?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study outlines the successful development and testing of an immunochromatographic test strip for the detection of the anticoccidial drug, nicarbazin in milk. The preparation and characterization of an anti-4,4′-dinitrocarbanilide monoclonal antibody was based on hapten re-4,4′-dinitrocarbanilide. The monoclonal antibody was highly specific for 4, 4′-dinitrocarbanilide, with a half-life inhibitory concentration (IC50) of 3.46 ng/mL. The detection limit was 0.86 ng/mL. The linear range was 1.71–7 ng/mL. The cut-off values of colloidal gold-based immunochromatographic test paper in phosphate buffered saline (PBS) and in milk were 10 ng/mL, 100 ng/mL, respectively. These results were in good agreement with ELISA analyses and suggest that immunochromatographic test strips are a fast and sensitive method for the semi-quantitative and qualitative analysis of nicarbazin in milk.
Introduction
Coccidiosis is a parasitic disease causing intestinal damage in poultry and is of primary concern to American commercial poultry farmers. The disease leads to serious losses in the poultry, meat, and egg production sectors (Chapman, Jeffers, & Williams, Citation2010), and it is very common for poultry mortality to exceed 20%. Coccidiosis is caused by the unicellular parasite Eimeria, which has a life cycle of approximately 4–7 days (Pellerdy, Citation1974). When active Eimeria oocysts are swallowed by poultry (chickens), coccidia become implanted in the gut and chimeric cells multiply between intestinal cells, seriously damaging surrounding tissue.
Current anticoccidial drugs are classified into three categories, depending on the source, chemical structure, and production process. One is a polyether ionophore antibiotic, the other is chemical synthesis, and the third is Chinese herbal medicine. Antibiotics mainly include include monensin, lasalocid, salinomycin, naslamycin, maduramycin, and so on, it has a wide anti-coccidial spectrum, there is cross-resistance and additive effect between these drugs, but no synerg, Chemicals mainly include sulfonamides, clopids, chlorpheniramine, amprolium, nicarbazin, and benzophenone. The use of chemical drugs and antibiotics can prolong the service life of some chemical drugs, such as nicarbazine. Traditional Chinese medicine preparations mainly include Artemisia annua, Dust Spike Net, Wucao Tang, etc. Most of these drugs are extracted from Chinese herbal medicines. (Chapman et al., Citation2010; Peek & Landman, Citation2011). Nicarbazin is the earliest internationally developed anticoccidial drug and is chemically synthesized. It comes from the anticoccidial drug family, which has several key, but variable characteristics; they are wide ranging in terms of activity and they are highly potent, but they have a greater potential for drug resistance, they produce limited immunity, they are sometimes toxic, and they are usually more expensive than ionophores. Nicarbazin has the advantages of being a wide spectrum, highly efficient, and slow resistance drug. However, the drug leads to anorexia in animals, and is used under high temperature and high humidity conditions, causing chickens to overheat. The resultant stress sensitivity is toxic to laying hens. In addition, the extensive use of veterinary drugs can lead to different levels of drug residue in animal-derived foods, causing harm to human health (Matus & Boison, Citation2016).
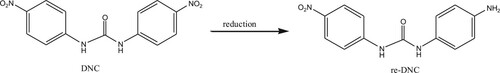
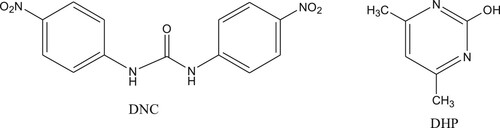
Nicarbazin, also known as nitrouracil, comprises two separate molecules; dinitrodiphenyl urea (DNC) and hydroxy dimethyl pyrimidine (HDP). DNC plays a major role as an insect repellent (Matabudul, Crosby, & Sumar, Citation1999) (). When used in the ratio 1:1, DNC:HDP is highly effective, therefore, these molecules compounds are usually used together. After administration, HDP is absorbed and eliminated faster, so is the main determination of DNC residues (Beier & Stanker, Citation2001).
Figure 1. Nicarbazin, complex of 4,4′-dinitrocarbanilide(DNC) and 2-hydroxy-4,6-dimethylpyrimidine (DHP).
In recent years, the U.S. Food and Drug Administration (FDA) announced a limitation on the use of nicarbazin in imported animal-derived foods.(Nász, Károlyné, Rikker, & Eke, Citation2012) Following the United States, China, Japan, New Zealand, Belgium, and other countries have proposed maximum limits for nicarbazin in animal-derived foods (Mortier, Daeseleire, & Van Peteghem, Citation2005; Ofclerk, Citation2015). In the United Kingdom, nicarbazin in eggs is limited to 0.1 mg/kg. The Japanese Ministry of Health, who oversee animal-derived food standards, stipulate the maximum nicarbazin residues in aquatic products as 0.02 mg/kg (Broekaert, Van Peteghem, Daeseleire, Sticker, & Van Poucke, Citation2011; Huet et al., Citation2005).
In recent years, several methods have been developed for the detection of anticoccidial drugs to include gas chromatography, capillary electrophoresis (Hong-Xiao, Tan, Shen, & Tian, Citation2013), high performance liquid chromatography (HPLC) (Holland, Munns, Roybal, Hurlbut, & Long, Citation1995; Hoshino, Horie, Nose, & Iwasaki, Citation2017), liquid chromatography/mass spectrometry (LC/MS)(Mortier, Daeseleire, Huyghebaert, Grijspeerdt, & Van Peteghem, Citation2005; Olejnik, Szprengier-Juszkiewicz, & Jedziniak, Citation2009)and the enzyme-linked immunosorbent assay (ELISA) (Wang et al., Citation2013). These methods, whether instrument based or ELISA based have several drawbacks; they require extensive time and professional labour inputs. In addition, instrument analyses are expensive and difficult, and accuracy can be low. Similarly, instrumentation approaches cannot be used for large sample screening and rapid on-site detection (Li, Liu, Song, & Kuang, Citation2018).
In contrast, the immunochromatographic test strip detection method is simple to operate, gives quick results (5–10 min), and it is portable and suitable for on-site rapid detection. In this study, we established a colloidal gold-based immunochromatographic test method to detect several anticoccidial drug residues in milk.
Materials and methods
Chemicals and materials
The chemicals, 4,4′-dinitrocarbanilide (DNC), halofuginone (HFG), moxidectin (MOX), Maduramicin (MD), metronidazole (MNZ), 4-aminophenylarsonicacid (ASA), and roxarsone (ROX) were purchased from J&K Scientific Ltd. (Beijing, China). The following chemicals were all obtained from Sigma-Aldrich (St Louis, MO, USA). bovine serum albumin (BSA; MW, 67000), ovalbumin (OVA; MW, 45000), CDI (N,N′-carbonyldiimidazole), Freund’s complete adjuvant (FCA), Freund’s incomplete adjuvant (FIA), 3,3′,5,5′-tetramethylbenzidine (TMB), and gold chloride trihydrate. RPMI-1640 cell culture medium, hypoxanthine aminopterin thymidine supplement (HAT), hypoxanthine thymidine supplement (HT), polyethylene glycol (PEG) 1500, and fetal calf serum were purchased from Gibco BRL (Paisley, UK). All reagents and other chemicals were analytical grade or higher. Other materials such as dodecyl polyglycol ether(Brij) for preparing test strips were obtained from JieYi Biotech. Co. Ltd. (Shanghai, China). Preparation of anti-DNC monoclonal cell line number 4A9.
Buffer solutions
The basic buffer was phosphate-buffered saline (PBS, 0.01 M, pH 7.4). The washing buffer (PBS-T) was made by adding 0.05% Tween-20 to PBS. The coating buffer was 0.05 M carbonate buffer (CBS, pH 9.6). The blocking buffer was prepared by adding 0.2% gelatin to CBS. Solution A comprised 9.33 g citric acid, 36.8 g Na2 HPO4, and 180 µL of 30% H2O2 per 1000 mL. Solution B was prepared by adding 0.06% (v/v) TMB in glycol. The substrate solution was made by adding solution A to B, in a ratio of 5:1 (v/v), and used at room temperature. Sulfuric acid (2 M) was used as a stop solution.
Preparation of the hapten and antigen
DNC was used as a marker of nicarbazin residue. When sodium sulfide is used as a reducing agent, with two nitro groups on the phenyl ring, one of the nitro groups can be selectively reduced to an amino group by controlling the reducing agent (Imamura, Hashimoto, & Kominami, Citation2012). The following procedure was performed: sodium sulfide (51.64 mg) and DNC (100 mg) were dissolved in methanol, stir at room temperature for 6 h to produce a brownish white precipitate which was filtered through a filter paper, and the precipitate was dried at 37°C. The product was confirmed by LC-MS analysis. One of the nitro groups of the DNC structure was reduced to an amino group and selected as a hapten (re-DNC) (). The hapten was conjugated with BSA, OVA by the diazotization method (DIA) (Guo et al., Citation2015; Kong, Xie, Liu, Song, & Kuang, Citation2017; Yu, Liu, Song, Kuang, & Xu, Citation2016). After this, 25 mg hapten was dissolved in 3 ml N,N-dimethylformamide and added to 0.2 mL 1M HCL. The solution was stirred gently at 4°C for 30 min, as 0.1 mL 30% sodium nitrite was added drop-wise. Activation at 4°C for 45 min. Finally, the activated solution was divided into two portions and added drop-wise to 65 mg BSA, and 50 mg OVA [each dissolved in 5 mL 0.1 M CB (pH 9.6)]. Solutions were stirred continuously for 4 h in an ice bath. The BSA conjugates were used to immunize BALB/c mice. The OVA conjugates were used as coating antigens. Once synthesized, all antigens were stored at −20°C. The final antigen products were characterized by UV/Visible spectroscopy (Bokin Instruments, Tsushima, Japan).
Monoclonal antibody (McAb) preparation
Female BALB/c mice (6–8 weeks) were chosen for experimentation (Zhang et al., Citation2013). The immunogen re-DNC-DIA-BSA(100 µg) was emulsified with FCA for the first immunization and subcutaneously injected into the backs of several mice. Fifty µg of the immunogen re-DNC-BSA was emulsified with FIA for booster immunizations after four weeks. Blood samples were collected 7–10 days after the third immunization and the titer and inhibition of each mouse were measured by ELISA. After four immunizations, high titer, good suppression positive BALB/c mice were chosen for cell fusion (Guo et al., Citation2015; Kong et al., Citation2017). Spleen cells from positive mice were fused with SP2/0 cells (mouse myeloma cells)by the hybridoma technique, and the cells were cultured in a medium prepared with RPMI-1640 in a 96-well plate. One week later, an indirect competitive (IC)-ELISA was performed, and high titer, good suppression effective wells were selected for serial dilution, cloning and re-cloning. The chosen cell line was expanded for culture and injected into BALB/c mice to produce ascites monoclonal antibodies (Peng et al., Citation2017). Ascites were purified using the caprylic acid and ammonium sulfate precipitation method(Yin, Liu, Song, Kuang, & Xu, Citation2015). Monoclonal antibody concentrations were quantified using UV/visible spectroscopy (Bokin Instruments, Tsushima, Japan).
Characterization of McAbs
Indirect competitive ELISAs were employed to characterize McAb sensitivity and affinity. Sensitivity was evaluated by IC50, which is defined as the concentration of DNC required to inhibit 50% of the maximum absorbance, the linear range to detect DNC defined as the concentration of DNC toward from 20% to 80% inhibition of the control(IC20-IC80), and the limit of detection value (LOD), which is equivalent to IC10.
Monoclonal antibody specificity was determined by evaluating the cross-reactivity (CR) of analytes compared to DNC. The CR of the McAb against DNC, HFG, MOX, MD, MNZ, ASA and ROX was calculated according to the following equation:
Preparation of colloidal gold particles
The colloidal gold used in these experiments was synthesized using a previous method(Zhang et al., Citation2013). The particle size was approximately 20–30 nm (Guo et al., Citation2015). Specifically, 50 mL of 0.01% chloroauric acid was boiled for 30 min. Then, 2 mL of 1% trisodium citrate solution was added while stirring, causing the solution to turn wine-red within 1 min. The solution was heated and stirred for a further 15 min, cooled to room temperature, and stored at 4°C for later use.
Labelling McAbs with colloidal gold particles
Purified antibodies were labelled. Briefly, 1 mL colloidal gold solution (adjusted to pH 8.8 with 0.1 mol/L K2CO3) was added to 8 μg antibody, diluted in PBS. The solution was mixed at room temperature for 45 min and free colloidal gold was blocked with 1 mL 10% aqueous BSA solution and incubated for 1 h at room temperature. After this, the solution was centrifuged for 30 min at 20000 rpm, at 4°C (D-37520 Osterode Made in Germany). Finally, the pellet was resuspended in 20 mmol/L Tris (pH 8.2), 0.1% Tween, 0.1% PEG, 5% trehalose, 5% sucrose, 5% Brij and 0.2% BSA) and stored at 4°C (Peng, Song, Liu, Kuang, & Xu, Citation2015; Xu et al., Citation2016).
Composition of immunochromatographic strips
Immunochromatographic strips consisted of a polyvinyl chloride (PVC) backing card, a nitrocellulose (NC) membrane, a sample pad and an absorbent pad. The NC membrane, with a test and control line was affixed to a PVC backing plate. Test and control lines were drawn by coated antigen and goat anti-mouse IgG, respectively. The test line was near one end of the sample pad and the control line was near one end of the absorbent pad. The sample pad was previously saturated in 0.01 M PBS, containing 1% BSA, 1% sucrose, 0.2% Tween-20 and dried at room temperature for 3 h. The absorbent pad was made of cotton linter filter paper.
The principle and test procedure of immunochromatographic strips
The sample solution and gold-labelled McAb were pre-mixed and allowed to react for 5 min. Then, the strip was inserted into the solution, allowing the solution to spread to the test strip. If the sample solution contained a test substance, the gold-labelled monoclonal antibody recognized this, and formed a complex. Due to competition, the coated antigen on the test line did not bind to the complex, and when it spread to the control line, goat anti-mouse IgG recognized the gold-labelled McAb test substance, and the control line showed red. In contrast, for negative samples, test and control lines showed red bands and the coating antigen on the test line recognized a gold-labelled monoclonal antibody.
As mentioned above, the analysis test procedure is simple. First, approximately 50 μL of the gold-labelled monoclonal antibody was mixed with 150 μL of the standard solution or sample extract for 5 min at room temperature. Then, the test strip was inserted into the mixed solution and the results were produced in 7–8 min.
Analysis of DNC in milk samples using immunochromatographic strips
Milk samples used for immunochromatographic strip analysis were negative, a series of DNC spiked milk samples were positive. Milk purchased from a local market was negative, as shown by LC/MS.
Results and discussion
Antigen characterization
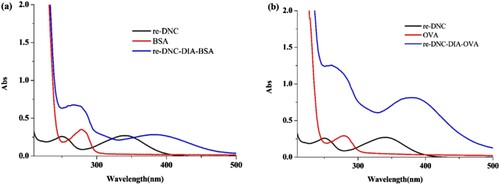
In general, small molecules are not immunogenic, for this they need to be coupled to carrier proteins e.g. BSA, OVA. The immunogen re-DNC was coupled to BSA, and the carrier protein OVA was used as a coating.
From , BSA and OVA is shown to have absorption peaks near 280 nm, and re-DNC had absorption peaks at 250 nm and 350 nm. Re-DNC-DIA-BSA/OVA clearly generated small molecule peaks and characteristic peaks from 350 nm to 400 nm in the diazotization method, indicating the small molecule was successfully coupled.
Characterization of the McAb
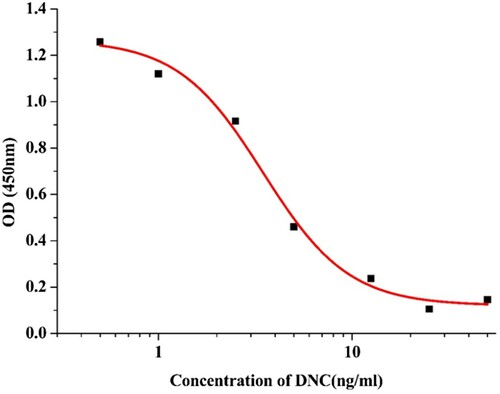
The anti-DNC monoclonal antibody 4A9 was purified by the caprylic acid ammonium sulfate precipitation method. shows that 4A9 was highly specific for DNC, and the cross-reactivity (CR) test of DNC analogues was <1%. As shown in , a standard curve for DNC detection was established. The equation for 4A9 was y = 0.12025 + 1.14671 / (1 + (x / 3.46954) 1.97267) and the correlation coefficient, R2 was 0.9907. From the above equation, the IC10, IC20, IC50, and IC80 against DNC were 0.859, 1.718, 3.469, and 7.006 ng/mL, respectively. The linear range of DNC detection was 1.718–7.006 ng/mL, and the LOD was 0.859 ng/mL. The above data indicates that the antibody has strong specificity for further experiments.
Table 1. Cross-reactivities of anti-dinitrocarbanilide monoclonal antibody with various coccidiostats.
Establishment of the immunochromatographic assay
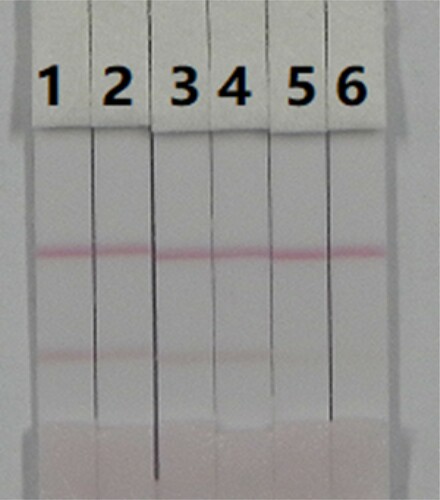
A series of DNC standard solutions in PBS were prepared and incubated with colloidal gold-labelled monoclonal antibodies for 5–7 min to ensure antibody recognition of small molecule DNC reaction time. The cut-off value was the concentration of the standard solution when only the control line appeared on the test strip. As the concentration of the standard solution increased, the colour of the test line gradually became lighter, until the colour disappeared. Therefore, the cut-off value was 10 ng/mL ().
Using immunochromatographic strips in milk samples
A series of concentrations of DNC standard solution were added to the blank milk sample for analysis using immunochromatographic strips. As shown in , the cut-off was 100 ng/mL. Therefore, this analytical approach can be used to detect anticoccidial drug residues in milk samples. It must be noted that complex components in milk samples can reduce antibody recognition efficiencies, thereby affecting the final cut-off value of test strips.
Analysis of coccidiostats in milk samples
To verify the accuracy of immunochromatographic test strips, an IC-ELISA assay was performed. As shown in , the DNC recovery was 99%–100%. The coefficient of variation (CV) was 7 × 10−5–2.9 × 10−2. The detection of DNC in milk samples by IC-ELISA was consistent with immunochromatographic strips, demonstrating these strips are a quick and easy method to detect anticoccidial drugs in milk samples.
Table 2. The DNC in the milk sample was analyzed by ic-ELISA and immunochromatographic strips (n = 3).
Conclusions
In this study, a DNC nitro group was reduced to an amino group and coupled to a protein (BSA ;OVA) using the diazotization method. Monoclonal antibodies were obtained by immunization and fusion. Highly sensitive monoclonal antibodies, labelled with colloidal gold, were successfully used for immunoassay strip detection of anticoccidial drugs in milk. In recovery experiments, the recovery rate was 99%–100%. The analytical results from test strips were consistent with IC-ELISA assays. Therefore, this immunochromatographic analytical method is a significant step forward in the detection of nicarbazin residues in milk samples.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Beier, R. C., & Stanker, L. H. (2001). An antigen based on molecular modeling resulted in the development of a monoclonal antibody-based immunoassay for the coccidiostat nicarbazin. Analytica Chimica Acta, 444(1), 61–67. doi: https://doi.org/10.1016/S0003-2670(01)01154-0
- Broekaert, N., Van Peteghem, C., Daeseleire, E., Sticker, D., & Van Poucke, C. (2011). Development and validation of an UPLC-MS/MS method for the determination of ionophoric and synthetic coccidiostats in vegetables. Analytical and Bioanalytical Chemistry, 401(10), 3335–3344. doi: https://doi.org/10.1007/s00216-011-5433-1
- Chapman, H. D., Jeffers, T. K., & Williams, R. B. (2010). Forty years of monensin for the control of coccidiosis in poultry. Poultry Science, 89(9), 1788–1801. doi: https://doi.org/10.3382/ps.2010-00931
- Guo, J., Liu, L., Xue, F., Xing, C., Song, S., Kuang, H., & Xu, C. (2015). Development of a monoclonal antibody-based immunochromatographic strip for cephalexin. Food and Agricultural Immunology, 26(2), 282–292. doi: https://doi.org/10.1080/09540105.2014.907242
- Guo, L., Song, S., Liu, L., Peng, J., Kuang, H., & Xu, C. (2015). Comparsion of an immunochromatographic strip with ELISA for simultaneous detection of thiamphenicol, florfenicol and chloramphenicol in food samples. Biomedical Chromatography, 29(9), 1432–1439. doi: https://doi.org/10.1002/bmc.3442
- Holland, D. C., Munns, R. K., Roybal, J. E., Hurlbut, J. A., & Long, A. R. (1995). Liquid chromatographic determination of the anticoccidial drug halofuginone hydrobromide in eggs. Journal of Aoac International, 78(1), 37.
- Hong-Xiao, S. U., Tan, H. R., Shen, H. Q., & Tian, C. Q. (2013). Simultaneous separation and determination of four anticoccidial drugs in soil by high performance capillary electrophoresis. Journal of Instrumental Analysis, 32(2), 156–161.
- Hoshino, Y., Horie, M., Nose, N., & Iwasaki, H. (2017). Determination of monensin in chicken meat by high performance liquid chromatography. Journal of the Food Hygienic Society of Japan, 26(6), 585–590_ 581. doi: https://doi.org/10.3358/shokueishi.26.585
- Huet, A. C., Mortier, L., Daeseleire, E., Fodey, T., Elliott, C., & Delahaut, P. (2005). Screening for the coccidiostats halofuginone and nicarbazin in egg and chicken muscle: Development of an ELISA. Food Additives & Contaminants, 22(2), 128–134. doi: https://doi.org/10.1080/02652030500038041
- Imamura, K., Hashimoto, K., & Kominami, H. (2012). Chemoselective reduction of nitrobenzenes to aminobenzenes having reducible groups by a titanium(IV) oxide photocatalyst under gas- and metal-free conditions. Cheminform, 48(36), 4356–4358.
- Kong, D., Xie, Z., Liu, L., Song, S., & Kuang, H. (2017). Development of ic-ELISA and lateral-flow immunochromatographic assay strip for the detection of citrinin in cereals. Food and Agricultural Immunology, 28(3), 414–426. doi: https://doi.org/10.1080/09540105.2017.1293014
- Li, Y., Liu, L., Song, S., & Kuang, H. (2018). Development of a gold nanoparticle immunochromatographic assay for the on-site analysis of 6-benzylaminopurine residues in bean sprouts. Food and Agricultural Immunology, 29(1), 14–26. doi:https://doi.org/10.1080/09540105.2017.1354359
- Matabudul, D. K., Crosby, N. T., & Sumar, S. (1999). A new and rapid method for the determination of nicarbazin residues in poultry feed, eggs and muscle tissue using supercritical fluid extraction and high performance liquid chromatography. The Analyst, 124(4), 499–502. doi: https://doi.org/10.1039/a808327d
- Matus, J. L., & Boison, J. O. (2016). A multi-residue method for 17 anticoccidial drugs and ractopamine in animal tissues by liquid chromatography-tandem mass spectrometry and time-of-flight mass spectrometry. Drug Testing and Analysis, 8(5–6), 465–476. doi: https://doi.org/10.1002/dta.2019
- Mortier, L., Daeseleire, E., Huyghebaert, G., Grijspeerdt, K., & Van Peteghem, C. (2005). Detection of residues of the coccidiostat diclazuril in poultry tissues by liquid chromatography-tandem mass spectrometry after withdrawal of medicated feed. Journal of Agricultural and Food Chemistry, 53(4), 905–911. doi: https://doi.org/10.1021/jf048468z
- Mortier, L., Daeseleire, E., & Van Peteghem, C. (2005). Liquid chromatographic tandem mass spectrometric determination of five coccidiostats in poultry eggs and feed. Journal of Chromatography B, 820(2), 261–270. doi: https://doi.org/10.1016/j.jchromb.2005.04.016
- Nász, S., Károlyné, E. M., Rikker, T., & Eke, Z. (2012). Determination of coccidiostats in milk products by LC–MS and its application to a fermentation experiment. Chromatographia, 75(11–12), 645–653. doi: https://doi.org/10.1007/s10337-012-2236-2
- Ofclerk, O. (2015). Food Act 1981, New Zealand (Maximum Residue Limits of Agricultural Compounds Food Standards 2015 pursuant to sections 11C and 11L of the said Act, dated 20 February 2015.
- Olejnik, M., Szprengier-Juszkiewicz, T., & Jedziniak, P. (2009). Multi-residue confirmatory method for the determination of twelve coccidiostats in chicken liver using liquid chromatography tandem mass spectrometry. Journal of Chromatography A, 1216(46), 8141–8148. doi: https://doi.org/10.1016/j.chroma.2009.04.097
- Peek, H. W., & Landman, W. J. M. (2011). Coccidiosis in poultry: Anticoccidial products, vaccines and other prevention strategies. Veterinary Quarterly, 31(3), 143–161. doi: https://doi.org/10.1080/01652176.2011.605247
- Pellerdy, L. P. (1974). Coccidia and coccidiosis. Transactions of the American Microscopical Society, 85(2), 325.
- Peng, J., Liu, L., Xu, L., Song, S., Kuang, H., Cui, G., & Xu, C. (2017). Gold nanoparticle-based paper sensor for ultrasensitive and multiple detection of 32 (fluoro)quinolones by one monoclonal antibody. Nano Research, 10(1), 108–120. doi: https://doi.org/10.1007/s12274-016-1270-z
- Peng, J., Song, S., Liu, L., Kuang, H., & Xu, C. (2015). Development of sandwich ELISA and immunochromatographic strip for the detection of peanut allergen Ara h 2. Food Analytical Methods, 8(10), 2605–2611. doi: https://doi.org/10.1007/s12161-015-0163-1
- Wang, Y., Wang, Z., Jiang, H., Xia, X., Shen, J., & Ding, S. (2013). Development of a monoclonal antibody-based enzyme-linked immunosorbent assay for the analysis of diclazuril in chicken tissues. Food Analytical Methods, 6(6), 1685–1692. doi: https://doi.org/10.1007/s12161-013-9582-z
- Xu, L., Song, Y., Liu, L., Song, S., Zhu, J., Kuang, H., & Xu, C. (2016). Sandwich ELISA and immunochromatographic strip of Kunitz trypsin inhibitor using sensitive monoclonal antibodies. Food and Agricultural Immunology, 27(6), 772–782. doi: https://doi.org/10.1080/09540105.2016.1160367
- Yin, Y., Liu, L., Song, S., Kuang, H., & Xu, C. (2015). Development of a highly sensitive icELISA to detect semicarbazide based on a monoclonal antibody. Food and Agricultural Immunology, 26(3), 356–365. doi: https://doi.org/10.1080/09540105.2014.914891
- Yu, L., Liu, L., Song, S., Kuang, H., & Xu, C. (2016). Development of an immunochromatographic test strip and ic-ELISA for tetrabromobisphenol: A detection in lake water and rice pudding samples. Food and Agricultural Immunology, 27(4), 460–470. doi: https://doi.org/10.1080/09540105.2015.1126234
- Zhang, X., Feng, M., Liu, L., Xing, C., Kuang, H., Peng, C., … Xu, C. (2013). Detection of aflatoxins in tea samples based on a class-specific monoclonal antibody. International Journal of Food Science & Technology, 48(6), 1269–1274. doi: https://doi.org/10.1111/ijfs.12086