of Lipid Research , 53 (10), 2069–2080. doi: https://doi.org/10.1194/jlr.M026179
- Bastida, S. , & Sanchez-Muniz, F. J. (2001). Thermal oxidation of olive oil, sunflower oil and a mix of both oils during forty discontinuous domestic fryings of different foods. Food Science and Technology International , 7 (1), 15–21. doi: https://doi.org/10.1106/1898-PLW3-6Y6H-8K22
- Cella, M. , Scheidegger, D. , Palmer-Lehmann, K. , Lane, P. , Lanzavecchia, A. , & Alber, G. (1996). Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. The Journal of Experimental Medicine , 184 (2), 747–752. doi: https://doi.org/10.1084/jem.184.2.747
- Cho, D. , Seung, K. J. , Hoon, P. J. , Kim, Y. I. , Hahm, E. , Lee, J. , … W, L. (2002). The enhanced IL-18 production by UVB irradiation requires ROI and AP-1 signaling in human keratinocyte cell line (HaCaT). Biochemical and Biophysical Research Communications, , 298 (2), 289–295. doi: https://doi.org/10.1016/S0006-291X(02)02433-6
- Denisov, E. T. , & Afanasev, I. B. (2005). Oxidation and antioxidants in organic chemistry and biology . New York, NY : CRC Press.
- Fong, T. A. , & Mosmann, T. R. (1989). The role of IFN-γ in delayed-type hypersensitivity mediated by Th1 clones. Journal of Immunology , 143 (9), 2887–2893.
- Guillen, M. D. , & Goicoechea, E. (2008). Formation of oxygenated α, β-unsaturated aldehydes and other toxic compounds in sunflower oil oxidation at room temperature in closed receptacles. Food Chemistry , 111 (1), 157–164. doi: https://doi.org/10.1016/j.foodchem.2008.03.052
- Hayam, I. , Cogan, U. , & Mokady, S. (1997). Enhanced peroxidation of proteins of the erythrocyte membrane and of muscle tissue by dietary oxidized oil. Bioscience, Biotechnology, and Biochemistry , 61 (6), 1011–1012. doi: https://doi.org/10.1271/bbb.61.1011
- Huang, W. C. , Kang, Z. C. , Li, Y. J. , & Shaw, H. M. (2009). Effects of oxidized frying oil on proteins related to α-tocopherol metabolism in rat liver. Journal of Clinical Biochemistry and Nutrition , 45 (1), 20–28. doi: https://doi.org/10.3164/jcbn.08-250
- Liu, P. , Kerr, B. J. , Weber, T. E. , Chen, C. , Johnson, L. J. , & Shurson, G. C. (2014). Influence of thermally oxidized vegetable oils and animal fats on intestinal barrier function and immune variables in young pigs. Journal of Animal Science , 92 (7), 2971–2979. doi: https://doi.org/10.2527/jas.2012-5710
- Lu, S. C. (2009). Regulation of glutathione synthesis. Molecular Aspects of Medicine , 30 (1-2), 42–59. doi: https://doi.org/10.1016/j.mam.2008.05.005
- Ma, X. , & Trinchieri, G. (2001). Regulation of interleukin-12 production in antigen-presenting cells. Advances in Immunology , 79 , 55–92. doi: https://doi.org/10.1016/S0065-2776(01)79002-5
- Mactonia, S. E. , Hosken, N. A. , Litton, M. , Vieira, P. , Hsieh, C. S. , Culpepper, J. A. , … O’Garra, A. (1995). Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. Journal of Immunology , 154 (10), 5071–5079.
- Min, D. B. , & Boff, J. M. (2002). Lipid oxidation of edible oil. In Food lipids (2nd ed, pp. 353–382). New York, NY : CRC Press.
- Nwanguma, B. C. , Achebe, A. C. , Ezeanyika, L. U. , & Eze, L. C. (1999). Toxicity of oxidized fats II: Tissue levels of lipid peroxides in rats fed a thermally oxidized corn oil diet. Food and Chemical Toxicology , 37 (4), 413–416. doi: https://doi.org/10.1016/S0278-6915(99)00023-X
- Official method and recommended practices of the American Chemist’ Society. 6th ed. Method Ja 8-87 . (2010). Champaign : AOCS Press.
- Ogino, H. , Sakazaki, F. , Okuno, T. , Arakawa, T. , & Ueno, H. (2015). Oxidized dietary oils enhance immediate- and/or delayed-type allergic reactions in BALB/c mice. Allergology International , 64 (1), 66–72. doi: https://doi.org/10.1016/j.alit.2014.07.004
- Okamura, H. , Tsutsui, H. , Komatsu, T. , Yutsudo, M. , Hakura, A. , Tanimoto, T. , … Kurimoto, M. (1995). Cloning of a new cytokine that induces IFN-γ production by T cells. Nature , 378 (6552), 88–91. doi: https://doi.org/10.1038/378088a0
- O’Shea, J. J. , & Paul, W. E. (2002). Regulation of T(H)1 differentiation – controlling the controllers. Nature Immunology , 3 (6), 506–508. doi: https://doi.org/10.1038/ni0602-506
- Robinson, D. , Shibuya, K. , Mui, A. , Zonin, F. , Murphy, E. , Sana, T. , … O’Garra, A. (1997). IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activated IRAK and NFkappaB. Immunity , 7 (4), 571–581. doi: https://doi.org/10.1016/S1074-7613(00)80378-7
- Sivaranjani, N. , Rao, S. V. , & Rajeev, G. (2013). Role of reactive oxygen species and antioxidants in atopic dermatitis. Journal of Clinical and Diagnostic Research , 7 (12), 2683–2685.
- Takeoka, G. R. , Full, G. H. , & Dao, L. T. (1997). Effect of heating on the characteristics and chemical composition of selected frying oils and fats. Journal of Agricultural and Food Chemistry , 45 (8), 3244–3249. doi: https://doi.org/10.1021/jf970111q
- Totani, N. , Burenjargal, M. , Yawata, M. , & Ojiri, Y. (2008). Chemical properties and cytotoxicity of thermally oxidized oil. Journal of Oleo Science , 57 (3), 153–160. doi: https://doi.org/10.5650/jos.57.153
- Varady, J. , Eder, K. , & Ringseis, R. (2011). Dietary oxidized fat activates the oxidative stress-responsive transcription factors NF-κB and Nrf2 in intestinal mucosa of mice. European Journal of Nutrition , 50 (8), 601–609. doi: https://doi.org/10.1007/s00394-011-0181-8
- Wakabayashi, T. , Hu, D. L. , Tagawa, Y. , Sekikawa, K. , Iwakura, Y. , Hanada, K. , & Nakane, A. (2005). IFN-gamma and THF-alpha are involved in urushiol-induced contact hypersensitivity in mice. Immunology and Cell Biology , 83 (1), 18–24. doi: https://doi.org/10.1111/j.1440-1711.2005.01310.x
- Yun, J. M. , & Surh, J. (2012). Fatty acid composition as a predictor for the oxidation stability of Korean vegetable oils with or without induced oxidative stress. Preventive Nutrition and Food Science , 17 (2), 158–165. doi: https://doi.org/10.3746/pnf.2012.17.2.158
