ABSTRACT
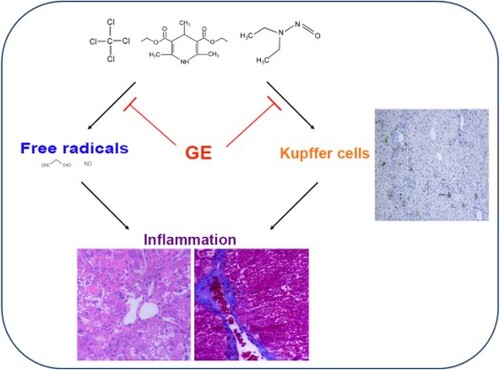
Glossy privet fruit and Ecliptae herba (GE) as formula was used as traditional Chinese medicine for liver diseases. However, systematic research of GE in liver injury has been explored rarely. Here, we comprehensively investigate the hepato-protective effects of the mixture of GE on different models. Our data indicated that GE administration decreased the levels of ALT and AST in serum compared with the model group. The histopathological changes were markedly alleviated in the GE treated groups. In addition, expression of cytokines including TNF-α, IL-6 and TGF-β in liver tissue associated with liver injury was elevated in the model groups, but significantly decreased after GE treatment. Furthermore, we explored that activation of Kupffer cells in liver tissue were suppressed after treatment with GE. Therefore, the major mechanism of the liver protective effects of GE may be attributed to the inhibition of Kupffer cell activation.
1. Introduction
Liver diseases were reported as one of the most serious health problems in the world (Williams, Citation2006), which were mainly caused by hepatitis virus infection, drugs and hepatic ischemia-reperfusion (Sun et al., Citation2011; Zhang, Pan, Jiang, & Mo, Citation2016). Liver diseases could destruct liver function and also cause a series of complications, such as diabetes, obesity, chronic kidney disease and cardiovascular disease (Chacko & Reinus, Citation2016). Liver diseases include acute and chronic liver disease, whereas chronic liver disease is a major cause of worldwide mortality and morbidity (Debray, Yousef, & Durand, Citation2006). Cirrhosis induced by chronic liver injury was considered to be at risk of a premalignant status during hepatocellular carcinoma development (Friedman, Citation2008; Lu, Chen, Ren, Yang, & Zhao, Citation2017; Schuppan & Afdhal, Citation2008), which is one of most frequent causes of death throughout the world (Siegel, Miller, & Jemal, Citation2016).
Traditional Chinese medicines, such as D. nipponica Makino (Yu et al., Citation2014), Quercetin (Ma, Li, Xie, Liu, & Liu, Citation2015), Echinacea (Zhai et al., Citation2007), Puerarin (Wang et al., Citation2016), Curcumin (Abdel-Wahhab et al., Citation2016), Eclipta (Chung et al., Citation2015), Glossy Privet Fruit (Wang, Hsu, & Yin, Citation2009), have been studied and reported to be effective on liver diseases. Among these herbs, Glossy privet fruit and Ecliptae herba constitute a famous traditional Chinese formulation Er-Zhi-Wan, which was firstly recorded in ‘Yi Bian’ in the Ming Dynasty of China (Yao et al., Citation2014). Glossy privet fruit and Ecliptae herba formula (GE) was reported to be effective in liver protection (Xu et al., Citation2012; Yao et al., Citation2014), however, the mechanism of GE in liver protection remains to discover.
In this study, mouse models of liver injury were induced to mimic different human liver disease process including acute liver injury, liver fibrosis, liver cirrhosis and hepatocellular carcinoma. We found that treatment with GE could reduce the expression of inflammatory cytokines and mitigate the histopathological changes of mouse liver. Besides, we discovered that the activation of Kupffer cells in liver tissue were suppressed in the GE treated groups. Furthermore, in vitro effects of GE decreasing inflammation were verified on RAW 264.7 cells stimulated by LPS. Therefore, we hypothesize that the protective effects may be attributed to the suppression of Kupffer cells activation.
2. Materials and methods
2.1. Materials
Ecliptae herba (Eclipta prostrata L., yerbadetajo herb) and Glossy privet fruit (Ligustrum lucidum Ait., Ligustri lucidi fructus) formula were obtained from Sichuan Neautus Traditional Chinese Medicine CO., LTD. The major component of GE formula is about Glossy privet fruit and Ecliptae herba by weight ratio (1:1) respectively. Paeonia lactiflora Pall. (Radix Paeoniae Alba, debark peony root), Corbicula fluminea (Asian Clam) and Curcuma longa L. (turmeric) were contained in the formula. Components of Glossy privet fruit and Ecliptae herba were detected according to the test items of ⟪Chinese pharmacopoeia (2015)⟫ (Supplementary Table 1). Carbon tetrachloride (CCl4) was purchased from Beijing Chemical Reagent Company (Beijing, China). Diethylnitrosamine (DEN) and 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) were purchased from TCI Development CO., Ltd. (Shanghai, China). The detection kits including superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), nitric oxide (NO) and malondialdehyde (MDA) were all purchased from Nanjing Jiancheng Institute of Biotechnology (Nanjing, China). Enhanced Bicinchoninic Acid (BCA) Protein Assay Kit was obtained from Beyotime Institute of Biotechnology (Shanghai, China). PCR kit was obtained from Takara (Takara Biotechnology, Shanghai, China).
2.2. Animals
Male C57BL/6J mice (5–7 weeks old, 20–25 g) were purchased from Hubei Center for Disease Control and Prevention, Wuhan, China (quality certification number: SCXK (E) 2015-0018). All animal studies were approved by the Animal Experimentation Ethics Committee of College of Life Science and Technology, Huazhong University of Science and Technology. The animal study was carried out in strict with guidelines approved by the Science and Technology Department of Hubei Province.
2.3. Animal treatment
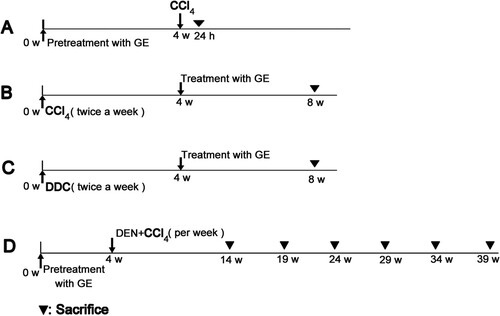
In the study, four different liver disease models were prepared. The mice in every model were randomly allocated into control group, model group, 600 mg/kg GE treated group and 1200 mg/kg GE treated group (n = 7). Twenty-four hours after last administration, the mice were sacrificed (). The sera were collected and stored at −20°C. Fresh liver tissues were stored at −80°C for experiments.
Scheme 1. The scheme of the experimental design. (A) Acute liver injury model; (B) Liver fibrosis model; (C) Liver cirrhosis model; (D) Hepatocellular carcinoma model.
2.3.1. Acute liver injury model
After pretreatment with GE for four weeks, the mice were injected intraperitoneally with 10 mL/kg CCl4 (0.2%, dissolved in olive oil) one time to induce acute liver injury model, whereas the animals in the control group received olive oil only (Scheme 1A).
2.3.2. Liver fibrosis model
The mice were injected intraperitoneally with 1 mL/kg CCl4 (10%, dissolved in olive oil) 2 times per week for 8 weeks, whereas the animals in the control group received olive oil only (i.p.). 4 weeks post first administration of CCl4, GE treatment groups were given at the dose of 600 or 1200 mg/kg orally for 4 weeks (Scheme 1B).
2.3.3. Liver cirrhosis model
The mice were administrated orally with 0.5 mg/kg DDC (10%, dissolved in olive oil) 2 times per week for 8 weeks, whereas the animals in the control group received olive oil only. 4 weeks post first administration of DDC, 600 or 1200 mg/kg dose were orally given in GE treatment groups for 4 weeks (Scheme 1C).
2.3.4. Hepatocellular carcinoma model
After GE pretreatment for 4 weeks, 165 mg/kg DEN (10%, dissolved in olive oil) and 1.5 mL/kg CCl4 (10%, dissolved in olive oil) were administrated once a week for 10 weeks. On day 3 after treatment of DEN, CCl4 was intraperitoneal injection. Twenty-four hours post the last injection, the mice (for first stage investigation) were sacrificed. The mice (2–6 stages) were then sacrificed every 5 weeks (Scheme 1D).
2.4. Biochemical assay
The levels of serum alanine transaminase (ALT) and aspartate transaminase (AST) were detected with automatic biochemical analyzer (Beckman, Germany). The contents of SOD, GSH-Px, NO and MDA in liver tissues were assayed based on the manufacturer’s instruction of the kits.
2.5. Histopathological examination
Pieces of liver tissues were fixed in 4% formaldehyde for 24 h. Paraffin sections (5μm) were prepared and subjected to haematoxylin and eosin staining for general morphological observations. Masson Trichrome staining was performed according to Wu’s report (Wu, Zhang, Yang, Hu, & Fallon, Citation2016). The images were captured using a light microscope (Nikon Eclipse TE2000-U, NIKON, Japan) and then photographed at 200× magnification.
2.6. Immunohistochemical staining of cytokines
Liver paraffin sections (5 μm) were deparaffinized and repaired using EDTA buffer and 3% H2O2. The slides were then incubated at 4°C overnight with antibody (1:100) against TNF-α (60291-1-Ig, Proteintech, China) and TGF-β (21898-1-AP, Proteintech, China), respectively. After that, the sections were incubated with HRP (DAKO, DK) for 50 min at room temperature. The final staining was developed using 3’,3’-diaminobenzidine (DAB) detection kits. The sections were washed in tap water and counterstained with haematoxylin, and the images were viewed and captured using the light microscope at 100× magnitude. Every immunostaining slide of liver sample was taken with a light microscope (Nikon Eclipse TE2000-U, NIKON, Japan). Three equal-sized fields of each section were randomly chosen for quantification. The number of positive cells and the integrated optical density (IOD) of positive areas were quantified by the software Image Pro Plus (Media Cybernetics, USA).
2.7. Expression of cytokines in liver tissue at the level of mRNA
Total RNA of the hepatic tissues was extracted using the RNAiso Plus reagent. The cDNA was synthesized according to the PrimeScript RT reagent Kit and the assay was performed using real-time PCR with SYBR Premix Ex TaqTM I and 7500 Real-Time PCR System (Applied Biosystems, USA). Sequences of the primers (Yu et al., Citation2014) used in the study were listed in Supplementary Table 2. The GAPDH gene was selected as the housekeeping gene in our study.
2.8. Cytokine assay in vitro
RAW 264.7 cells were obtained from BeNa Culture Collection (Beijing, China) and maintained in our lab. The cells were cultured in RPMI 1640 medium (HyClone) with 10% foetal bovine serum, 1% penicillin and streptomycin (Gibco). RAW 264.7 cells were incubated in 96 well culture plates and treat with GE (10 μg/mL). Cell supernatants from each well were collected after 24 h of incubation for cytokines analysis. Cytokines (IL-6 and TNF-α) in the supernatant were analysed through flow cytometer (BD Biosciences, USA) using Cytometry Bead Array Kit (BD, USA).
2.9 Western blot
Liver tissues were lysed in RIPA buffer containing protease inhibitors, and centrifuged at 12,000 g for 20 min at 4°C. After quantification with BCA kit (P0010, Beyotime, China), 10 μL protein of each group were electrophoresed on 12% SDS-acrylamide gels. Blots were incubated at 4 °C overnight with primary antibodies (1:1000) against GAPDH (60004-1-Ig, Proteintech, China), TNF-α (60291-1-Ig, Proteintech, China), TGF-β (21898-1-AP, Proteintech, China) and NF-κB (10745-1-AP, Proteintech, China), respectively. And then washed three times with TBST (0.5% Tween-20 in TBS) and incubated with the secondary antibody (A0208, A0216, 1:10000, Beyotime, China) for 2 h. After washed three times with TBST, the images were acquired with ChemiDoc XRS+ (BIO-RAD, USA).
2.10. Statistical analysis
All data were expressed as mean and standard error of mean (SEM). Statistical differences were determined by Student’s test or one-way ANOVA. All data analyses were conducted using the SPSS 22 statistical analysis software (SPSS Inc, Chicago, USA). In any case, p-value less than 0.05 was considered to be statistically significant.
3. Results
3.1. Levels of ALT and AST decreased in the GE treated groups
Evaluation of the status of liver injury in the mice was based on the analysis of serum ALT and AST activities (Zhang, Chen, Sun, & Zhao, Citation2014). As shown in (A), the levels of ALT and AST in the GE pretreated groups were lower than that in the model group. The similar results were presented in the other three models ((B–D). And the levels of ALT and AST in the GE (600 mg/kg) group were markedly decreased than that in the model group. However, without chemical damage from the second to fifth stages (Figure S1), the levels of ALT and AST had no significant change among all groups. Until the sixth stage, the ALT level in the model group was markedly increased than that in the control group. Therefore, the activities of ALT and AST were decreased after GE treatment, especially at the dose of 600 mg/kg.
Figure 1. Serum levels of ALT and AST in different models. (A) acute liver injury model, (B) liver fibrosis model, (C) liver cirrhosis model, (D) the first stage in hepatocellular carcinoma. All values are expressed as mean ± S.E.M. (n = 7). ## p < .01, vs. control group; ###p < .001 vs. control group; *p < .05, vs. model group.
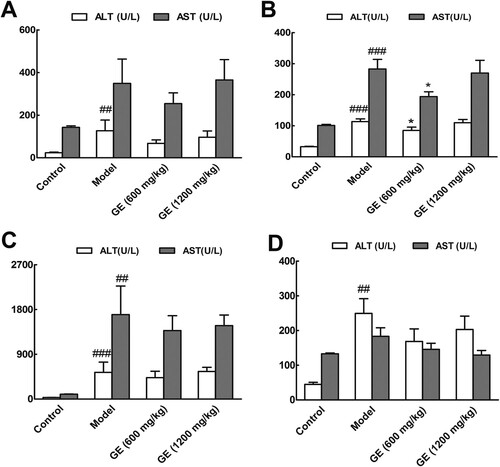
3.2. Histopathological changes alleviated in the liver of GE treated groups
Liver histopathological study was used to determine the protective effect of GE. The morphological changes in liver tissues through H&E and Masson’s trichrome staining were shown in . Compared with the control group, the stained sections in the model groups revealed extensive liver injuries characterized by moderate to severe loss of hepatic architecture, condensed nuclei around the central vein and plenty of deposition of collagen fibres. However, the histopathological changes were markedly alleviated in the GE treated groups, especially at the dose of 600 mg/kg. And in the liver fibrosis model ((B)), pseudolobuli were presented in liver damaged severely. Similarly, from the second to sixth stages of hepatocellular carcinoma (Figure S2), the status of inflammation and fibrosis in the GE treated groups were alleviated in contrast to that in the model groups. The histopathological analysis is in agreement with the results of biochemical assay.
Figure 2. Histopathological changes of liver tissue in different models. Representative pictures (×200) of H&E-staining and Masson′s trichrome-staining liver sections from mice were shown. (A) acute liver injury model, (B) liver fibrosis model, (C) liver cirrhosis model, (D) the first stage in hepatocellular carcinoma.
3.3. Kupffer cells activation decreased in the GE treated groups
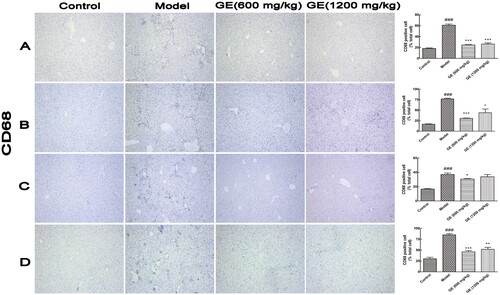
Resident Kupffer cells and monocyte/macrophages were often activated during liver injury (Liaskou, Wilson, & Oo, Citation2012; Lin, Liu, Piao, & Song, Citation2018). CD68 is used as a marker for the various cell lines of the macrophage lineage, like Kupffer cells, and CD68 overexpression is indicative of macrophages/ monocytes dysfunction and hyperproliferation (Holness & Simmons, Citation1993). In , CD68 were overexpressed of the liver tissues in the model groups, whereas levels of CD68 expression were decreased significantly after treatment with GE. From the second to fifth stages during hepatocellular carcinoma development, expression level of CD68 displayed no significant change among groups (Figure S3). Therefore, GE protected liver from damage through decreasing Kupffer cells activation.
Figure 3. Activation of Kupffer cells in different models. Representative images (×100) of immunohistochemical staining for CD68 in liver tissue. (A) acute liver injury model, (B) liver fibrosis model, (C) liver cirrhosis model, (D) the first stage in hepatocellular carcinoma. All values are expressed as mean ± S.E.M. (n = 7). ###p < .001 vs. control group; *p < .05, vs. model group; **p < .01, vs. model group; ***p < .001, vs. model group.
3.4. The expression of inflammatory cytokines decreased in the GE groups
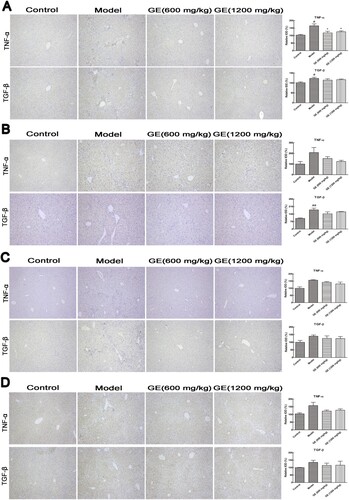
When the liver was injured, immune cells were induced to secrete pro-inflammation cytokines, including TNF-α, TGF-β, IL-1β and IL-6 to promote inflammation (Bieghs & Trautwein, Citation2013). To preliminarily investigate the effect of GE on liver disease, the expression levels of TNF-α, TGF-β, and IL-6 were detected through immunohistochemistry and analysed using computer imaging system. showed that the expression levels of TNF-α and TGF-β were significantly higher in the model group after chemical injury, compared to that in the control group. However, after treatment with GE, the expression levels of these cytokines were remarkably decreased than that in the model group. Moreover, the results of Western Blot experiments were in line with immunohistochemical results. GE suppressed the protein expression of TNF-α and TGF-β in the injured liver tissue of mice, as well as NF-κB (Ganai, Khan, Malik, & Farooqi, Citation2015) (Figure S4).
Figure 4. Cytokines expression in different models. Representative images (×100) of immunohistochemical staining for cytokines in liver tissue were shown. (A) acute liver injury model, (B) liver fibrosis model, (C) liver cirrhosis model, (D) the first stage in hepatocellular carcinoma. All values are expressed as mean ± S.E.M. (n = 7). #p < .05, vs. control group; *p < .05, vs. model group.
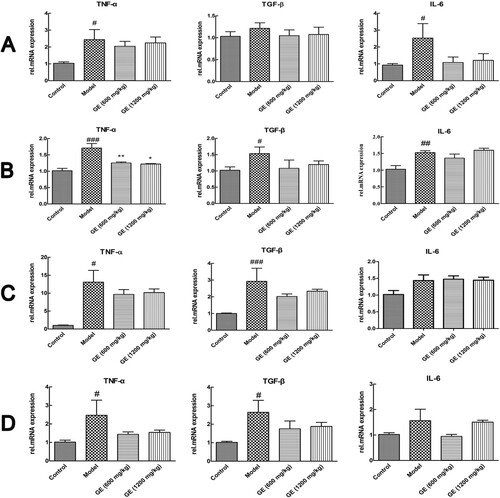
The expression levels of these cytokines were further investigated at mRNA level. As shown in , the mRNA levels of TNF-α, TGF-β and IL-6 were increased in the model group compared with that in the control group, whereas decreased after pretreatment with GE. The results were in accordance with liver immunohistochemical results, which indicated the pro-inflammatory cytokines decreased after pretreatment with GE.
Figure 5. Cytokines in different models in the level of mRNA. (A) acute liver injury model, (B) liver fibrosis model, (C) liver cirrhosis model, (D) the first stage in hepatocellular carcinoma. All values are expressed as mean ± S.E.M. (n = 7). #p < .05, vs. control group; ##p < .01 vs. control group; ###p < .001 vs. control group; *p < .05, vs. model group; **p < .01, vs. model group.
At the different stages of liver cancer development (Figure S5 A–E), the expressions of TNF-α and TGF-β were higher in the model groups, and decreased in the GE groups, although the changes were not significant. Figure S6 showed that TNF-α and TGF-β were markedly increased in the model groups, but these cytokines were decreased after treatment of GE. Therefore, liver protective effects of GE may attribute to reduce the expression level of inflammatory cytokines.
4. Discussion
Er Zhi Wan, a traditional Chinese medicine formulation, has been developed as a nourishing formula for hundreds of years, which contains two herbs by weight ratio (1:1), Glossy privet fruit and Ecliptae herba (Yao et al., Citation2014). Glossy privet fruit is a common edible plant in Asian countries such as China and Japan (Gao et al., Citation2015). Ecliptae herba, is widespread throughout China and India, and considered as a nutritious herbal medicine (Xi et al., Citation2014). Both of them have pleiotropic effects including anti-inflammatory, hepatoprotective, and antioxidant (Ding, Ma, & Shang, Citation2006; He et al., Citation2001; Xi et al., Citation2014). Even though, the hepatoprotective mechanism of Glossy privet fruit and Ecliptae herba formula still needs in-depth study. In the present study, GE pretreatment and treatment both have protective effects on different models of liver injury. Treatment with GE decreased levels of transaminase and pro-inflammatory cytokines, as well as alleviated histopathological changes. And activation of Kupffer cells was also suppressed.
Inflammation is considered as the main driver of hepatic tissue damage leading to fibrogenesis and hepatocellular carcinoma (Berasain et al., Citation2009; Park et al., Citation2010). In our study, the levels of inflammatory cytokines in liver tissue decreased remarkably in the GE treated groups. And in vitro, the levels of IL-6 and TNF-α decreased in RAW 264.7 cells induced by LPS after treatment of GE (Figure S7). Therefore, the mixture of extract from GE formula was suggested to prevent inflammation and contribute to the protection of liver.
Inflammatory factors, such as TNF-α, IL-6 and TGF-β, can be secreted by activated Kupffer cells and then activated Kupffer cells produce more cytokines inversely, which subsequently induces liver injury (Dong et al., Citation2016; McClain, Song, Barve, Hill, & Deaciuc, Citation2004). Our exploration showed that the expression levels of TNF-α, IL-6 and TGF-β in mice decreased after pretreatment with GE. Our findings suggested that the hepatoprotective effect of GE against chemical-induced liver injury is probably owing to a decrease of pro-inflammatory cytokines related to Kupffer cells. As an accompanying process of inflammation, the generation of free radicals including MDA and NO increased in the model group, whereas decreased in the GE treated groups. Activities of GSH-Px and SOD were just reversed (Figure S8).
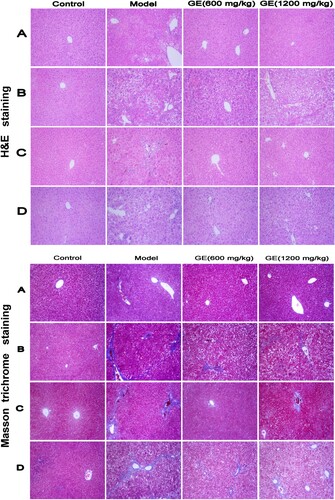
Kupffer cells (KCs), the major innate immune cells, are the largest population of resident macrophages in the liver (Tacke, Citation2012). They have the capacity to phagocytose and release the cytokines IL-1, IL-6 and TNF-α as well as chemokines CCl2-4 upon activation (Tacke, Citation2012). Overactive KCs have been identified to elicit liver injury (Qiao, Qian, Wang, Ma, & Wang, Citation2014). In this study, Kupffer cells were activated and CD68 overexpressed after chemical injury in the different liver disease models, whereas treatment with GE decreased activation of Kupffer cells and the inflammation. To our best knowledge, that GE protected the liver by partly inactivating Kupffer cells was rarely reported. The pro-inflammatory cytokines released from activated Kupffer cells can also directly interact with hepatic stellate cells via TGF-β, thereby contributing to collagen production (Tacke, Citation2012). In our study, distinct collagen fibre or pseudolobule in the liver tissue were observed in the model group after Masson staining (), which may be attributed to overexpression of TGF-β in liver tissue of the model group with that in the GE groups.
5. Conclusion
In conclusion, the results presented here suggest that Glossy privet fruit and Ecliptae herba formula can protect the liver from damage induced by chemical reagents. The protection mechanisms may be attributed to reduce the activation of Kupffer cells and then decrease the expression levels of transaminase and pro-inflammatory cytokines, and alleviate histopathological changes.
Supplemental Material
Download ()Acknowledgements
We are thankful to the Analysis and Test Center of Huazhong University of Science and Technology. Lijun Zhao, Yuanyuan Wang, Chungwah Ma, Hailong Li and Fangli Ma designed the experiment; Lijun Zhao, Qi Wang, Xiaoqiang Zhu and Yun Wan performed the research and analysed the results; Lijun Zhao wrote the draft manuscript; Xiangliang Yang and Yanhong Zhu revised and approved the submitted version.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abdel-Wahhab, M. A., Salman, A. S., Ibrahim, M. I., El-Kady, A. A., Abdel-Aziem, S. H., Hassan, N. S., Waly Ahmed I. (2016). Curcumin nanoparticles loaded hydrogels protects against aflatoxin B1-induced genotoxicity in rat liver. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 94, 159–171. doi: 10.1016/j.fct.2016.06.005
- Berasain, C., Castillo, J., Perugorria, M. J., Latasa, M. U., Prieto, J., & Avila, M. A. (2009). Inflammation and liver cancer. Annals of the New York Academy of Sciences, 1155, 206–221. doi: 10.1111/j.1749-6632.2009.03704.x
- Bieghs, V., & Trautwein, C. (2013). The innate immune response during liver inflammation and metabolic disease. Trends in Immunology, 34, 446–452. doi: 10.1016/j.it.2013.04.005
- Chacko, K. R., & Reinus, J. (2016). Extrahepatic complications of nonalcoholic Fatty liver disease. Clinics in Liver Disease, 20, 387–401. doi: 10.1016/j.cld.2015.10.004
- Chung, I. M., Rahuman, A. A., Marimuthu, S., Kirthi, A. V., Anbarasan, K., & Rajakumar, G. (2015). An investigation of the cytotoxicity and caspase-mediated apoptotic effect of Green synthesized zinc oxide nanoparticles using Eclipta prostrata on human liver carcinoma cells. Nanomaterials (Basel, Switzerland), 5, 1317–1330. doi: 10.3390/nano5031317
- Debray, D., Yousef, N., & Durand, P. (2006). New management options for end-stage chronic liver disease and acute liver failure: Potential for pediatric patients. Pediatric Drugs, 8, 1–13. doi: 10.2165/00148581-200608010-00001
- Ding, Y. Q., Ma, F. Q., & Shang, X. Y. (2006). Effect of Erzhiwan on anti-oxidation of skin tissue in aged mice resulting from D-galactose. Chinese Journal of Clinical Rehabilitation, 10, 141–143.
- Dong, D., Zhong, W., Sun, Q., Zhang, W., Sun, X., & Zhou, Z. (2016). Oxidative products from alcohol metabolism differentially modulate pro-inflammatory cytokine expression in Kupffer cells and hepatocytes. Cytokine, 85, 109–119. doi: 10.1016/j.cyto.2016.06.014
- Friedman, S. L. (2008). Mechanisms of hepatic fibrogenesis. Gastroenterology, 134, 1655–1669. doi: 10.1053/j.gastro.2008.03.003
- Ganai, A. A., Khan, A. A., Malik, Z. A., & Farooqi, H. (2015). Genistein modulates the expression of NF-kappaB and MAPK (p-38 and ERK1/2), thereby attenuating d-Galactosamine induced fulminant hepatic failure in Wistar rats. Toxicology and Applied Pharmacology, 283, 139–146. doi: 10.1016/j.taap.2015.01.012
- Gao, L., Li, C., Wang, Z., Liu, X., You, Y., Wei, H., Guo, Tao. (2015). Ligustri lucidi fructus as a traditional Chinese medicine: A review of its phytochemistry and pharmacology. Natural Product Research, 29, 493–510. doi: 10.1080/14786419.2014.954114
- He, Z. D., But, P. P. H., Chan, T. W., Dong, H., Xu, H. X., Lau, C. P., Sun, Han-Dong. (2001). Antioxidative glucosides from the fruits of Ligustrum lucidum. Chemical & Pharmaceutical Bulletin, 49, 780–784. doi: 10.1248/cpb.49.780
- Holness, C. L., & Simmons, D. L. (1993). Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood, 81, 1607. doi: 10.1182/blood.V81.6.1607.1607
- Liaskou, E., Wilson, D. V., & Oo, Y. H. (2012). Innate immune cells in liver inflammation. Mediators of Inflammation, 2012, 1–21. doi: 10.1155/2012/949157
- Lin, R., Liu, Y., Piao, M., & Song, Y. (2018). Magnesium isoglycyrrhizinate positively affects concanavalin A-induced liver damage by regulating macrophage polarization. Food and Agricultural Immunology, 29, 1041–1052. doi: 10.1080/09540105.2018.1508424
- Lu, Y., Chen, J., Ren, D., Yang, X., & Zhao, Y. (2017). Hepatoprotective effects of phloretin against CCl4-induced liver injury in mice. Food and Agricultural Immunology, 28, 211–222. doi: 10.1080/09540105.2016.1258546
- Ma, J. Q., Li, Z., Xie, W. R., Liu, C. M., & Liu, S. S. (2015). Quercetin protects mouse liver against CCl(4)-induced inflammation by the TLR2/4 and MAPK/NF-kappaB pathway. International Immunopharmacology, 28, 531–539. doi: 10.1016/j.intimp.2015.06.036
- McClain, C. J., Song, Z., Barve, S. S., Hill, D. B., & Deaciuc, I. (2004). Recent advances in alcoholic liver disease. IV. Dysregulated cytokine metabolism in alcoholic liver disease. American Journal of Physiology Gastrointestinal and Liver Physiology, 287, G497–G502. doi: 10.1152/ajpgi.00171.2004
- Park, E. J., Lee, J. H., Yu, G.-Y., He, G., Ali, S. R., Holzer, R. G., … Karin Michael. (2010). Dietary and Genetic obesity promote liver inflammation and Tumorigenesis by Enhancing IL-6 and TNF expression. Cell, 140, 197–208. doi: 10.1016/j.cell.2009.12.052
- Qiao, Y. L., Qian, J. M., Wang, F. R., Ma, Z. Y., & Wang, Q. W. (2014). Butyrate protects liver against ischemia reperfusion injury by inhibiting nuclear factor kappa B activation in Kupffer cells. The Journal of Surgical Research, 187, 653–659. doi: 10.1016/j.jss.2013.08.028
- Schuppan, D., & Afdhal, N. H. (2008). Liver cirrhosis. The Lancet, 371, 838–851. doi: 10.1016/S0140-6736(08)60383-9
- Siegel, R. L., Miller, K. D., & Jemal, A. (2016). Cancer statistics, 2016. CA: A Cancer Journal for Clinicians, 66, 7–30.
- Sun, H., Chen, L., Zhou, W., Hu, L., Li, L., Tu, Q., … Wang HongYang. (2011). The protective role of hydrogen-rich saline in experimental liver injury in mice. Journal of Hepatology, 54, 471–480. doi: 10.1016/j.jhep.2010.08.011
- Tacke, F. (2012). Functional role of intrahepatic monocyte subsets for the progression of liver inflammation and liver fibrosis in vivo. Fibrogenesis & Tissue Repair, 5, S27. doi: 10.1186/1755-1536-5-S1-S27
- Wang, Z.-h., Hsu, C.-c., & Yin, M.-c. (2009). Antioxidative characteristics of aqueous and ethanol extracts of glossy privet fruit. Food Chemistry, 112, 914–918. doi: 10.1016/j.foodchem.2008.06.078
- Wang, S., Shi, X. L., Feng, M., Wang, X., Zhang, Z. H., Zhao, X., … Ding Yi-Tao (2016). Puerarin protects against CCl4-induced liver fibrosis in mice: Possible role of PARP-1 inhibition. International Immunopharmacology, 38, 238–245. doi: 10.1016/j.intimp.2016.06.008
- Williams, R. (2006). Global challenges in liver disease. Hepatology, 44, 521–526. doi: 10.1002/hep.21347
- Wu, W., Zhang, J., Yang, W., Hu, B., & Fallon, M. B. (2016). Role of splenic reservoir monocytes in pulmonary vascular monocyte accumulation in experimental hepatopulmonary syndrome. Journal of Gastroenterology and Hepatology, 31, 1888–1894. doi: 10.1111/jgh.13388
- Xi, F. M., Li, C. T., Han, J., Yu, S. S., Wu, Z. J., & Chen, W. S. (2014). Thiophenes, polyacetylenes and terpenes from the aerial parts of Eclipata prostrate. Bioorganic & Medicinal Chemistry, 22, 6515–6522. doi: 10.1016/j.bmc.2014.06.051
- Xu, H., Su, Z. R., Huang, W., Choi, R. C., Zheng, Y. Z., Lau, D. T., … Tsim Karl Wah-Keung (2012). Er Zhi Wan, an ancient herbal decoction for woman menopausal syndrome, activates the estrogenic response in cultured MCF-7 cells: An evaluation of compatibility in defining the optimized preparation method. Journal of Ethnopharmacology, 143, 109–115. doi: 10.1016/j.jep.2012.06.009
- Yao, W., Gu, H., Zhu, J., Barding, G., Cheng, H., Bao, B., … Li Wei. (2014). Integrated plasma and urine metabolomics coupled with HPLC/QTOF-MS and chemometric analysis on potential biomarkers in liver injury and hepatoprotective effects of Er-Zhi-Wan. Analytical and Bioanalytical Chemistry, 406, 7367–7378. doi: 10.1007/s00216-014-8169-x
- Yu, H., Zheng, L., Yin, L., Xu, L., Qi, Y., Han, X., … Peng Jinyong. (2014). Protective effects of the total saponins from Dioscorea nipponica Makino against carbon tetrachloride-induced liver injury in mice through suppression of apoptosis and inflammation. International Immunopharmacology, 19, 233–244. doi: 10.1016/j.intimp.2014.01.019
- Zhai, Z., Haney, D., Wu, L., Solco, A., Murphy, P. A., Wurtele, E. S., … Cunnick Joan E. (2007). Alcohol extracts of Echinacea inhibit production of nitric oxide and tumor necrosis factor-alpha by macrophages in vitro. Food and Agricultural Immunology, 18, 221–236. doi: 10.1080/09540100701797363
- Zhang, S., Chen, J., Sun, A., & Zhao, L. (2014). Protective effects and antioxidant mechanism of bamboo leaf flavonoids on hepatocytes injured by CCl4. Food and Agricultural Immunology, 25, 386–396. doi: 10.1080/09540105.2013.810709
- Zhang, M., Pan, L.-J., Jiang, S.-T., & Mo, Y.-W. (2016). Protective effects of anthocyanins from purple sweet potato on acute carbon tetrachloride-induced oxidative hepatotoxicity fibrosis in mice. Food and Agricultural Immunology, 27, 157–170. doi: 10.1080/09540105.2015.1079589