ABSTRACT
Zearalenone (ZEA) is a mycotoxin that is mainly produced by Fusarium fungi in food and feed. It causes many adverse effects on mammals, but there is little research on the effects of ZEA on intestinal mucosal immunity and intestinal flora in animals or humans. In this study, we aimed to explore the effects of short-term ZEA exposure on mucosal immunity and the microecological balance of the intestine. We found that the morphological structure of the intestinal mucosa in mice was severely destroyed after ZEA was administered by gavage for one week, and the mRNA expression levels of mucosal β-defensin, Mucin-1, Mucin-2, interleukin-1beta (IL-1β), and tumour necrosis factor-α (TNF-α) and secretory immunoglobulin A (sIgA) levels were significantly increased. In addition, the intestinal microflora was altered by ZEA. Our study showed that ZEA not only caused inflammation of the mucous membrane but also disturbed the microecological balance of the intestine in mice.
Introduction
Environmental pollutants have always been a major threat to human health, and they can easily damage the immune system of human beings and cause some epidemics. Zearalenone (ZEA) is a kind of mycotoxin that exerts an oestrogen effect (Kiang, Kennedy, Pathre, & Mirocha, Citation1978), and it is a common environmental pollutant in grain crops around the world. ZEA is widely found in food, feed and raw materials and causes serious harm to the health of animals and human beings (Aiko & Mehta, Citation2015; Bennett & Klich, Citation2003; Kuiper-Goodman, Scott, & Watanabe, Citation1987). Research confirms that ZEA and its metabolites mainly act on oestrogen target organs and that it easily induces reproductive disorders in animals and high oestrogen syndrome in humans (Kuiper-Goodman et al., Citation1987). However, what we often ignore is that the small intestine (SI) is the main organ that absorbs ZEA (Biehl et al., Citation1993) and that the intestinal mucosal barrier will be the first to be affected by ZEA.
The morphology and abundance of the intestinal microflora are influenced by many external environmental factors (Hullar, Burnett-Hartman, & Lampe, Citation2014; Phillips, Citation1900), and the intestinal microflora play an essential role in regulating the changes in animal physiology and pathophysiology (D’Aversa et al., Citation2013; Ojeda, Bobe, Dolan, Leone, & Martinez, Citation2016). As we all know, the intestinal mucosa is the first line of defence of the intestinal barrier. Specifically, the intestinal epithelial cells (IECS), mucus, sIgA and antimicrobial peptides (AMPs) constitute the “mucosal firewalls”, which protect the body from microbial flora, viruses and environmental pollutants (Belkaid & Hand, Citation2014; Macpherson, Slack, Geuking, & Mccoy, Citation2009). Indeed, intestinal microbes are adjuvants of the immune system, and there is a complex reciprocal relationship between intestinal microbes and the healthy intestinal tract. Intestinal microbes possess an excellent ability to regulate intestinal infection and immunity (Molloy, Bouladoux, & Belkaid, Citation2012). Healthy gut microbes are conducive to enhancing the body’s immunity, while intestinal dysbacteriosis is related to host inflammation and autoimmune disorders (Quigley, Citation2013; Shen & Wong Connie, Citation2016).
Previous studies have shown that environmental pollutants can induce IECS inflammation and damage intestinal health by destroying the intestinal mucosal barrier (Seonghwan et al., Citation2010). In recent years, researchers found that most mycotoxins were closely related to intestinal inflammatory diseases in humans (Maresca & Fantini, Citation2010). However, there are few studies on the toxic effects of ZEA on the intestinal microflora and intestinal mucosal immune barrier in mammals, and the exact mechanism of its action is unclear (Gajęcka, Zielonka, & Gajęcki, Citation2017).
How ZEA affects the intestinal microecological balance and mucosal immunity, and what is its mechanism are still questions that need to be answered. Therefore, we systematically explored the effects of ZEA on the intestinal microbes and mucosal immune barrier in mice by using 16S rDNA sequencing technology. The aim of this study was to reveal the potential mechanism of ZEA on the intestinal health of mammals or humans and to provide a theoretical basis for the prevention and treatment of ZEA exposure.
Materials and methods
Animals and treatments
ZEA was purchased from Sigma-Aldrich (St Louis, MO, USA), and it was dissolved in ethanol and made into 100 mg/ml storage solutions. Then, it was dissolved in olive oil at 10 mg/ml before use.
Twenty-four immature male BALB/C mice (Laboratory Animal Center of Jilin University, China) were randomly divided into two groups. Each group had three replicates, and each replicate contained four mice. A weekend for the trial period, all the mice were reared under standard conditions (temperature: 22 ± 2°C; humidity: 50%∼70%) and were provided an adequate diet and sterile drinking water.
All the mice were fed a basal diet (Liaoning Changsheng Biological Company, China). The experimental group (ZEA group) was administered ZEA by gavage at a dosage of 20 mg/kg body weight once daily for one week, and the control group (CON group) was given an equal volume of vector. All the experiments were approved by the Institutional Animal Care and Use Committee of Jilin University.
At the end of the experiment, the mice were sacrificed by cervical dislocation. The jejunum was separated, and the intermediate segment (3 cm long) was taken. The faeces was washed away with saline, and the tissue was fixed with 4% polyoxymethylene. The remaining jejunum and intestinal faeces were carefully collected, quickly placed in liquid nitrogen, and then transferred to −80°C.
Morphological analysis of the intestinal mucosa
The intestinal tissues were fixed with 4% polyoxymethylene for 24 h, embedded in petrolin and sectioned into 2–3 µm sections. The sections were stained with haematoxylin first, counterstained with eosin, and finally observed under an optical microscope.
Determination of the expression of the genes involved in intestinal mucosal immunity by real-time PCR
Total RNA was extracted from the jejunum. The A260/A280 nm ratio of the RNA sample was in the usable range of 1.8–2.1. cDNA was synthesized using a Reverse Transcription Kit, and a SYBR Green Mix Kit (Trans, Beijing, China) was used for the real-time PCR. The relative expression levels of the genes were calculated using the 2−ΔΔCt method (Livak & Schmittgen, Citation2001). The primer sequences are listed in .
Table 1. Primer sequences for real-time PCR.
Detection of mucosal IgA in the jejunum
Equal amounts of jejunal faeces were dissolved with 0.01 M PBS + PMSF, broken down at a low temperature, and centrifuged for 10 min at 3000 r at 4°C. The supernatant was then collected. Next, the absorbance was detected at 562 nm according to the instructions in the mouse IgA ELISA kit (eBioscience, California, USA), and the IgA content (ng/ml) was calculated.
Composition and diversity of the bacterial communities
Total genomic bacterial DNA was extracted from the faeces of the jejunum in BALB/C mice. Then, the qualified genomic DNA was used for 16S rDNA V3-V4 region PCR amplification. The primers were 341F (5’-ACTCCTACGGGAGGCAGCAG-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’). All the procedures were performed according to the manufacturer’s protocol (BGI, Shengzheng, China). Two hypervariable regions of 16S rDNA, the V3 and V4 regions, were used to identify the vast majority of the bacteria based on the 16S rDNA sequencing. The qualified PCR products were purified by 16S V3-V4 amplification, and the DNA library was constructed. The qualified DNA library was sequenced using an Illumina MiSeq 2*300, and information was acquired for the bioinformatic analysis.
The raw data were filtered to eliminate adapter pollution and low quality to obtain clean reads. Then, the paired-end reads with overlapping regions were merged to raw tags with FLASH software (v1.2.11) (Magoč & Salzberg, Citation2011). Paired-end reads without overlaps were removed. Following removal of the primer sequences, the forward and reverse amplification primers were mapped to the two ends of the tags. The tags were clustered to the operational taxonomic unit (OUT) at 97% sequence similarity by scripts using the software USEARCH (v7.0.1090) (Edgar, Citation2013). The PCR-generated chimaera from the OTU representation sequence was removed, and effective tags were obtained by UCHIME software (v4.2.40) (Edgar, Haas, Clemente, Christopher, & Rob, Citation2011). The database used for species annotation was Greengene (V201305) (Desantis et al., Citation2006).
Statistical analysis
All the data were analysed using SPSS statistical software (version 20.0). The variation between the control group and the ZEA group was analysed by a t-test, and the results are expressed as the mean ± SEM. The differences were judged as statistically significant at P values < 0.05.
Results
Damaging effects of ZEA on the intestinal mucosal morphology in mice
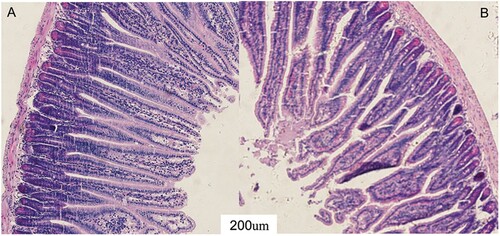
To objectively assess the effect of ZEA on intestinal mucosa in mice, the mucosal morphology of the jejunum was observed (). The intestinal villi in the CON group were arranged neatly, and the microvilli structure formed a brush border ((a)). While the integrity of the jejunum intestinal villi in the ZEA group was severely destroyed ((b)), the mucosal epithelium microvilli were dispersed, and some of them were defluvium defluxion.
Effect of ZEA on the intestinal mucosal immune system
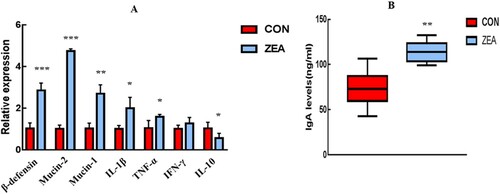
To further evaluate the effect of ZEA on intestine mucosal immunity in mice, we detected the mRNA expression of key genes involved in mucosal immunity (β-defensin, Mucin-1, and Mucin-2) and inflammatory cytokines (IL-1β, TNF-α, IFN-γ, and IL-10) in the jejunum ((a)). The expression of β-defensin, Mucin-1, and Mucin-2 was significantly upregulated (P < 0.001, P < 0.001, and P < 0.01, respectively), and the expression of IL-1β and TNF-α was remarkably upregulated (P < 0.05 and P < 0.05, respectively). In contrast, the expression of the anti-inflammatory cytokine IL-10 was significantly downregulated (P < 0.05) after ZEA exposure. There was no change in the expression of IFN-γ (P > 0.05) compared with the CON.
ZEA induces the secretion of mucosal sIgA in the jejunum
To evaluate the effect of ZEA on the sIgA contents in the intestinal mucosa, we detected the faecal IgA levels in the jejunum ((B)). The results showed that short-term ZEA exposure can significantly induce faecal IgA levels in the jejunum and that the faecal IgA in the ZEA group increased more than 1.5-fold (P < 0.01) compared to that in the CON group.
Effect of ZEA on the microflora in the jejunum
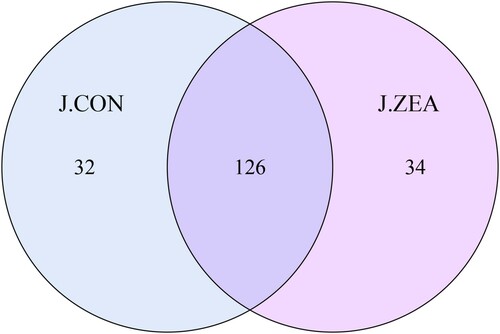
We further detected changes in the intestinal microflora in the mice exposed to ZEA. A total of 169685 effective tags were obtained, with an average of 28280 effective tags for each sample and an average length of 426 bp (). At 97% similarity, the jejunal bacterial community of the CON and ZEA groups shared 126 OTUs ().
Table 2. Effect of ZEA on bacterial community diversity in the jejunal digesta.
Using the ACE index () to assess bacterial richness, short-term ZEA exposure led to a tendency to reduce the richness of the jejunal microflora. At the same time, the Simpson index was used to evaluate changes in the intestinal microbial diversity (). In the ZEA group, the bacterial diversity was almost similar to that of the CON group.
Short-term ZEA exposure had no significant effect on the predominant bacteria of the jejunum, but the stability of the intestinal microecology was altered. As shown in , compared with the CON group, the relative abundance of Cyanobacteria in the ZEA group decreased from 0.10% to 0.006%. At the class level, the Epsilonproteobacteria in the ZEA group disappeared. At the order level, the Campylobacter disappeared. At the family level, the helicobacteraceae disappeared and the Comamonadaceae was reduced by almost half (0.015%–0.008%). At the genus level, the Helicobacter disappeared. At the species level, [Clostridium]_leptum_DSM_753 completely vanished. It was remarkable that the Lactobacillus_intestinalis decreased nearly 18-fold (18.2%–1.9%). In addition, there was an increase in unidentified strains (71.6%–84.4%).
Table 3. Common changes in the intestinal microbiome community.
Discussion
Mycotoxin is a class of environmental pollutant that easily causes an intestinal ecological imbalance and induces some intestinal diseases, such as irritable bowel syndrome (IBS) (Choung & Locke, Citation2011), allergic enteritis (Compare & Nardone, Citation2014), and inflammatory bowel disease (IBD) (Maresca & Fantini, Citation2010). However, the toxic effects of ZEA on the intestinal microecological balance are still lacking.
Our study showed that short-term ZEA exposure affected the mucosal morphology and structural integrity of the jejunum. It resulted in a significant upregulation of the expression of β-defensin, Mucin-1, and Mucin-2 in the jejunal mucosa, while the sIgA in the jejunum also increased. Similarly, current research suggests that ZEA increases the content of Mucins in Caco-2/HT29-MTX cells (Wan, Allen, Turner, & El-Nezami, Citation2014), and the mucin abnormalities may indicate intestinal infection. Wan (Wan, Woo, Allen, Turner, & El-Nezami, Citation2013) found that ZEA induced the upregulation of β-defensin in IPEC-J2 cells from swine. Another study revealed that some mycotoxins can increase sIgA in intestinal epithelial cells (Bouhet & Oswald, Citation2005). Mucosal sIgA not only protects intestinal epithelial cells from environmental toxins and pathogenic bacteria but also maintains the homeostasis of the intestinal mucosal environment (Kato, Kawamoto, Maruya, & Fagarasan, Citation2014; Mantis, Rol, & Corthésy, Citation2011). Lower levels of sIgA may reflect the low intestinal mucosal immunity, while higher levels of sIgA may reflect the high microbial pressure caused by chronic infection or continuous exposure to environmental toxins (Okumura & Takeda, Citation2018). Indeed, β-defensin, Mucins and sIgA are the main components of mucosal immunity, and they play an essential role in the immune defence of the intestinal mucosa (Derrien, , & van Hylckama Vlieg, Citation2015). Their elevation indicates intestinal mucosa abnormalities.
Thus, we also detected the inflammatory markers of the jejunum mucosa. The results showed that the mRNA expression levels of IL-1β and TNF-α were upregulated and that the expression of the anti-inflammatory factor IL-10 was downregulated by ZEA exposure. Wan (L. Y. M. Wan, Woo, Turner, Wan, & El-Nezami, Citation2013) found that ZEA could induce the upregulation of IL-1β and TNF-α in IPEC-J2 cells from piglets. However, Liu (Liu et al., Citation2014) revealed that different doses of ZEA showed a downward trend in the gene expression of IL-1β and TNF-α in the jejunum of rats. According to current research, the effect of ZEA on the changes in the intestinal inflammatory response is still controversial. The main reason may be the differences in the experimental model and dosage. However, an increase in pro-inflammatory cytokines and a decrease in anti-inflammatory cytokines indicate mild inflammation in the intestinal mucosa (Urara & Hiroya, Citation2015). Therefore, all the conclusions indicate that ZEA can alter the normal mucosal inflammatory response.
Current research shows that intestinal microbes play a key role in the health of animals and that changes in microbial communities may induce certain diseases (Horai et al., Citation2015; Sun & Chang, Citation2014). The results of our further study indicated that short-term ZEA exposure did not cause significant changes in the overall richness and diversity of the intestinal flora, but it caused a mild local disorder of the intestinal flora. The most obvious change was a significant decrease in Lactobacillus_intestinalis. It is generally known that Lactobacillus has a mutually beneficial relationship with the human body. It not only provides the host with a nutrient source but also protects the host against potential invasion of pathogens (Martín et al., Citation2013). The decline in Lactobacillus may cause the growth of harmful intestinal bacteria and may even induce IBS (Chassard et al., Citation2012; Fan et al., Citation2017). Clostridium leptum cooperates with other intestinal microbiota to ferment unabsorbed dietary carbohydrates and produce short-chain fatty acids (SCFAs). SCFAs are the main source of energy for the intestinal epithelium and profoundly affect the mucosal immunity of the jejunum. A reduction in Clostridium leptum is observed in Crohn’s disease (CD) patients and ulcerative colitis (UC) patients (Kabeerdoss, Sankaran, Pugazhendhi, & Ramakrishna, Citation2013; Manichanh et al., Citation2006). Thus, the decline of [Clostridium]_leptum_DSM_753 reflected that ZEA may possess the possibility of driving gut disease. Studies suggest that most mycotoxins modify the intestinal microflora and affect the microecological balance of the intestinal mucosa. Our findings further confirmed that ZEA can also change the abundance of probiotics in the intestine and that the changes in probiotics indicates an unstable state of the intestinal flora. If the state continues, some intestinal diseases, such as IBS, will be induced.
In conclusion, we found that short-term ZEA exposure caused toxic effects on SI in BALB/C mice. In particular, ZEA altered the normal microecology by inhibiting the growth of beneficial bacteria in the intestine. For example, Lactobacillus_intestinalis and [Clostridium]_leptum_DSM_753 not only inhibited pathogenic bacteria but also drove beneficial metabolism to maintain an intestinal microecological balance. In addition, we found that ZEA induced a mucosal immune response in SI and drove the intestinal mucosa to produce a severe inflammatory response, which further caused the histomorphological change in SI. Therefore, we believe that short-term ZEA exposure changes the intestinal flora imbalance and normal intestinal mucosal immune function, thus inducing mucosal inflammation. Ultimately, this drives the damage to the morphological structure of the intestinal mucosa. Thus, we believe that ZEA may be a potential factor in inducing inflammatory diseases in the human intestinal tract.
Acknowledgements
This research was financially supported by the Jilin Science and Technology Development Project (20170307018NY); the Special Project of the Province-University Co-constructing Program of Jilin Province (SXGJXX2017-4); the Northeast Agricultural University/Scientific Observing and Experimental Station of Animal Nutrition and Feed Science in Northeast, Ministry of Agriculture, P.R. China; and the Jilin Modern Agricultural Technology Demonstration and Extension Project.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aiko, V., & Mehta, A. (2015). Occurrence, detection and detoxification of mycotoxins. Journal of Biosciences, 40(5), 943–954. doi: https://doi.org/10.1007/s12038-015-9569-6
- Belkaid, Y., & Hand, T. W. (2014). Role of the microbiota in immunity and inflammation. Cell, 157(1), 121–141. doi: https://doi.org/10.1016/j.cell.2014.03.011
- Bennett, J. W., & Klich, M. (2003). Mycotoxins. Clinical Microbiology Reviews, 16(3), 497–516. doi: https://doi.org/10.1128/CMR.16.3.497-516.2003
- Biehl, M. L., Prelusky, D. B., Koritz, G. D., Hartin, K. E., Buck, W. B., & Trenholm, H. L. (1993). Biliary excretion and enterohepatic cycling of zearalenone in immature pigs. Toxicology & Applied Pharmacology, 121(1), 152–159. doi: https://doi.org/10.1006/taap.1993.1140
- Bouhet, S., & Oswald, I. P. (2005). The effects of mycotoxins, fungal food contaminants, on the intestinal epithelial cell-derived innate immune response. Veterinary Immunology & Immunopathology, 108(1–2), 199–209. doi: https://doi.org/10.1016/j.vetimm.2005.08.010
- Chassard, C., Dapoigny, M., Scott, K. P., Crouzet, L., Del’Homme, C., Marquet, P., … Eschalier, A. (2012). Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Alimentary Pharmacology & Therapeutics, 35(7), 828–838. doi: https://doi.org/10.1111/j.1365-2036.2012.05007.x
- Choung, R. S., & Locke, G. R. (2011). Epidemiology of IBS. Gastroenterology Clinics of North America, 40(1), 1–10. doi: https://doi.org/10.1016/j.gtc.2010.12.006
- Compare, D., & Nardone, G. (2014). The role of gut microbiota in the pathogenesis and management of allergic diseases. European Review for Medical & Pharmacological Sciences, 18(17), 11–17.
- D’Aversa, F., Tortora, A., Ianiro, G., Ponziani, F. R., Annicchiarico, B. E., & Gasbarrini, A. (2013). Gut microbiota and metabolic syndrome. Internal & Emergency Medicine, 8(1), 11–15. doi: https://doi.org/10.1007/s11739-013-0916-z
- Derrien, M., & van Hylckama Vlieg, J. E. T. (2015). Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends in Microbiology, 23(6), 354–366. doi: https://doi.org/10.1016/j.tim.2015.03.002
- Desantis, T. Z., Hugenholtz, P., Larsen, N., Rojas, M., Brodie, E. L., Keller, K., … Andersen, G. L. (2006). Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Applied & Environmental Microbiology, 72(7), 5069–5072. doi: https://doi.org/10.1128/AEM.03006-05
- Edgar, R. C. (2013). UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nature Methods, 10(10), 996–998. doi: https://doi.org/10.1038/nmeth.2604
- Edgar, R. C., Haas, B. J., Clemente, J. C., Christopher, Q., & Rob, K. (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics, 27(16), 2194–2200. doi: https://doi.org/10.1093/bioinformatics/btr381
- Fan, W. T., Ding, C., Xu, N. N., Zong, S., Ma, P., & Gu, B. (2017). Close association between intestinal microbiota and irritable bowel syndrome. European Journal of Clinical Microbiology & Infectious Diseases Official Publication of the European Society of Clinical Microbiology, 36(1), 1–15. doi: https://doi.org/10.1007/s10096-016-2778-6
- Gajęcka, M., Zielonka, Ł, & Gajęcki, M. (2017). Activity of zearalenone in the porcine intestinal tract. Molecules, 22(1), 18. doi: https://doi.org/10.3390/molecules22010018
- Horai, R., Zárate-Bladés, C., Dillenburg-Pilla, P., Chen, J., Kielczewski, J., Silver, P., … Honda, K. (2015). Microbiota-dependent activation of an autoreactive T cell receptor provokes autoimmunity in an immunologically privileged site. Immunity, 43(2), 343–353. doi: https://doi.org/10.1016/j.immuni.2015.07.014
- Hullar, M. A. J., Burnett-Hartman, A. N., & Lampe, J. W. (2014). Gut microbes, diet, and cancer. Cancer Treatment & Research, 159, 377–399. doi: https://doi.org/10.1007/978-3-642-38007-5_22
- Kabeerdoss, J., Sankaran, V., Pugazhendhi, S., & Ramakrishna, B. S. (2013). Clostridium leptum group bacteria abundance and diversity in the fecal microbiota of patients with inflammatory bowel disease: A case–control study in India. Bmc Gastroenterology, 13(1), 20–20. doi: https://doi.org/10.1186/1471-230X-13-20
- Kato, L. M., Kawamoto, S., Maruya, M., & Fagarasan, S. (2014). Gut TFH and IgA: Key players for regulation of bacterial communities and immune homeostasis. Immunology & Cell Biology, 92(1), 49–56. doi: https://doi.org/10.1038/icb.2013.54
- Kiang, D. T., Kennedy, B. J., Pathre, S. V., & Mirocha, C. J. (1978). Binding characteristics of zearalenone analogs to estrogen receptors. Cancer Research, 38(11 Pt 1), 3611.
- Kuiper-Goodman, T., Scott, P. M., & Watanabe, H. (1987). Risk assessment of the mycotoxin zearalenone. Regulatory Toxicology & Pharmacology, 7(3), 253–306. doi: https://doi.org/10.1016/0273-2300(87)90037-7
- Liu, M., Gao, R., Meng, Q., Zhang, Y., Bi, C., & Shan, A. (2014). Toxic effects of maternal zearalenone exposure on intestinal oxidative stress, barrier function, immunological and morphological changes in rats. PLoS ONE, 9(9), e106412. doi: https://doi.org/10.1371/journal.pone.0106412
- Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods, 25(4), 402–408. doi: https://doi.org/10.1006/meth.2001.1262
- Macpherson, A. J., Slack, E., Geuking, M. B., & Mccoy, K. D. (2009). The mucosal firewalls against commensal intestinal microbes. Seminars in Immunopathology, 31(2), 145–149. doi: https://doi.org/10.1007/s00281-009-0174-3
- Magoč, T., & Salzberg, S. L. (2011). FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics, 27(21), 2957–2963. doi: https://doi.org/10.1093/bioinformatics/btr507
- Manichanh, C., Rigottier-Gois, L., Bonnaud, E., Gloux, K., Pelletier, E., Frangeul, L., … Marteau, P. (2006). Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Digest of the World Core Medical Journals, 55(2), 205–211.
- Mantis, N. J., Rol, N., & Corthésy, B. (2011). Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunology, 4(6), 603–611. doi: https://doi.org/10.1038/mi.2011.41
- Maresca, M., & Fantini, J. (2010). Some food-associated mycotoxins as potential risk factors in humans predisposed to chronic intestinal inflammatory diseases. Toxicon, 56(3), 282–294. doi: https://doi.org/10.1016/j.toxicon.2010.04.016
- Martín, R., Miquel, S., Ulmer, J., Kechaou, N., Langella, P., & Bermúdez-Humarán, L. G. (2013). Role of commensal and probiotic bacteria in human health: A focus on inflammatory bowel disease. Microbial Cell Factories, 12(1), 71–71. doi: https://doi.org/10.1186/1475-2859-12-71
- Molloy, M. J., Bouladoux, N., & Belkaid, Y. (2012). Intestinal microbiota: Shaping local and systemic immune responses. Seminars in Immunology, 24(1), 58–66. doi: https://doi.org/10.1016/j.smim.2011.11.008
- Ojeda, P., Bobe, A., Dolan, K., Leone, V., & Martinez, K. (2016). Nutritional modulation of gut microbiota — the impact on metabolic disease pathophysiology. Journal of Nutritional Biochemistry, 28, 191–200. doi: https://doi.org/10.1016/j.jnutbio.2015.08.013
- Okumura, R., & Takeda, K. (2018). Maintenance of intestinal homeostasis by mucosal barriers. Inflammation & Regeneration, 38(1), G1168. doi: https://doi.org/10.1186/s41232-018-0063-z
- Phillips, M. L. (1900). Reacción visceral: Efectos ambientales sobre la microbiota humana. Salud Pública De México, 51(4), 343–352. doi: https://doi.org/10.1590/S0036-36342009000400012
- Quigley, E. M. (2013). Gut bacteria in health and disease. Gastroenterology & Hepatology, 9(9), 560.
- Seonghwan, P., Hyejin, C., Yang, H., Keehun, D., Juil, K., & Yuseok, M. (2010). Repression of peroxisome proliferator-activated receptor γ by mucosal ribotoxic insult-activated CCAAT/enhancer-binding protein homologous protein. Journal of Immunology, 185(9), 5522–5530. doi: https://doi.org/10.4049/jimmunol.1001315
- Shen, S., & Wong Connie, H. Y. (2016). Bugging inflammation: Role of the gut microbiota. Clinical & Translational Immunology, 5(4), e72. doi: https://doi.org/10.1038/cti.2016.12
- Sun, J., & Chang, E. B. (2014). Exploring gut microbes in human health and disease: Pushing the envelope. Genes & Diseases, 1(2), 132–139. doi: https://doi.org/10.1016/j.gendis.2014.08.001
- Urara, N., & Hiroya, K. (2015). The nonsteroidal mycoestrogen zearalenone and its five metabolites suppress LH secretion from the bovine anterior pituitary cells via the estradiol receptor GPR30 in vitro. Theriogenology, 84(8), 1342–1349. doi: https://doi.org/10.1016/j.theriogenology.2015.07.014
- Wan, L. Y. M., Allen, K. J., Turner, P. C., & El-Nezami, H. (2014). Modulation of mucin mRNA (MUC5AC and MUC5B) expression and protein production and secretion in caco-2/HT29-MTX Co-cultures following exposure to individual and combined fusarium mycotoxins. Toxicological Sciences, 139(1), 83–98. doi: https://doi.org/10.1093/toxsci/kfu019
- Wan, L. Y. M., Woo, C. S. J., Turner, P. C., Wan, M. F., & El-Nezami, H. (2013). Individual and combined effects of fusarium toxins on the mRNA expression of pro-inflammatory cytokines in swine jejunal epithelial cells. Toxicology Letters, 220(3), 238–246. doi: https://doi.org/10.1016/j.toxlet.2013.05.003
- Wan, M. L., Woo, C. S., Allen, K. J., Turner, P. C., & El-Nezami, H. (2013). Modulation of porcine β-defensins 1 and 2 upon individual and combined fusarium toxin exposure in a swine jejunal epithelial cell line. Applied & Environmental Microbiology, 79(7), 2225–2232. doi: https://doi.org/10.1128/AEM.03277-12