ABSTRACT
Procaine (PRC) is a local anaesthetic used in the treatment of several disease related to ageing, asthma, and arthritis. In this study, we describe a rapid, sensitive, simple, and accurate method for the determination of PRC in milk, using an immunochromatographic test strip. For the immunochromatographic test, antigen (PRC-bovine serum albumin) and goat anti-mouse IgG were drawn onto a nitrocellulose membrane representing the control line and test line. The test was developed with a sensitive monoclonal antibody specific for PRC, generated by immunizing BALB/c mice with a well-characterized PRC- Keyhole limpet hemocyanin conjugate, produced in our laboratory. Under optimized conditions, the semi-quantitative test strip was able to detect PRC at concentrations down to 1 ng/mL in both 0.01 M phosphate buffer saline (pH 7.4) and milk samples. All results were obtained within 5 min. Our data indicate that the immunochromatographic assay is capable of sensitive, rapid, and specific on-site screening of PRC.
Introduction
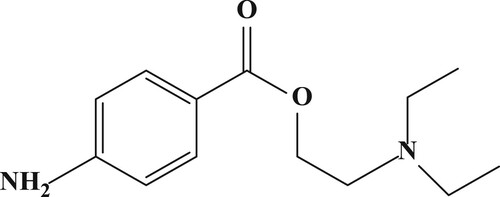
Procaine, 2-diethylaminoethyl 4-aminobenzoate (PRC), also known as novocaine or neocaine is a local anaesthetic drug of the amino ester group (). It is widely used in the injectable form as an injection with antibiotics and can relieve pain associated with medical procedures used in gynaecology, dentistry, surgery, and anaesthesia block therapy. It can however, also be used as a stimulating drug (Guan et al., Citation2016; Silva et al., Citation2016). PRC is less toxic and less expensive than both cocaine and tetracaine. However, because of its poor penetration into intact mucous membranes, it is less effective for surface application (Fijalek, Baczynski, Piwonska, & Warowna-Grzeskiewicz, Citation2005; Liu, Peng, et al., Citation2017). It is widely used in veterinary medicine to treat anti-inflammatory drug with fatty liver and clinical ketosis diseases, which result in a loss of milk production. The detrimental effects of differing doses of PRC (both high and low doses) include dizziness, chills, convulsions, and coma. Therefore, it is vital that an accurate and sensitive detection method for PRC is established.
Several methods for the detection of PRC in pharmaceutical preparations and biological samples have been reported. In both the British and US Pharmacopoeia, methods for assaying PRC hydrochloride are based on the solvent extraction, followed by spectrophotometric measurement at 280 nm or titration with sodium hydroxide. In the Chinese Pharmacopoeia, however, PRC is titrated with sodium hydroxide and determined by thin layer chromatography (Liu et al., Citation2012). Other analytical methods have also been used, including colorimetry (Lee & Livett, Citation1967; Salama & Omer, Citation1980), spectrophotometry (Liu et al., Citation2000), gas chromatography (Baniceru, Croitoru, & Popescu, Citation2004; Ohshima & Takayasu, Citation1999), high performance liquid chromatography (He, Peng, Tang, & Zhang, Citation2013; Liu, Peng, et al., Citation2017; Lu, Citation2012), and liquid chromatography tandem mass spectrometry (Babic et al., Citation2010; Dhananjeyan et al., Citation2007). However, these methods are time consuming and expensive because they require long pre-treatment procedures and sophisticated analytical instruments (Ge, Suryoprabowo, Zheng, & Kuang, Citation2017; Liu, Suryoprabowo, Zheng, Song, & Kuang, Citation2017; Wang, Zheng, et al., Citation2017).
Immunoassays have been widely used as screening methods due to their high sensitivity, specificity, fast detection speed, and low cost. The electrochemical immunoassay based on the use of gold nanoparticle and immunochromatographic strips offer attractive approaches for the detection and identification of biomolecules. It had been widely used as a diagnosis tool to detect and identify infectious diseases, cancer, cardiovascular problems, and biological warfare agents (Mao, Baloda, Gurung, Lin, & Liu, Citation2008; Weller, Citation2000). Gold nanoparticles of different coloured latex particles also have been used in formats such as the dot blot immunoassay. This approach simplifies interpretation of the results of a multiparameter assay, as each binding zone and the corresponding analyte can be identified based on the colour of the maker (Taranova, Berlina, Zherdev, & Dzantiev, Citation2015). Chemiluminescence immunoassay (CLIA) also has been widely used in different fields including clinical diagnosis, food safety, and environmental. CLIA is a technique of chemiluminescence (CL) combined with immunoassay (IA). It has high sensitivity, good specificity, rapid analysis, and simple operation (Wang, Liu, et al., Citation2017; Xiao & Lin, Citation2015)
Immunoassays based on the highly specific interaction between antigen and antibody could be a sensitive tool for the detection of PRC. The lateral-flow immunochromatographic (LFIC) strip allows the semi-quantitative analysis of several drug residues and has become increasingly popular because it is simple, rapid (can be performed in 5–10 min), specific, and sensitive. A further advantage of this method this is that all of the reagents required are included on the strip (Jiang, Zeng, Liu, Song, & Kuang, Citation2018; Li, Liu, Song, & Kuang, Citation2018; Song, Zou, Zhu, Liu, & Kuang, Citation2018; Zeng, Jiang, Liu, Song, & Kuang, Citation2018).
Although LFIC testing is widely used, to the best of our knowledge, no LFIC device for the detection of PRC residues has been described. Therefore, the aim of this study is to develop an LFIC method for the detection of PRC in milk sample.
Materials and methods
Chemicals and reagents
PRC was purchased from J&K Scientific (Shanghai, China). Complete Freund's adjuvant (FCA), incomplete Freund's adjuvant (FIA), and enzyme immunoassay-grade horseradish peroxidase (HRP)-labelled goat anti-mouse immunoglobulin were all obtained from Sigma-Aldrich (St. Louis, MO, USA). Gelatin was supplied by Beijing Biodee Biotechnology Co., Ltd. (Beijing, China). Tetramethylbenzidine and HRP were from Aladdin Chemistry Co., Ltd. (Shanghai, China). All reagents used for cell fusion were purchased from Sunshine Biotechnology Co., Ltd. (Nanjing, China). Bovine serum albumin (BSA) and keyhole-limpet hemocyanin (KLH) were obtained from Solarbio Science & Technology, Co., Ltd. (Beijing, China). All other reagents and chemicals were supplied by the National Pharmaceutical Group Chemical Reagent Co., Ltd. (Shanghai, China).
Nitrocellulose (NC) high-flow plus membranes (Pura-bind RP) were obtained from Whatman-Xinhua Filter Paper Co., Ltd. (Hangzhou, China). The glass fibre membrane (CB-SB08) used for the sample pad, the polyvinylchloride (PVC) backing material and the absorbance pad (SX18), were supplied by Goldbio Tech Co., Ltd. (Shanghai, China). The conjugated coating antigens (PRC-BSA) and specific monoclonal antibody (anti-PRC mAb) were generated in our laboratory.
All buffer solutions were prepared with ultrapure water (Milli-Q Purification System, Millipore Co., Bedford, MA, USA). The strip-cutting instrument was a CM-4000 Guillotine Cutting System from Gene, Shanghai, China, and the dispensers used were Airjet Quanti 3000TM and Biojet Quanti 3000TM (XinqidianGene Technology Co., Ltd., Beijing, China).
Preparation and characterization of anti-PRC monoclonal antibody (mAb)
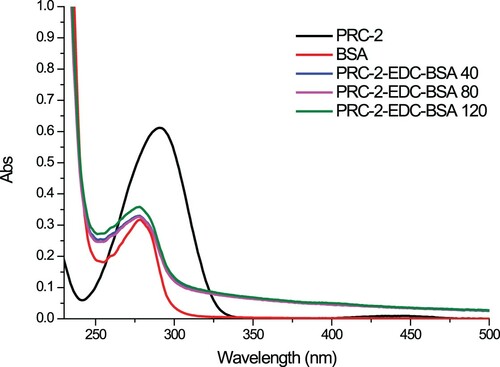
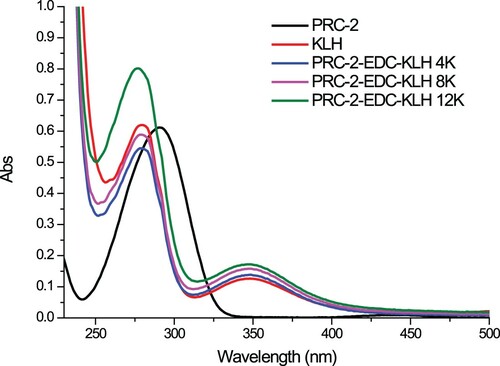
PRC was conjugated to BSA () or KLH () using the ester method (Ge et al., Citation2017; Suryoprabowo, Liu, Peng, Kuang, & Xu, Citation2014). Briefly, a mixture of PRC, carboxyl-reactive carbodiimide cross linker (1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride) (EDC), and N-hydroxysuccinimide were added to N,N-dimethylformamide and incubated for 24 h (solution 1). KLH or BSA was mixed with 0.01 M phosphate buffer saline (PBS) (solution 2). Solution 1 was slowly added to solution 2 under constant stirring for 8 h at room temperature. The resulting conjugates were dialysed against 0.01 M PBS for 3 days and subsequently with distilled water for 3 days. The conjugation of protein and PRC was assessed by UV absorption.
Female BALB/c mice (6–8 weeks of age) were used to produce the anti-PRC mAb by subcutaneous immunization with PRC-KLH. FCA was used in the first immunization, and FIA used in subsequent immunizations. The mice were immunized every 3 weeks with 100 µg for the first immunization and 50 µg for the remainder (2–5 times). Blood samples from mice were measured by enzyme-linked immunosorbent assay (ELISA). The mouse demonstrating the highest titer was sacrificed and its splenocytes were fused with Sp 2/0 murine myeloma cells. The hybridomas were screened by indirect ELISA by using PRC-BSA as the coating antigen. The selected hybridoma cells were expanded and injected into BALB/c mice to produce mAbs (Deng et al., Citation2012; Suryoprabowo et al., Citation2014). The ascites were harvested and purified using the caprylic acid-ammonium sulfate precipitation method (Kong et al., Citation2017; Kuang et al., Citation2013). The purified antibody solution was stored at –20°C until further use.
Development of the LFIC test strip device
Preparation of colloidal gold nanoparticles
All solvents were prepared with ultrapure water and then filtered through a transfer membrane (0.22 µm). Chloroauric acid (25 mL of 0.1 g/L solution) was heated to boiling with constant stirring, and then 1% (w/v) sodium citrate tribasic dihydrate solution (1.0 mL) was added. The mixture was stirred for 30 min until the colour of the solution turned wine-red, then cooled to room temperature, and stored at 4 °C. Transmission electron microscopy demonstrated that the gold nanoparticles had an almost uniform particle size of 15 nm.
Preparation of colloidal gold-labelled mAb
The anti-PRC mAb (4G6) which had a half-maximal inhibitory concentration (IC50) of 0.1 ng/mL was used. Colloidal gold solution (10 mL) was adjusted to pH 7.0 with 0.1 M K2CO3 (40 µL). Briefly, 0.5 mL of 0.2 mg/mL mAb solution was added dropwise to the colloidal gold solution, and 35 min later, 1 mL of 10% (w/v) BSA was added, and the mixture stirred for 2 h. The product was centrifuged for 45 min at 8000 rpm to remove residual gold aggregates. The solution was then separated into two layers, and the lower layer (red gold-labelled mAb) was collected and washed with 0.02 M phosphate buffer (pH 7.4) containing 5% sucrose, 1% BSA, and 0.5% polyethylene glycol (PEG) 6000. The conjugation product was reconstituted to 1 mL in gold-labelling resuspension buffer (0.02 M PBS, 5% sucrose, 2% sorbitol, 1% mannitol, 0.1% PEG, 0.1% Tween, and 0.04% NaN3) and stored at 4°C until use.
Preparation of NC capture membranes
The coating antigen (PRC-BSA) and goat anti-mouse IgG antibody were sprayed onto the NC membrane at a concentrations of 1 µL/cm using a dispenser, resulting in the formation of the test and control lines. The capture and control reagents were sprayed onto the glass fibre membrane to prepare the conjugate pad, which was dried at 37°C for 2 h. The NC membrane containing the capture reagents was pasted onto the centre of the plastic backing plate (PVC) and the conjugate pad, sample pad, and absorbent pad were laminated and pasted onto the plate. Finally, the plate was cut into strips (2.8 mm wide) with a strip cutter.
Test procedure and principle
The gold-labelled mAb (50 µL) was mixed with 150 µL of sample solution, allowed to react for 5 min, and then applied to the sample pad. The solution migrated along the absorbent pad and the test result was obtained within 5 min. PRC, if present in the sample, would compete with the PRC-BSA conjugates embedded in the test line, for the finite amount of anti-PRC mAb added to the sample. When a detectable amount of PRC was present in the sample, the free PRC bound all of the gold-labelled mAb, preventing the mAb binding to the PRC-BSA in the test line. Therefore, the larger the amount of PRC present in the sample, the weaker the colour of the test line. If no PRC present, the limited amount of colloidal-gold-labelled mAb would be trapped by the immobilized PRC-BSA conjugate, and a clearly visible red test line would appear.
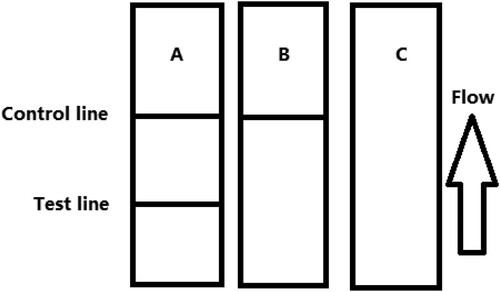
When the sample flow reached the control line, which contained the goat anti-mouse IgG antibody, it produced a detectable reaction. Therefore, the control line will always appear in a successful test, whereas the test line will only appear when the sample is negative ((A)). The appearance of the control line alone is a positive result ((B)), whereas if no lines appear, the test procedure was performed incorrectly, or the strip was invalid, indicating that the test should be repeated with a new strip ((C)).
Determination of performance
Sensitivity of the test strip
The sensitivity of the test strip was determined by assaying PRC samples against a PRC standard curve. First, PRC standards were diluted to the following concentrations, 0, 0.05, 0.1, 0.25, 0.5, and 1 ng/mL in 0.01 M PBS (pH7.4) and the detection limits determined. The sample solution (150 µL) was mixed with gold-labelled mAb (50 µL), allowed to react for 5 min, and then added to the sample pad. After a further 5 min, the colour intensities of the different strips were recorded with a test strip reader. The lowest detection limit (LDL) using the naked eye, was defined as the amount of PRC which could produce a visibly different colour reaction between the positive samples and the negative samples on the strip. Six replicate samples for each concentration were analysed on the same day.
Detection of PRC in milk
The detection method for PRC in milk was developed by using spiked samples. The spiked samples were prepared by adding PRC standard concentrations to the negative milk sample at final concentrations of 0.05, 0.1, 0.25, 0.5, and 1 ng/mL, respectively. The PRC standard solutions at a concentration of 10 µg/mL were prepared with, 0.01 M PBS (pH 7.4). A non-spiked sample (blank) was used as the control. Six replicates of each concentration were analysed with the test strips.
Results and discussion
Optimization of the test strip
The sensitivity of the immunochromatography strip is mainly affected by the concentration of the coating antigen and colloidal-gold-labelled mAb. The coating antigen was synthesized in our laboratory and its concentration optimized. Strips with different coating antigen concentrations (0.5, 1, or 2 mg/mL) on the test line were evaluated with 0.01 M PBS (pH 7.4) containing either 0 or 5 ng/mL PRC. The coating antigen concentration resulted in colour development in both the control and test lines. There were significant differences in colour intensity when 0.5, 1, or 2 mg/mL coating antigen was used (). Specifically, 1 and 2 mg/mL coating antigen have deeper colour intensity than 0.5 mg/mL coating antigen. No colour was found on the test line when using a concentration of 0.5 mg/mL coating antigen and a concentration of 5 ng/mL PRC standard. Therefore, 0.5 mg/mL coating antigen was selected for the preparation of test line on the LFIC strip.
Figure 5. Optimization of the immunochromatographic strip. Concentration of coating antigen (A) 0.5 mg/mL; (B) 1 mg/mL; (C) 2 mg/mL. The amount of the mAb that add in GNP: (a) 10 µg/mL; (b) 8 µg/mL. The standard concentration: (1) 0 ng/mL; (2) 5 ng/mL.
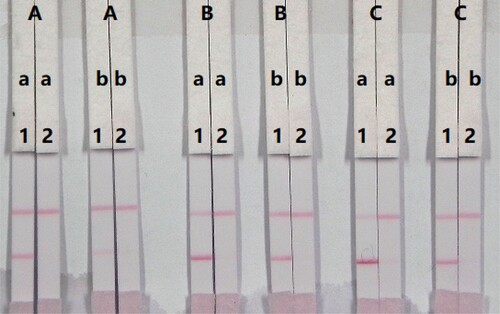
The concentration of mAb added to gold nanoparticle affects the sensitivity of the LFIC strip. A reaction system (50 µL) containing different concentrations of mAb (8 or 10 µg/mL) was allowed to react with PRC-negative (0 ng/mL) and PRC-positive (5 ng/mL) samples. Based on , there were significant differences in colour intensity between 8 and 10 µg/mL. We observed that the optimum concentration of mAb in gold nanoparticle was 10 µg/mL of colloidal gold because it contributed to strong colour intensities on both lines with the negative sample and more sensitive detection with the positive sample. Therefore, the optimal system for our developed immunochromatographic test strip consisted of 0.5 mg/mL coating antigen and 10 µg/mL of mAb in gold nanoparticle.
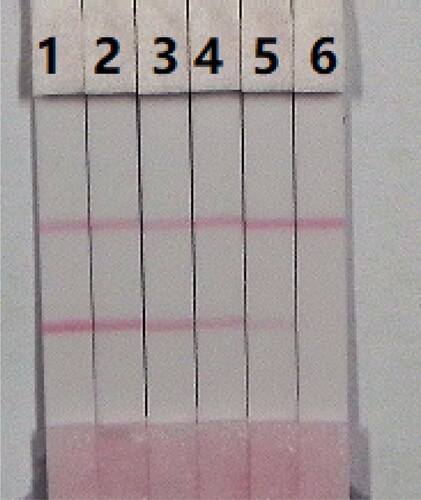
The assay sensitivity was determined using a series of diluted PRC standards. The LDL using the naked eye, was set as the amount of PRC that could produce a clearly visible change in the colour intensity of the test strip, when compared to the negative control sample. As shown in , the signal colour on the test line changed from strong to weak and disappeared completely at 10 ng/mL of PRC in 0.01 M PBS (pH 7.4). The assay was determined by testing PRC at concentrations of 0, 0.05, 0.1, 0.25, 0.5, and 1 ng/mL in 0.01 M PBS (pH 7.4). Strong colour was observed on the test line when testing the spiked sample using concentration of 0–0.5 ng/mL of PRC. However, it disappeared completely when the concentration of PRC reached 1 ng/mL.
Detection of PRC in milk
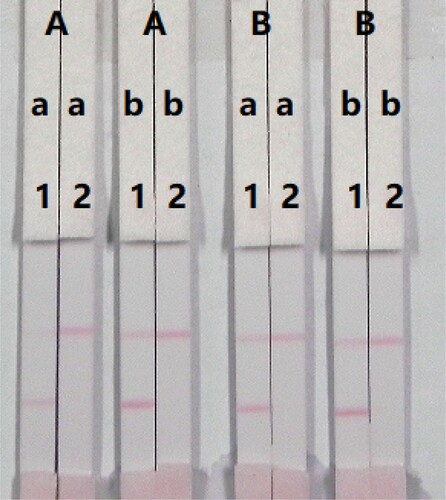
The concentration of mAb in the gold nanoparticles and coating antigen affected the sensitivity of the assay. Two different concentrations of mAb (8 or 10 µg/mL) were allowed to react with PRC-negative samples (0 ng/mL) and PRC-positive samples (1 ng/mL). The difference in the colour intensity produced with 0.5 and 1 mg/mL coating antigen was significant. The colour intensity of the test line with 0.5 mg/mL coating antigen was weaker than with 1 mg/mL coating antigen, suggesting that a greater sensitivity of detection could be achieved at 1 mg/mL coating antigen. As shown in , the optimum conditions for the detection of PRC in the milk samples were 1 mg/mL coating antigen and 10 µg/mL mAb in the gold nanoparticles.
Figure 7. Optimization of the immunochromatographic strip for PRC in milk sample. Concentration of coating antigen (A) 0.5 mg/mL; (B) 1 mg/mL. The ammount of the mAb that add in GNP: (a) 8 µg/mL; (b) 10 µg/mL. The standard concentration: (1) 0 ng/mL; (2) 1 ng/mL.
To evaluate the feasibility of our developed method, we measured PRC in milk samples. Two of the major advantages of the immunochromatographic test strip assay are its rapidity and ease of use. The milk samples were spiked with 10 µg/mL of the PRC standard, prepared with 0.01 M PBS (pH 7.4). The final concentrations of PRC in the milk samples were 0.05, 0.1, 0.25, 0.5, and 1 ng/mL. Under optimal conditions (), the colour intensity decreased as the PRC concentration increased. The signal colour changed from strong to weak, and disappeared completely at 1 ng/mL for both samples.
Conclusions
A simple, sensitive, and rapid analytical method for the determination of PRC was developed using an LFIC assay. Under optimal conditions, the cut-off detection limit for PRC using the semi-quantitative test strip was as low as 1 ng/mL in both the 0.01 M PBS (pH 7.4) and milk samples. All results were obtained within 5 min.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Babic, S., Pavlovic, D. M., Aperger, D., Perisa, M., Zrncic, M., Horvat, A. J. M., & Kastelan-Macan, M. (2010). Determination of multi-class pharmaceuticals in wastewater by liquid chromatography-tandem mass spectrometry (LC-MS-MS). Analytical and Bioanalytical Chemistry, 398(3), 1185–1194. doi: https://doi.org/10.1007/s00216-010-4004-1
- Baniceru, M., Croitoru, O., & Popescu, S. M. (2004). Determination of some local anesthetics in human serum by gas chromatography with solid-phase extraction. Journal of Pharmaceutical and Biomedical Analysis, 35(3), 593–598. doi: https://doi.org/10.1016/j.jpba.2004.02.012
- Deng, X., Liu, L., Ma, W., Xu, C., Wang, L., & Kuang, H. (2012). Development and validation of a sandwich ELISA for quantification of peanut agglutinin (PNA) in foods. Food and Agricultural Immunology, 23(3), 265–272. doi: https://doi.org/10.1080/09540105.2011.617358
- Dhananjeyan, M. R., Bykowski, C., Trendel, J. A., Sarver, J. G., Ando, H., & Erhardt, P. W. (2007). Simultaneous determination of procaine and para-aminobenzoic acid by LC-MS/MS method. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences, 847(2), 224–230. doi: https://doi.org/10.1016/j.jchromb.2006.10.004
- Fijalek, Z., Baczynski, E., Piwonska, A., & Warowna-Grzeskiewicz, M. (2005). Determination of local anaesthetics and their impurities in pharmaceutical preparations using HPLC method with amperometric detection. Journal of Pharmaceutical and Biomedical Analysis, 37(5), 913–918. doi: https://doi.org/10.1016/j.jpba.2004.09.025
- Ge, W., Suryoprabowo, S., Zheng, Q., & Kuang, H. (2017). Development of an immunochromatographic test strip for the detection of papaverine in pure ginger powder. Food and Agricultural Immunology, 28(6), 1304–1314.
- Guan, X. W., Li, X. Y., Chai, S. G., Zhang, X. H., Zou, Q. C., & Zhang, J. Z. (2016). A sensitive electrochemical sensor based on solution polymerized molecularly imprinted polymers for procaine detection. Electroanalysis, 28(9), 2007–2015. doi: https://doi.org/10.1002/elan.201600007
- He, Y. T., Peng, J. D., Tang, J. X., & Zhang, C. (2013). Incorporation of high performance liquid chromatography with resonance Rayleigh scattering detection for determination of procaine and lidocaine in human plasma. Analytical Methods, 5(24), 7110–7116. doi: https://doi.org/10.1039/c3ay40725j
- Jiang, W., Zeng, L., Liu, L., Song, S., & Kuang, H. (2018). Immunochromatographic strip for rapid detection of phenylethanolamine A. Food and Agricultural Immunology, 29(1), 182–192. doi: https://doi.org/10.1080/09540105.2017.1364709
- Kong, D., Xie, Z., Liu, L., Song, S., Kuang, H., Cui, G., & Xu, C. (2017). Development of indirect competitive ELISA and lateral-flow immunochromatographic assay strip for the detection of sterigmatocystin in cereal products. Food and Agricultural Immunology, 28(2), 260–273. doi: https://doi.org/10.1080/09540105.2016.1263985
- Kuang, H., Xing, C., Hao, C., Liu, L., Wang, L., & Xu, C. (2013). Rapid and highly sensitive detection of lead ions in drinking water based on a strip immunosensor. Sensors, 13(4), 4214–4224. doi: https://doi.org/10.3390/s130404214
- Lee, R. M., & Livett, B. H. (1967). A method for the colorimetric estimation of local anaesthetics containing an ester link, and its use in the determination of esterase activity. Biochemical Pharmacology, 16(9), 1757–1765. doi: https://doi.org/10.1016/0006-2952(67)90251-1
- Liu, A.-L., Wang, J.-D., Chen, W., Xia, X.-H., Chen, Y.-Z., & Lin, X.-H. (2012). Simultaneous and sensitive determination of procaine and its metabolite for pharmaceutical quality control and pharmacokinetic research by using a graphite paste electrode. Journal of Solid State Electrochemistry, 16(4), 1343–1351. doi: https://doi.org/10.1007/s10008-011-1517-2
- Li, Y., Liu, L., Song, S., & Kuang, H. (2018). Development of a gold nanoparticle immunochromatographic assay for the on-site analysis of 6-benzylaminopurine residues in bean sprouts. Food and Agricultural Immunology, 29(1), 14–26. doi: https://doi.org/10.1080/09540105.2017.1354359
- Liu, L. D., Liu, Y., Wang, H. Y., Sun, Y., Ma, L., & Tang, B. (2000). Use of p-dimethylaminobenzalhyde as a colored reagent for determination of procaine hydrochloride by spectrophotometry. Talanta, 52(6), 991–999. doi: https://doi.org/10.1016/S0039-9140(00)00470-7
- Liu, D., Peng, J. D., Liu, S. P., Zhou, M. Q., Zhang, J., & Li, A. P. (2017). Resonance Rayleigh scattering technique as a detection method for the RP-HPLC determination of local anaesthetics in human urine. Luminescence, 32(1), 4–10. doi: https://doi.org/10.1002/bio.3140
- Liu, L., Suryoprabowo, S., Zheng, Q., Song, S., & Kuang, H. (2017). Rapid detection of aldicarb in cucumber with an immunochromatographic test strip. Food and Agricultural Immunology, 28(3), 427–438. doi: https://doi.org/10.1080/09540105.2017.1293015
- Lu, Y. (2012). Content determination of procaine in procaine hydrochloride for injection by HPLC. China Pharmacy, 23(4), 369–371.
- Mao, X., Baloda, M., Gurung, A. S., Lin, Y., & Liu, G. (2008). Multiplex electrochemical immunoassay using gold nanoparticle probes and immunochromatographic strips. Electrochemistry Communications, 10(10), 1636–1640. doi: https://doi.org/10.1016/j.elecom.2008.08.032
- Ohshima, T., & Takayasu, T. (1999). Simultaneous determination of local anesthetics including ester-type anesthetics in human plasma and urine by gas chromatography mass spectrometry with solid-phase extraction. Journal of Chromatography B, 726(1–2), 185–194. doi: https://doi.org/10.1016/S0378-4347(98)00510-6
- Salama, R. B., & Omer, A. I. (1980). Colorimetric determination of procaine hydrochloride in pharmaceutical preparations. Journal of Pharmaceutical Sciences, 69(3), 346–348. doi: https://doi.org/10.1002/jps.2600690326
- Silva, T. G., de Araujo, W. R., Munoz, R. A. A., Richter, E. M., Santana, M. H. P., Coltro, W. K. T., & Paixao, T. R. L. C. (2016). Simple and sensitive paper-based device coupling electrochemical sample pretreatment and colorimetric detection. Analytical Chemistry, 88(10), 5145–5151. doi: https://doi.org/10.1021/acs.analchem.6b00072
- Song, S., Zou, S., Zhu, J., Liu, L., & Kuang, H. (2018). Immunochromatographic paper sensor for ultrasensitive colorimetric detection of cadmium. Food and Agricultural Immunology, 29(1), 3–13. doi: https://doi.org/10.1080/09540105.2017.1354358
- Suryoprabowo, S., Liu, L. Q., Peng, J., Kuang, H., & Xu, C. L. (2014). Development of a broad specific monoclonal antibody for fluoroquinolone analysis. Food Analytical Methods, 7(10), 2163–2168. doi: https://doi.org/10.1007/s12161-014-9863-1
- Taranova, N. A., Berlina, A. N., Zherdev, A. V., & Dzantiev, B. B. (2015). 'Traffic light' immunochromatographic test based on multicolor quantum dots for the simultaneous detection of several antibiotics in milk. Biosensors & Bioelectronics, 63, 255–261. doi: https://doi.org/10.1016/j.bios.2014.07.049
- Wang, X., Liu, F., Shao, Q., Yin, Z., Wang, L., & Fu, Z. (2017). A novel chemiluminescent immunochromatographic assay strip for rapid detection of mercury ions. Analytical Methods, 9(16), 2401–2406. doi: https://doi.org/10.1039/C7AY00231A
- Wang, Z., Zheng, Q., Guo, L., Suryoprabowo, S., Liu, L., & Kuang, H. (2017). Preparation of an anti-dexamethasone monoclonal antibody and its use in development of a colloidal gold immunoassay. Food and Agricultural Immunology, 28(6), 958–968.
- Weller, M. G. (2000). Immunochromatographic techniques - a critical review. Fresenius Journal of Analytical Chemistry, 366(6-7), 635–645. doi: https://doi.org/10.1007/s002160051558
- Xiao, Q., & Lin, J.-M. (2015). Advances and applications of chemiluminescence immunoassay in clinical diagnosis and foods safety. Chinese Journal of Analytical Chemistry, 43(6), 929–938. doi: https://doi.org/10.1016/S1872-2040(15)60831-3
- Zeng, L., Jiang, W., Liu, L., Song, S., & Kuang, H. (2018). development of IC-ELISA and lateral-flow immunochromatographic strip for detection of vitamin B-2 in an energy drink and vitamin tablets. Food and Agricultural Immunology, 29(1), 121–132. doi: https://doi.org/10.1080/09540105.2017.1360257