ABSTRACT
A polyclonal antibody-based indirect ELISA for the sensitive and specific detection of osmotolerant Zygosaccharomyces rouxii was established. The reactivity of the polyclonal antibodies (pAbs) with Z. rouxii was confirmed by immunofluorescence. The ELISA detection limit was 5 × 104 CFU/mL. For an evaluation of the specificity of pAbs, reactivity to 10 fungi species and 2 bacteria species commonly found in food environments was analysed by indirect ELISA. ELISA reacts with all tested Z. rouxii, whereas no cross-reactions or weakly reactions with 9 fungi and 2 bacteria were observed. The immunoassay staining confirmed the ELISA. Further on, the assay applicability was tested with concentrated apple juice, wine, and honey. Food samples were inoculated with 100 CFU/mL of Z. rouxii. After enrichment for 24 h, the established ELISA can successfully detect contaminated wine and concentrated apple juice samples, while only 30 h was required for honey samples.
1. Introduction
Zygosaccharomyces rouxii is known for its ability to survive in the face of stress caused by high concentrations of ionic (salt) and non-ionic (sugar and polyols) (Dakal, Solieri, & Giudici, Citation2014). Combined with its ability to grow at low pH conditions and preservatives, it can carry out fermentation thus increases the risk of juice industries or cause high sugary and salty foods spoilage. For instance, in the traditional fermented foods, Z. rouxii produce aromatic flavours and alcohol (Watanabe, Uehara, & Mogi, Citation2013). In addition, the flor formation spoilage caused by Z. rouxii in the surface of soy sauce (Watanabe et al., Citation2013) or wine (Zuehlke, Petrova, & Edwards, Citation2013) have been reported. More recently, the spoilage ability of Z. rouxii has been also investigated by our laboratory (Wang, Hu, Long, Niu, et al., Citation2015). The Z. rouxii but not the other 22 species of osmotolerant isolates could survive in concentrated apple juice. Although the spoilage of food industries caused by Z. rouxii can be noticed by swelling of packaging or flor formation, the products would have already been seriously spoiled at that point time, resulting in serious economic losses (Martorell, Stratford, Steels, Fernandez-Espinar, & Querol, Citation2007).
Therefore, it is essential for food manufacturers to implement routine inspection to detect potential Z. rouxii contamination in raw material, finished products and processing environment. Traditional microbiological technique used for quality control tests is cost-effective but laborious and time consuming, especially for Z. rouxii because it often takes a long time (48–72 h) to grow under high sugar conditions (Wang, Hu, Long, Guo, et al., Citation2015). And the culturing method has an obvious drawback that the actual number of microbial communities is usually underestimated (Gobbi et al., Citation2010). Researchers have made great efforts in the rapid identification of Z. rouxii and most reports rely on genetic-based methods. Pearson and McKee (Citation1992) used two primers from Z. rouxii plasmid in a multiplex polymerase chain reaction (PCR) to identify this yeast. Identification of Zygosaccharomyces species including Z. rouxii species is normally done by sequencing the IST or D1/D2 rDNA regions (Harrison, Muir, Stratford, & Wheals, Citation2011; Hulin & Wheals, Citation2014). However, these genetic-based assays require skilled personnel and may not be easily adapted to routine testing. Other available detection methods, including gas chromatography–mass spectrometry (GC-MS) (Zhang, Yue, & Yuan, Citation2013), high-performance liquid chromatography (HPLC) (Arroyomanzanares, Huertaspérez, Gámizgracia, & Garcíacampaña, Citation2015), and electronic nose (Gobbi et al., Citation2015; Wang et al., Citation2016) are expensive and/or require well-trained laboratory technicians. Therefore, a more affordable, rapid, and sensitive method for detection of Z. rouxii is required.
Enzyme-linked immunosorbent assay (ELISA) have been used to detect food-borne microorganisms such as Vibrio parahaemolyticus, Alicyclobacillus spp., Escherichia coli (Kumar et al., Citation2011; Li, Huang, Xia, & Liu, Citation2014; Li, Xia, & Yu, Citation2013; Wang, He, & Shi, Citation2007). Due to its sensitivity, specificity, rapidity, and simplicity, it may become a promising alternative of plating detection method. More recently, ELISA was applied to detect spores of A. acidoterrestris in complex food matrix (Mast, Dietrich, Didier, & Martlbauer, Citation2016). These ELISAs based on polyclonal, monoclonal antibodies or even antibody fragments can be well applied to the detection of bacteria in complex food matrices.
However, fungi share conserved glycoprotein immunogens, which means that the antibodies were often unable to differentiate the target fungus from non-target fungus (Thornton & Wills, Citation2015). The immuno-detection of fungi usually presents the cross-reaction problem between pAbs and targets (Schmechel, Green, Blachere, Janotka, & Beezhold, Citation2008; Vojdani, Citation2004). Hoffmann et al. (Citation2016) and Wang et al. (Citation2013) have suggested that structures of antigens or analytes would influence the selectively of the antibodies, resulting cross-reactions. Maybe for this reason, there are very few reports focused on the pAbs-based immunoassays for food fungi testing. Here in, we used Z. rouxii B-WHX-12-54 grown under high sugar (600 g/L) stress as an immunogens to obtain rabbit pAbs. The reactivity of pAbs to 9 fungis and 2 bacteria commonly found in food environments was analysed by Indirect ELISA. We found that pAbs only obviously recognise Z. rouxii, suggesting polyclonal antibody-based ELISA can be used in the detection of Z. rouxii in food. Since Z. rouxii leads to spoilage of sugar containing or even high sugar foods (apple juice concentrated, wine and honey) (Dakal et al., Citation2014; Loureiro & Malfeitoferreira, Citation2003), we established the indirect ELISA to detect Z. rouxii in enriched samples of these foods.
2. Materials and methods
2.1. Chemicals and reagents
Bovine serum albumin (BSA), Goat anti-rabbit IgG (H + L)-horseradish peroxidase (Goat anti-rabbit IgG (H + L)-HRP), and 3,3′5,5′-tetramethyl benzidine (TMB) were purchased from Sigma (St. Louis, MO, USA). The BCA protein assay kit and ECL western blotting detection kit were purchased from Pierce (Rockford, IL., USA). Tween-20, H2SO4 and commonly used reagents were purchased from local supplies at high purity. The coating buffer was Phosphate-buffered saline (PBS, pH 7.4). Phosphate-buffered saline (PBS, pH 7.4) with 0.05% (v/v) Tween-20 (PBST) was used for washing buffer and 2 mol/L H2SO4 was used as the halting solution. Concentrated apple juice (70° Brix) were purchased from a local juice factory (Shaanxi Haisheng Fresh Fruit Juice Co., Ltd.). Milk powder, Wine and honey were purchased from local supermarket.
2.2. Microorganisms and culture
All of the yeast strains and bacterial strains used are listed in . Fourteen wild-type isolates of different Z. rouxii, Saccharomyces cerevisiae, Pichia kluyveri, P. kudriavzevii, Kluyveromyces marxiauns, Candida tropicalis, C. orthopsilosis, Hanseniaspora uvarum, Debaryomyces hansenii as well as Wickerhamomyces anomalus were obtained from Microbiology Laboratory of the College of Food Science and Engineering, Northwest A & F Univ. (NWSUAF) (Wang et al., Citation2012; Wang, Hu, Long, Niu, et al., Citation2015). Three strains of different Z. rouxii as well as two strains of S. cerevisiae and E. coli were purchased from the American Type Culture Collection (ATCC). From the German Collection of Microorganisms and Cell Cultures (DSMZ), single strain of Alicyclobacillus acidoterrestris was acquired. Single strain of D. hansenii was purchased from Agricultural Culture Collection of China (ACCC). In brief, all yeast strains were inoculated in Yeast extract peptone dextrose broth (YPD, 1% yeast extract, 2% peptone and 2% glucose) liquid medium and cultured at 28 °C for 48 h. A. acidoterrestris was cultured in A. acidoterrestris medium (AAM, 4.0 g yeast extract, 2.0 g glucose, 0.4 g ammonia sulphate, 0.5 g calcium chloride, 1 g magnesium sulphate, 1.2 g monopotassium phosphate and 0.5 g manganese sulphate per litre of deionised water). E. coli was cultured in luria broth (LB, 1% tryptone, 0.5% yeast extract and 1% NaCl).
Table 1. Strains used in this study.
2.3. Polyclonal antibodies production
Z. rouxii B-WHX-12-54 was cultured on 60% YPD liquid medium at 30°C until the late-exponential-phase. The strain was collected by concentrated and strain density was more than 3 × 109 CFU/mL. Stressed Z. rouxii B-WHX-12-54 cells were washed three times with PBS by centrifugation and resuspension in PBS and then were inactivated by sterilisation. Sterilised strain and Freund’s complete adjuvant were mixed (1:1) and fully emulsified as antigens. Two New Zealand White Rabbit were inoculated by subcutaneous intravenous injection with 1 mL antigens (5 × 108 CFU/mL), followed by injection of 2 mL on 5 further occasions at 10 day intervals. Then, the serum of the inoculated rabbits was collected and purified using protein A. The obtained anti-Z. rouxii polyclonal antibodies (pAbs) were stored at −20°C (Wang et al., Citation2012).
The concentration of obtained pAbs was measured using the BCA protein quantification kit (Rockford, IL, USA). Using B-WHX-12-54 as antigen, the titer of pAbs was tested by ELISA.
2.4. Western blot analysis
For the extraction of yeast proteins, the method was according to (Curto et al., Citation2010; Guo et al., Citation2016) without the acetone purification step. For bacteria proteins, 0.1 g cell pellets were added with 1 mL PBS, and then performed on an ultrasonic cell disruption system at 4°C for 30 minutes. All protein samples were quantified by BCA protein quantification kit. About 12 μL (8 μg) protein samples then mixed with 3 μL 5× SDS-PAGE sample buffers and boiled for 5 min and separated on 10% polyacrylamide gel. The separated proteins were transferred onto polyvinylidene (PVDF) membranes, the PVDF membrane was blocked for 2 h with 5% milk powder in TBST buffer. The pAbs (6.3 mg/mL) in a dilution of 1:1000 (diluted in TBST) reacted with the immobilised proteins overnight at 4°C. Plates were then washed four times with TBST, and GAR-HRP (1:2000 diluted in TBST) was added. After 2.5 h, the PVDF members were washed and developed by chemiluminescence using a ECL western blotting detection kit (Rockford, IL, USA), then recorded using a ChemiDoc XRS System (Bio-Rad Laboratories. Inc. Hercules, CA, USA) and quantified using the software Quantity One 4.6.2 software (Wang et al., Citation2012).
2.5. Immunofluorescence studies
The method of immunolabeling of yeast was according to Beaussart et al. (Citation2012) and Coleman et al. (Citation2009) with little modification. Yeast cells were washed twice by centrifugation in PBS buffer. Cells (106 CFU/mL) were resuspended in 15 μg/mL normal goat serum for 15 min at room temperature to block nonspecific antibody binding and washed twice in PBS. They were further incubated in a 18 μg/mL solution of anti-Z. rouxii antibodies for 30 min at 37°C and then in a 3 μg/mL fluorescein isothiocyanate FITC-AffiniPure Goat Anti-Rabbit IgG (H + L) for 30 min at 37°C. Images were obtained using an Andor CSU-W confocal microscopy. An 100× Oil objective with a DU-888U3-CSO was used for all images that were captured with Andor iQ3 live cell imaging software. The treatment of flow cytometry samples was the same as that of immunostaining samples. Flow cytometry analysis was performed with a 488-nm laser, and data were analysed using flowjo 8.5 software.
2.6. Established indirect ELISA to the detection of Z. rouxii
To develop a sensitive and specific ELISA, assay conditions must be optimised, including the selection of the blocking reagent (0.5% BSA, 1% BSA, 5% BSA and 5% skim milk powder in PBS), the first antibody (pAbs from Rabbit1 or Rabbit2) and the second GAR-HRP (diluted 1:1000, 1:2000, 1:3000, 1:4000 and 1:5000). The incubation times were according to Wang et al. (Citation2012) and Li et al. (Citation2013) reported.
The ELISA procedure used was as follows: 96-well microtiter plates (Nunc, Roskilde, Denmark) were coated with 100 μL antigens (target or non-target strain) in PBS (pH 7.4) for 1 h at 37 °C. After incubation, plates were washed three times with 200 μL 10 mM (PBS) with 0.05% Tween-20. Then, the plates were incubated with blocking buffer (5% skim milk in 10 mM PBS) at 37 °C for 2 h with 200 μL per well. After three washes, the wells were blotted on absorbent paper. 100 μL of diluted pAbs (1:4000 in PBS, 1.575 μg/mL) were subsequently added. Following additional incubation for 1 h at 37 °C, the plate was washed three times again. Goat anti-rabbit IgG (H + L)-HRP was then added (1:2000 in PBS; 100 μL per well) and the plate was incubated at 37°C for 1 h. After three washes, substrate solution-A TMB (1 mg/mL) and substrate solution-B (0.03% H2O2) were mixed (1:1) and then added to each well (150 μL) to allow colour development for 15 min under dark conditions at 37°C. The reaction was stopped by adding 2 M of sulphuric acid (50 μL). The OD value of each well was measured at 450 nm by a microtiter plate reader (Bio-Rad Laboratories. Inc. Hercules. CA. USA).
2.7. Cross-reaction and detection limit
Five strains of Z. rouxii (ATCC 2623, ATCC 8383, ATCC 66351, B-WHX-12-53 and ATCC 38531) were used for inclusivity detection. Further on, three strains of H. uvarum (B-WHX-12-63, B-WHX-12-14 and B-WHX-12-61), a single strain of Z. bailli (ATCC 8766), a single strain of P. kluyveri (B-WHX-12-16), a single strain of C. orthopsilosis (B-WHX-12-30), a single strain of K. marxianus (B-WHX-12-21), a single strain of P. kudriavzevii (B-WHX-12-36), a single strain of C. tropicalis (B-WHX-12-52), a single strain of D. hansenii (ACCC 20310), a single strain of W. anomalus (B-WHX-12-11), a single strain of E. coli (ATCC 25922) and a single strain of A. acidoterrestris (DSM 3923) were used as exclusivity detection strains to test the potential cross-reactivity.
ELISA signal intensity value of Z. rouxii B-WHX-12-54 thrice as high as background values was considered to or be regarded to be detection limit.
2.8. Detection of Z. rouxii in food with different sugar concentration
In order to investigate the practical applicability of the Indirect ELISA, three Z. rouxii (B-WHX-12-54, ATCC 2623 and ATCC 8383) artificially contaminated three food matrices (wine, apple juice concentrate and honey).
Three strains were first activated in YPD medium. 10 mL of low sugar wines were inoculated with 100 CFU/mL of the three strains. Simultaneously, three activated strains were transferred to 60% YPD medium (1% yeast extract, 2% peptone and 60% glucose) for the growth at 30 °C until the late-exponential-phase. 10 mL of high sugar of concentrated apple juice or honey were inoculated with 100 CFU/mL of the three strains. The obtained contaminated food samples were diluted 10 fold using YPD medium and cultured at 28 °C for enrichment. Then samples were taken 0, 14 and 24 h after inoculation and analysed by the developed indirect ELISA and conventional plating methods. The test was repeated three times.
3. Results and discussion
3.1. Characterisation of pAbs
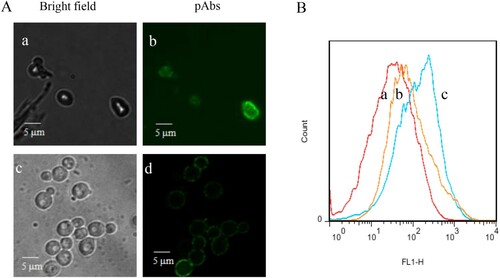
The concentration of obtained pAbs was determined to be 6.1 mg/mL of R1 (Rabbit1) and 6.3 mg/mL of R2 (Rabbit2). As indicated in Supplementary Figure S1, the titer of the pAbs was 4000, resulting OD450 values > 1. R2 is the better pAbs with high ELISA value. Therefore, pAbs of R2 was used for the establishment of an indirect ELISA system. The reactivity of the pAbs with Z. rouxii in different sugar stress was further confirmed by immunofluorescence studies and flow cytometry analysis (). Both stressed-Z. rouxii (grown in 60% YPD) and unstressed-Z. rouxii (grown in YPD) stained by the pAbs exhibited distinct fluorescence ((A)). Similar to the results in (A), flow cytometry showed fluorescence on stressed-Z. rouxii and unstressed-Z. rouxii when the strains stained by the pAbs ((B)). No fluorescence was observed in control sample without pAbs labelling. These results suggested that the reactivity of pAbs with Z. rouxii.
Figure 1. (A) Immunofluorescence images of Z. rouxii B-WHX-12-54 grown at different sugar concentrations. Fluorescence (b, d) and phase-contrast (a, c) images of Z. rouxii BW-12-54 grown in 2% and 60% YPD media, labeled with anti-Z. rouxii pAbs, followed FITC-conjugated secondary antibodies. (B) Histograms of negative control Z. rouxii B-WHX-12-54 (a), stressed-Z. rouxii B-WHX-12-54 (b) and unstressed-cells Z. rouxii B-WHX-12-54 (c) labeled with anti-Z. rouxii pAbs and a FITC-conjugated secondary Ab.
3.2. Establishment of indirect ELISA
Optimum results could be achieved when applying antiserum of R2 and the HRP labeled antibodies were used in a dilution of 1:4000 and 1:2000 in PBS, respectively. The optimum condition of blocking buffer was 5% milk powder in PBS. Based on these conditions, the ELISA system was very stable (repeat several times) and the positive samples at 106 CFU/mL showed ideal absorbance values higher than 1.0 and PBS blank sample is lower than 0.2.
3.3. Sensitivity, specificity, and reliability
As shown in (A), the detection limits of the indirect ELISA for Z. rouxii B-WHX-12-54 was 5 × 104 CFU/mL, which is comparable to the previously reported ELISA methods for the detection of A. acidoterrestris (105 CFU/mL) (Li et al., Citation2013; Wang et al., Citation2012), E. coli (107 CFU/mL) (Wang et al., Citation2007) as well as Staphylococcus aureus (104 CFU/mL) (Pastells et al., Citation2015).
Few studies reported ELISA method to detect fungal, which may due to fungal share conserved antigens (Thornton & Wills, Citation2015). Therefore, polyclonal antibodies-based immunoassays are suitable to detect bacteria that are usually cross-reactive in the fungal detection (Schmechel et al., Citation2008; Vojdani, Citation2004). Therefore, the specificity of pAbs is particularly important to achieve ELISA detection of fungal. The specificity of the method was evaluated using 19 strains of yeast, a single strain of E. coli, and a single strain of Alicyclobacillus, part of the results are shown in and .
Figure 2. (A) ELISA detection of Z. rouxii B-WHX-12-54 and cross-reacting analytes. Data represent the mean of three replications ± SD. (B) Western blot analysis with the extracts of Z. rouxii B-WHX-12-54 and cross-reacting analytes.
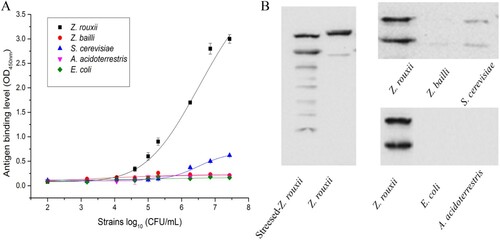
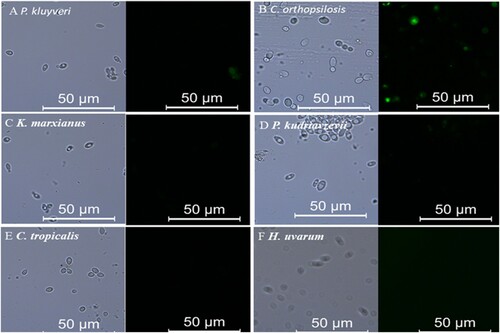
Figure 3. Representative images of immunostaining of fungis using an anti-Z. rouxii pAbs and a FITC-conjugated secondary Ab.
As shown in , the test strain density ranged from 102 to 2.7 × 107 CFU/mL. When the strain density reaches the detection limit, Z. rouxii B-WHX-12-54 was positive. In the experimental concentration range, there was no obvious cross-reaction between the antibody and the reference strains (only Z. bailli ATCC 8766, S. cerevisisae ATCC 38531, A. acidoterrestris DSM 3924 and E. coli ATCC 25922 were shown in the (A)). The reactivity between pAbs and the corresponding strain extracts in (B) was also analysed by western blot. These data may explain the different affinity of pAbs with stressed-Z. rouxii and unstressed-Z. rouxi. The pAbs reacted to multiple bands in stressed-Z. rouxii extract and two bands in unstressed Z. rouxii and to two bands in S. cerevisiae extracts. Interestingly, S. cerevisiae that was weakly or nonreactive in the ELISA was cross-reactive in the western blot. The western blot uses significantly more antigen than the ELISA, and this could show reactivity to minor or underrepresented antigens. In the western blot we observed multiple antigens, some of which are to be common, but we did not specifically identify these molecules.
Moreover, the specificity of the pAbs was investigated by inclusivity detection and exclusivity evolution of the indirect ELISA system. All of three Z. rouxii type strains (ATCC 2623 isolated from grape must and ATCC 8383 and ATCC 66351 isolated from honey) and two Z. rouxii isolates (B-WHX-12-53 and B-WHX-12-55 isolated from apple juice processing) not included in have positive results (OD450>1) in the test, whereas no reactivity or weakly reactivity (OD450 < 0.4) with P. kluyveri (B-WHX-12-16), K. marxianus (B-WHX-12-21), P. kudriavzevii (B-WHX-12-36), D. hansenii (Accc 20310), C. tropicalis (B-WHX-12-52), W. anomalus (B-WHX-12-11), 2 strains of S. cerevisiae (1917 and 1845) and 2 strains of H. uvarum (B-WHX-12-14 and B-WHX-12-61) were observed. The fluorescence images of part strains are shown in , only C. orthopsilosis (B-WHX-12-30) showed weakly fluorescence. The immunofluorescence studies confirmed the ELISA results. Most of the tested strains used in this study are isolated from apple orchards and apple juice processing which have the potential for spoilage (Wang et al., Citation2015a). In total, these results suggested that the pAbs-based ELISA can recognise the common food fungus contamination, and Z. rouxii contribute the most important to ELISA signal. Thus, pAs-based ELISA for detection of Z. rouxii has high sensitivity and specificity and has application value.
3.4. Detection of Z. rouxii in food with different sugar conditions
A preliminary measurement of the ELISA for the direct detection of Z. rouxii in concentrated apple juice revealed that high density of Z. rouxii in high soluble solids content (SSC) samples resulted in lower absorbance values. Therefore, the SSC of the sample is an important factor affecting the ELISA results. The result may be due to the Z. rouxii cells in the high SSC concentrated apple juice can’t bind to the bottom of microtiter plates due to the strong viscosity of the juice. The spoilage of Z. rouxii usually found on the surface of concentrated apple juice (Wang et al., Citation2015a) also supports this result. This issue will be solved since the SSC of diluted food samples changed ranging from (4 °Brix to 75 °Brix) to (5 °Brix to 15 °Brix) (Supplementary Table S1), which is suitable for ELISA detection.
An indirect ELISA developed in food (concentrated apple juice, wine, and honey) was evaluated for the detection of Z. rouxii in contaminated samples. In preliminary tests, the food samples (uncontaminated or spiked with 107 CFU/mL Z. rouxii) were serially diluted in PBS and analysed by the indirect ELISA. Detection limits for Z. rouxii were in the range of 105 CFU/mL, thus confirming the ability of the indirect ELISA method for the sensitive detection of Z. rouxii in food samples. However, the background absorbance values measured for the uncontaminated food samples varied depending on the kinds of the foods (). Analysis of wine and concentrated apple juice resulted in low background values of about 0.07-0.19, while the honey exhibited higher background values of about 0.53-0.63. One reason could be that the composition in honey should be consideration to interfere the results. However, the background values of honey could be diminished to proper values by a simple dilution 4 fold in PBS. Therefore, the dilution step results in a decrease of target concentration, which could be solved by an extended Z. rouxii enrichment time of honey samples.
Table 2. ELISA results for different drinks (diluted 10 fold in YPD medium) contaminated artificially with about 100 CFU/mL of isolate B-WHX-12-54 or ATCC 2623 and ATCC 8383.
In practical ELISA, for each tested strains, samples after contamination for 0, 14 and 24 h were simultaneously analysed by ELISA system and conventional plating methods. As expected, concentrated apple juice and wine samples could be detected within 24 h (). At this time, the plating results showed that the strain concentration was ranging from about 5 × 104 CFU/mL to 2 × 105 CFU/mL (Supplementary Table S2) and the ELISA signal intensity of food samples was at least higher than 3 fold of the samples at 0 h. While for Honey, samples could be detected positive within 30 h as indicated in . The developed indirect ELISA possesses the capacity of detecting Z. rouxii directly from contaminated food samples after a 24 h or 30 h incubation step. As low as 100 CFU/mL of Z. rouxii can be detected. The strain density is equal to previously reported e-nose combined with chemometrics method for the detection of Z. rouxii (102 CFU/mL). But the e-nose method is limited to predicting the concentration of Z. rouxii in concentrated apple juice. Besides, the method requires that the sample pretreatment is not suitable for routine testing. It is worth noting that detection of Z. rouxii in high sugar selective medium requires a very long time (96-120 h). The established indirect ELISA here only requires 24-30 h enrichment time and simple to operate. These results demonstrated established indirect ELISA can be used to rapid detect Z. rouxii in food samples.
Table 3. ELISA results for honey (diluted 10 fold in YPD medium) contaminated artificially with about 100 CFU/mL of isolate B-WHX-12-54 or ATCC 2623 and ATCC 8383.
4. Conclusions
We observed the anti-Z. rouxii pAbs could be obviously recognise Z. rouxii and did not or weakly react to spoilage yeast or bacteria in different food. Here in, we reported a highly sensitive and reliable polyclonal antibodies-based indirect ELISA for Z. rouxii detection in different food. It could be demonstrating that pAbs recognise Z. rouxii growth in different food containing different sugar stress condition, probably due to the conserved expression of Z. rouxii surface proteins. As far as we know, the detection of osmotolerant yeast depending on the high sugar selective medium is a long time-consuming classical microbiological method. Due to the high affinity of Abs and the ability to bind with various nanomaterials, the antibodies-based bioanalytical techniques could be used to ultimately replace classical methods to ensure food quality.
Acknowledgements
The authors would like to acknowledge Mrs Wang, who is responsible for the Life science research platform laser confocal chamber of Northwest A & F University. She generously provided the microscopy technology.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Arroyomanzanares, N., Huertaspérez, J. F., Gámizgracia, L., & Garcíacampaña, A. M. (2015). Simple and efficient methodology to determine mycotoxins in cereal syrups. Food Chemistry, 177, 274–279. doi: https://doi.org/10.1016/j.foodchem.2015.01.040
- Beaussart, A., Alsteens, D., El-Kirat-Chatel, S., Lipke, P. N., Kucharíková, S., Van Dijck, P., & Dufrêne, Y. F. (2012). Single-molecule imaging and functional analysis of Als adhesins and mannans during Candida albicans morphogenesis. ACS Nano, 6, 10950–10964. doi: https://doi.org/10.1021/nn304505s
- Coleman, D. A., Oh, S. H., Zhao, X. M., Zhao, H. Y., Hutchins, J. T., Vernachio, J. H., … Hoyer, L. L. (2009). Monoclonal antibodies specific for Candida albicans Als3 that immunolabel fungal cells in vitro and in vivo and block adhesion to host surfaces. Journal of Microbiological Methods, 78, 71–78. doi: https://doi.org/10.1016/j.mimet.2009.05.002
- Curto, M., Valledor, L., Navarrete, C., Gutierrez, D., Sychrova, H., Ramos, J., & Jorrin, J. (2010). 2-DE based proteomic analysis of Saccharomyces cerevisiae wild and K+ transport-affected mutant (trk1,2) strains at the growth exponential and stationary phases. Journal of Proteomics, 73, 2316–2335. doi: https://doi.org/10.1016/j.jprot.2010.07.003
- Dakal, T. C., Solieri, L., & Giudici, P. (2014). Adaptive response and tolerance to sugar and salt stress in the food yeast Zygosaccharomyces rouxii. International Journal of Food Microbiology, 185, 140–157. doi: https://doi.org/10.1016/j.ijfoodmicro.2014.05.015
- Gobbi, E., Falasconi, M., Concina, I., Mantero, G., Bianchi, F., Mattarozzi, M., & Sberveglieri, G. (2010). Electronic nose and Alicyclobacillus spp. spoilage of fruit juices: an emerging diagnostic tool. Food Control, 21, 374–382. doi: https://doi.org/10.1016/j.foodcont.2010.04.011
- Gobbi, E., Falasconi, M., Zambotti, G., Sberveglieri, V., Pulvirenti, A., & Sberveglieri, G. (2015). Rapid diagnosis of Enterobacteriaceae in vegetable soups by a metal oxide sensor based electronic nose. Sensors and Actuators B: Chemical, 207, 1104–1113. doi: https://doi.org/10.1016/j.snb.2014.10.051
- Guo, H., Niu, C., Liu, B., Wei, J. P., Wang, H. X., Yuan, Y. H., & Yue, T. L. (2016). Protein abundance changes of Zygosaccharomyces rouxii in different sugar concentrations. International Journal of Food Microbiology, 233, 44–51. doi: https://doi.org/10.1016/j.ijfoodmicro.2016.05.003
- Guo, H., Yuan, Y. H., Niu, C., Wang, Z. L., Qiu, Y., & Yue, T. L. (2017). Wash-free colorimetric homogeneous immunoassay for Zygosaccharomyces rouxii. RSC Advances, 7, 34307–34314. doi: https://doi.org/10.1039/C7RA02791E
- Harrison, E., Muir, A., Stratford, M., & Wheals, A. (2011). Species-specific PCR primers for the rapid identification of yeasts of the genus Zygosaccharomyces. FEMS Yeast Research, 11, 356–365. doi: https://doi.org/10.1111/j.1567-1364.2011.00724.x
- Hoffmann, H., Baldofski, S., Hoffmann, K., Flemig, S., Silva, C. P., Esteves, V. I., & Schneider, R. J. (2016). Structural considerations on the selectivity of an immunoassay for sulfamethoxazole. Talanta, 158, 198–207. doi: https://doi.org/10.1016/j.talanta.2016.05.049
- Hulin, M., & Wheals, A. (2014). Rapid identification of Zygosaccharomyces with genus-specific primers. International Journal of Food Microbiology, 173, 9–13. doi: https://doi.org/10.1016/j.ijfoodmicro.2013.12.009
- Kumar, B. K., Raghunath, P., Devegowda, D., Deekshit, V. K., Venugopal, M. N., & Karunasagar, I. (2011). Development of monoclonal antibody based sandwich ELISA for the rapid detection of pathogenic Vibrio parahaemolyticus in seafood. International Journal of Food Microbiology, 145, 244–249. doi: https://doi.org/10.1016/j.ijfoodmicro.2010.12.030
- Li, J. K., Huang, R., Xia, K., & Liu, L. (2014). Double antibodies sandwich enzyme-linked immunosorbent assay for the detection of Alicyclobacillus acidoterrestris in apple juice concentrate. Food Control, 40, 172–176. doi: https://doi.org/10.1016/j.foodcont.2013.11.037
- Li, J. K., Xia, K., & Yu, C. Z. (2013). Detection of Alicyclobacillus acidoterrestris in apple juice concentrate by enzyme-linked immunosorbent assay. Food Control, 30, 251–254. doi: https://doi.org/10.1016/j.foodcont.2012.07.009
- Loureiro, V., & Malfeitoferreira, M. (2003). Spoilage yeasts in the wine industry. International Journal of Food Microbiology, 86, 23–50. doi: https://doi.org/10.1016/S0168-1605(03)00246-0
- Martorell, P., Stratford, M., Steels, H., Fernandez-Espinar, M. T., & Querol, A. (2007). Physiological characterization of spoilage strains of Zygosaccharomyces bailii and Zygosaccharomyces rouxii isolated from high sugar environments. International Journal of Food Microbiology, 114, 234–242. doi: https://doi.org/10.1016/j.ijfoodmicro.2006.09.014
- Mast, S., Dietrich, R., Didier, A., & Martlbauer, E. (2016). Development of a polyclonal antibody-based sandwich enzyme-linked immunosorbent assay for the detection of spores of alicyclobacillus acidoterrestris in various fruit juices. Journal of Agriculture and Food Chemistry, 64, 497–504. doi: https://doi.org/10.1021/acs.jafc.5b03841
- Pastells, C., Acosta, G., Pascual, N., Albericio, F., Royo, M., & Marco, M. P. (2015). An immunochemical strategy based on peptidoglycan synthetic peptide epitopes to diagnose Staphylococcus aureus infections. Analytica Chimica Acta, 889, 203–211. doi: https://doi.org/10.1016/j.aca.2015.07.049
- Pearson, B., & McKee, R. (1992). Rapid identification of Saccharomyces cerevisiae, Zygosaccharomyces bailii and Zygosaccharomyces rouxii. International Journal of Food Microbiology, 16, 63–67. doi: https://doi.org/10.1016/0168-1605(92)90126-N
- Schmechel, D., Green, B. J., Blachere, F. M., Janotka, E., & Beezhold, D. H. (2008). Analytical bias of cross-reactive polyclonal antibodies for environmental immunoassays of Alternaria alternata. Journal of Allergy Clinical Immunology, 121, 763–768. doi: https://doi.org/10.1016/j.jaci.2007.09.046
- Thornton, C. R., & Wills, O. E. (2015). Immunodetection of fungal and oomycete pathogens: Established and emerging threats to human health, animal welfare and global food security. Critical Reviews in Microbiology, 41, 27–51. doi: https://doi.org/10.3109/1040841X.2013.788995
- Vojdani, A. (2004). Cross-reactivity of Aspergillus, Penicillium, and Stachybotrys antigens using affinity-purified antibodies and immunoassay. International Archives Occupational Environmental Health, 59, 256–265. doi: https://doi.org/10.3200/AEOH.59.5.256-265
- Wang, H. X., Hu, Z. Q., Long, F. Y., Guo, C. F., Yuan, Y. H., & Yue, T. L. (2015). Detection of Zygosaccharomyces rouxii and Candida tropicalis in a high-sugar medium by a metal oxide sensor-based electronic nose and comparison with test panel evaluation. Journal of Food Protection, 78, 2052–2063. doi: https://doi.org/10.4315/0362-028X.JFP-15-196
- Wang, H. X., Hu, Z. Q., Long, F. Y., Niu, C., Guo, C. F., Yuan, Y. H., & Yue, T. L. (2015). Characterization of osmotolerant yeasts and yeast-like molds from apple orchards and apple juice processing plants in China and investigation of their spoilage potential. Journal of Food Science, 80, M1850–M1860. doi: https://doi.org/10.1111/1750-3841.12946
- Wang, H. X., Hu, Z. Q., Long, F. Y., Guo, C. F., Yuan, Y. H., & Yue, T. L. (2016). Early detection of Zygosaccharomyces rouxii – spawned spoilage in apple juice by electronic nose combined with chemometrics. International Journal of Food Microbiology, 217, 68–78. doi: https://doi.org/10.1016/j.ijfoodmicro.2015.10.010
- Wang, N., He, M., & Shi, H. C. (2007). Novel indirect enzyme-linked immunosorbent assay (ELISA) method to detect total E. coli in water environment. Analytica Chimica Acta, 590, 224–231. doi: https://doi.org/10.1016/j.aca.2007.03.041
- Wang, Z., Beier, R. C., Sheng, Y., Zhang, S., Jiang, W., Wang, Z., & Shen, J. (2013). Monoclonal antibodies with group specificity toward sulfonamides: Selection of hapten and antibody selectivity. Analyticacl Bioanalytical Chemistry, 405, 4027–4037. doi: https://doi.org/10.1007/s00216-013-6785-5
- Wang, Z. L., Yue, T. L., Yuan, Y. H., Cai, R., Guo, C. F., Wang, X., & Niu, C. (2012). Development of polyclonal antibody-based indirect enzyme-linked immunosorbent assay for the detection of Alicyclobacillus strains in apple juice. Journal of Food Science, 77, M643–M649. doi: https://doi.org/10.1111/j.1750-3841.2012.02961.x
- Watanabe, J., Uehara, K., & Mogi, Y. (2013). Adaptation of the osmotolerant yeast Zygosaccharomyces rouxii to an osmotic environment through copy number amplification of FLO11D. Genetics, 195, 393–405. doi: https://doi.org/10.1534/genetics.113.154690
- Zhang, J. B., Yue, T. L., & Yuan, Y. H. (2013). Alicyclobacillus contamination in the production line of kiwi products in China. PloS One, 8, e67704. doi: https://doi.org/10.1371/journal.pone.0067704
- Zuehlke, J. M., Petrova, B., & Edwards, C. G. (2013). Advances in the control of wine spoilage by Zygosaccharomyces and Dekkera/Brettanomyces. Annual Review of Food Science and Technology, 4, 57–78. doi: https://doi.org/10.1146/annurev-food-030212-182533
