ABSTRACT
This study aimed to evaluate the analgesic efficacy of astilbin and its underlying mechanisms. Astilbin suppressed the symptoms of acetic acid–induced writhing, lengthened the latency period on the hot plate, and reduced the licking and biting response of formalin-injected mice, suggesting its analgesic efficacy on the central nervous system. Astilbin suppressed neuronal nitric oxide synthase and enhanced serotonin and norepinephrine in serum and brains of mice after 30 s of thermal stimulation, but it failed to influence their levels before thermal stimulation. Among six chosen antagonists, only nimodipine, a calcium channel blocker, strongly enhanced the abirritation of astilbin, as indicated by the lengthened latency period of mice treated with nimodipine plus astilbin in the hot plate test. The expression levels of c-Fos and phosphorylated calmodulin-dependent protein kinase II and c-Jun N-terminal kinase in the brains were reduced in the astilbin-treated mice. Astilbin-mediated analgesia is partially related to Ca2+ channels.
1. Introduction
As a series of pathological and physiologic symptoms, pain is caused by intense or damaging stimuli, and recurring pain always influences quality of life (Woolf & Mannion, Citation1999). According to research, pain affects 64% of patients with metastatic malignancy and 33% of cancer survivors (Van den Beuken-van Everdingen et al., Citation2007). Due to the requirement of in-depth patient evaluation and multimodal therapy, the management of pain has attracted research attention worldwide (McCarberg, Citation2011). The peripheral nervous system hypothesis and the central nervous system hypothesis are the two major pharmacologic theories of pain. Nonsteroidal anti-inflammatory drugs and narcotic analgesics are the two main types of analgesic agents currently used; however, their adverse effects, especially after long-term use, including gastrointestinal bleeding (Lapeyre-Mestre, Grolleau, & Montastruc, Citation2013), the renal function destruction (Allegaert, De Hoon, Debeer, & Gewillig, Citation2010), and clinical tolerance and dependence (Trang et al., Citation2015), strongly limit their application.
In recent years, natural medicinal agents with fewer adverse effects have attracted considerable research interest and have been recognized as a valuable reservoir for pharmacological compound screening. Encouragingly, more than 40% of cancer patients now receive auxiliary natural productsfor pain relief (Gozum, Tezel, & Koc, Citation2003). An herbal formula called the Jia-Yuan-Qing Pill relieves pain from bone cancer viamodulation of the peripheral nerve, without physical dependence (Tian et al., Citation2014). Albiflorin (AF), mainly obtained from Paeonia lactiflora Radix, is an isomer of paeoniflorin and shows analgesic activities in experimental studies of mice, partially via regulation of the Ca2+ channel (Zhang et al., Citation2016). These studies provide experimental support for the development of analgesics from natural products.
Astilbin (AB), a flavonoid compound ((A)) widely distributed in Rhizoma Smilacis Glabrae and Astragalus membranaceus, has attracted considerable attention due to its clinically relevant bioactivities, especially anti-inflammation (Chen, Zhu, Sun, & Ma, Citation2018), anti-oxidation (Zhang, Zhang, & Cheung, Citation2009), and immune regulation (Meng et al., Citation2016). Studies have reported that AB possesses antidepressant properties, mainly by increasing the levels of monoaminergic neurotransmitters, such as 5-hydroxytryptamine (5-HT) and dopamine (DA) (Lv et al., Citation2014). In lipopolysaccharide-induced RAW 264.7 cells, AB suppressed the production of nitric oxide (NO) and inducible nitric oxide synthase (iNOS) via regulation of the expression levels of phosphor-c-Jun N-terminal kinase (P-JNK) (Lu et al., Citation2015). However, no research has directly reported the potential analgesic activities of AB. On the basis of previous pharmacological research into AB, we hypothesized that it may show analgesic efficacy via its regulation of neurotransmitters.
Figure 1. (A) Chemical structure of astilbin. (B) The writhing and stretching times of mice within 15 min in acetic acid-induced writhing test. (C) The latency period of each mouse on the hot plate was recorded at 30 min, 60 min and 90 min after agents treatment. (D) The period of licking paws of each mouse were recorded in formalin test at the first phase (0–5 min) and the second phase (15–30 min). Results were expressed as mean ± S.E.M. (n = 12). *P < 0.05, **P < 0.01 and ***P < 0.001 versus control mice.
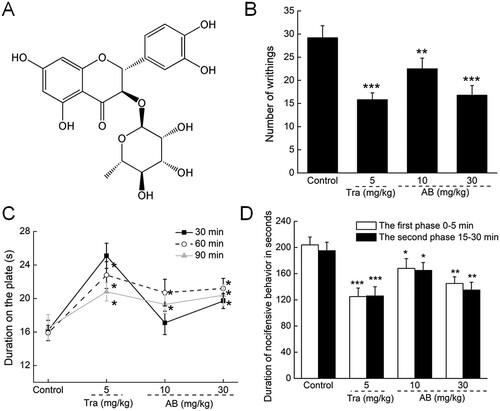
Based on the above hypothesis, the biological effects of AB on pain and its underlying mechanisms were successfully evaluated with behavioural tests combined with measurement of biochemical indexes and western blot analysis.
2. Materials and Methods
2.1. Animal care and experimental design for evaluation of analgesic effects
BALB/c female mice (8 weeks, 21–25 g) (License No.: 2016-0003) (purchased from YiSi Animal Institution, Changchun, China) were maintained at 23°C ± 1°C with a 12-h light/dark cycle (lights on 07:00–19:00). All mice were supplied with water and food available ad libitum.
The protocol was approved by the Animal Ethics Committee of Jilin University (20161202). The mice were randomly separated into four groups and orally treated with double-distilled water (n = 12; control mice), or 5 mg/kg of tramadol (Tra) (purchased from CSPC Pharmaceutical Group, China) (n = 12; positive control mice), 10 mg/kg, or 30 mg/kg of AB (Shanghai Yuanye Biotechnology Co. Ltd., Shanghai, China) (n = 12/group) for 18 consecutive days.
2.1.1 Acetic acid-induced writhing test
On the 15th day, 30 min after the designated treatments, the acetic acid-induced writhing test, a common screening tool for painkillers, was conducted to evaluate the analgesic effects of AB (Islam, Shajib, & Ahmed, Citation2016). Briefly, all mice received intraperitoneal injections of 10 mL/kg (bodyweight) of 0.5% acetic acid aqueous solution, and the writhing responses, including abdominal muscle contraction and hind paw stretching, were recorded within 15 min.
2.1.2 Hotplate test
On the 16th day, 30, 60, and 90 min after the designated treatments, the hot plate test, a commonly used procedure for screening pharmacodynamic activities and mechanisms, was performed (Choi & Koo, Citation2005; Sudo et al., Citation2015; Tian et al., Citation2014). The experimental mice were placed separately on a hot plate at 55°C ± 0.5°C. The period from the beginning of the test to the appearance of the first sign of jumping and/or hind paw licking to avoid thermal nociception was recorded as the thermal nociceptive threshold.
2.1.3. Formalin test
On the 18th day, 30 min after the designated treatments, each mouse was injected with 20 μL of 2% formalin (37% formaldehyde diluted in 0.9% NaCl) in the right hind paw and placed in an elevated transparent observation chamber. The licking and biting responses were recorded during the first phase (0–5 min) and the second phase (15–30 min).
2.1.4. Analysis of biochemical indexes
On the 20th day, blood was collected from the caudal vein of each mouse. After 6 h of relaxation, all mice were subjected to 30 s thermal stimulation on a hot plate at a 55°C ± 0.5°C, and blood samples and brain tissues were collected immediately after decapitation. The levels of neuronal nitric oxide synthase (nNOS), norepinephrine (NE), and 5-HT in the serum and brain tissues were detected using the corresponding enzyme-linked immunosorbent assay kit (Shanghai Yuanye Bio-Technology Co., Ltd, Shanghai, China) following the manufacturer’s instructions.
2.2. Mechanistic investigation of the analgesic effects
One hundred sixty-eight BALB/c female mice (8 weeks, 21–25 g) (License No.: 2016-0003) (purchased from YiSi Animal Institution, Changchun, China) were cared for under the same protocol as described in Section 2.1.
All mice were randomly separated into 14 groups of 12. The control mice received deuterium-depleted water (0.2 mL), and the mice in the other groups received oral AB at 30 mg/kg for 14 consecutive days. Six inhibitors/blockers purchased from Sigma-Aldrich (USA) were chosen for preliminary investigation of the underlying mechanism of the analgesic effects of AB. Methylene blue (10 mg/kg; guanylate cyclase inhibitor), naloxone (1 mg/kg; non-selective opioid receptor antagonist), glibenclamide (2 mg/kg; α2 adrenaline antagonist), yohimbine (2 mg/kg; α2 adrenergic antagonist), atropine (5 mg/kg; M-acetylcholine receptor antagonist), and nimodipine (10 mg/kg; Ca2+ channel blocker) were administrated orally alone or 15 min before AB administration (30 mg/kg) for 14 consecutive days. On the 14th day, 30 min after the last administration, the hotplate test was performed.
2.3. Western blot
The whole brain tissues were homogenized with lysis buffer (Sigma-Aldrich, USA) containing protease inhibitor cocktail (Sigma-Aldrich, USA) and 1-mM phenylmethanesulfonyl fluoride. The total protein concentration of the samples was detected using a BCA protein assay kit (Merck Millipore, USA). SDS-PAGE (10%) was used to separate the protein samples (30 μg), and the separated proteins were transferred onto a nitrocellulose membrane (0.45 µm) (Bio Basic, Inc., USA). The transferred membranes were blocked using 5% bull serum albumin at 4°C for 4 h and incubated at 4°C with the primary antibodies, including P-JNK, total (T)-JNK, P-calmodulin–dependent protein kinase II (P-CaMKII), T-CaMKII, c-Fos, and glyceraldehyde-3-phosphate dehydrogenase (Cell Signaling Technology, Beverly, USA) at a dilution of 1:2000. After washing, the transferred membranes were exposed to horseradish peroxidase–conjugated secondary antibodies at dilution of 1:2000 (Santa Cruz, USA) for 4 h at 4°C. The ECL detection system (GE Healthcare, UK) was used to visualize the bands, and their intensities were analyzed with ImageJ 1.8.0 (National Institutes of Health, Bethesda, MD).
2.4. Statistical analysis
The experimental data were expressed as mean ± SEM and calculated using the software SPSS 16.0 (IBM Corporation, Armonk, NY). The statistical significance was analyzed by a one-way of variance followed by post-hoc multiple comparisons (Dunn’s test), and was defined as P values of less than 0.05.
3. Results
3.1. Analgesic effects of AB
A strong reduction of writhing times (P < 0.001; (B)) and enhanced tolerance to the hot plate (P < 0.05; (C)) were noted in the Tra-treated mice. In the formalin test, Tra significantly suppressed pain symptoms in the mice in both the first (0–5 min) and second phases (15–30 min) (P < 0.001; (D)).
AB likewise suppressed acetic acid–induced writhing and stretching in a dose-dependent manner. Specifically, these were inhibited by 42.5% in the mice treated with 30 mg/kg of AB (P < 0.001; (B)). AB exhibited favourable activities against thermal stimulation, and a >21.6% enhancement of the latency period was observed 90 min after administration (P < 0.05; (C)), suggesting the central antinociceptive activity of AB.
AB, specifically at 30 mg/kg, inhibited the pain scores by 28.9% at the first phase (P < 0.01; (D)) and 30.8% at the second phase (P < 0.01; (D)) compared with the control mice in the formalin test, which further confirmed the central antinociceptive activity of AB.
3.2. Regulatory effect of AB on nNOS, 5-HT and NE
As a factor regulating the synthesis of NO, nNOS influences the symptoms of chronic pain (Lee et al., Citation2015). Both Tra and AB failed to influence the levels of nNOS in the serum and brains before 30s of thermal stimulation ((A,D)). After the mice received 30s of thermal stimulation, 30 mg/kg of AB suppressed the serum levels of nNOS by 18.3% (P < 0.05; (A)), and the cerebral levels of nNOS by 18.5% (P < 0.01; (D)), similar to the effects of Tra.
Figure 2. The levels of (A) nNOS, (B) 5-HT and (C) NE in serum, and the levels of (D) nNOS, (E) 5-HT and (F) NE in brain tissues were analysed before and after 30-s thermal stimulus of mice received 18-day AB and Tra administration. Data were expressed as mean ± S.E.M. (n = 12). *P < 0.05 and **P < 0.01 versus control mice.
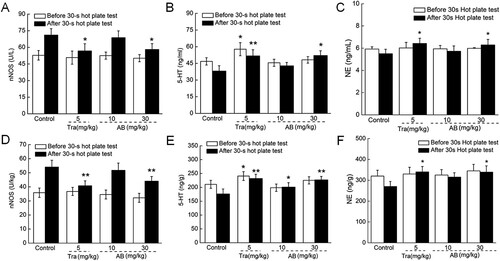
5-HT, a monoamine neurotransmitter, helps relieve nerve injury-induced pain (Sommer, Citation2004). Eighteen-day Tra administration enhanced the levels of 5-HT in both serum (P < 0.05; (B)) and brains (P < 0.001; (E)) both before and after thermal stimulation compared with the control mice. For comparison, 18-day AB (30 mg/kg) administration likewise resulted in a 13.5% enhancement of the serum 5-HT levels (P < 0.05; (B)) and a 31.2% enhancement of the cerebral 5-HT levels (P < 0.05; (E)) of the mice that received 30 s of thermal stimulation.
NE contributes to inflammation- and nerve injury-induced pain by affecting the level of alpha-2-adrenoceptor (Pertovaara, Citation2006). Tra and AB failed to influence the serum and cerebral levels of NE before the 30 s thermal stimulation ((C,F)). After the 30 s thermal stimulation, 14.4% and 25.6% enhancements of the NE levels in the serum (P < 0.05, (C)) and brains (P < 0.05, (F)), respectively, were observed in the mice treated with 30 mg/kg of AB. Eighteen-day Tra administration likewise significantly enhanced the levels of NE in both the serum and brains of the mice that received thermal stimulation (P < 0.05, (C,F)).
3.3. Effects of various antagonists on the abirritation of AB
Opioid receptors, choline receptors, Na+/K+ ion channels, and Ca2+ ion channels are reported to be targets for analgesic drugs (Malmberg & Yaksh, Citation1994; Millan, Citation2002; Nielsen, Mathiesen, & Blackburn-Munro, Citation2004). Among the six chosen antagonists, pretreatment with methylene blue (10 mg/kg), naloxone (1 mg/kg), glibenclamide (2 mg/kg), yohimbine (2 mg/kg), and atropine (10 mg/kg) showed no significant influences on the AB-mediated analgesic effects (). Encouragingly, 18-day administration of nimodipine (10 mg/kg) combined with AB (30 mg/kg) strongly enhanced the abirritation of AB, indicated by the enhanced latency period of the mice treated with nimodipine plus AB in the hot plate test compared with the mice receiving AB alone (P < 0.05; ).
Table 1. The influences of six chosen antagonist on the analgesic efficacies of AB in hot plate test.
3.4. AB regulated protein expression levels in brains
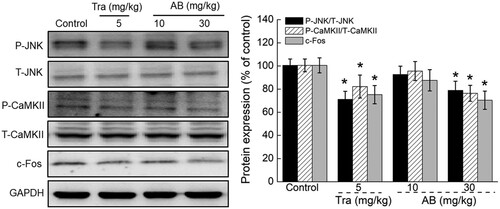
After 18-day administration, Tra and AB (specifically at 30 mg/kg) significantly reduced the expression levels of P-JNK, P-CaMKII, and c-Fos in the brains of the mice (P < 0.01; ) compared with the control mice.
Figure 3. The effects of AB and Tra on the expression levels of P-JNK, P-CaMKII and c-Fos in brains of mice received 30-s thermal stimulus. Quantification data of the expression of P-JNK and P-CaMKII were normalized by corresponding T-JNK and T-CaMKII respectively, and the expression of c-Fos were normalized by GAPDH. Data were expressed as mean ± S.E.D. (n = 6). *P < 0.05 versus control mice.
4. Discussion
This study provides the first support for the analgesic properties of AB, through classical mice models. As a nonselective screening model for analgesia, the acetic acid–induced writhing test was performed to evaluate the analgesic properties of AB (Shajib, Akter, Ahmed, & Imam, Citation2015). It was combined with the hot plate test to distinguish the central antinociceptive effect from the peripheral effect (Srinivasan et al., Citation2003). Morphine prolongs the thermal pain threshold due to its binding to opioid receptors in the hot plate test (Donnerer & Liebmann, Citation2013). AB suppressed the writhing and stretching of the mice after acetic acid injection and prolonged the latency period on the hot plate, confirming its central analgesia. In addition, the formalin test was performed to confirm these findings. In the formalin test, the first phase (0–5 min) reflects the mediated effects of formalin on nociceptors via the central nervous system (non-inflammatory pain), where as the second phase (15–30 min) reflects the inflammatory pain perception that accompanies the release of various inflammatory mediators (Khan et al., Citation2011). AB reduced the reaction time in both the first and second phases of the formalin test, similar to the effects of Tra, a typical central antinociceptive medicine, thus confirming AB’s analgesic properties.
Neurotransmitters such as serotonin are reported to influence the nociception threshold (Farhanchi et al., Citation2018). In this study, AB exhibited regulatory activities on the levels of nNOS, 5-HT and NE in the serum and brains. Hyper-levels of nNOS are typically noted during nerve injury, which further activate ion channels and protein kinase to exhibit modulatory effects on pain. 5-HT, involved in the pathophysiology of migraines, is suppressed in patients with headaches (Ren et al., Citation2018). A previous study also supported the use of 5-HT to regulate inflammation-induced pain (Kim et al., Citation2015). Dysfunctional NE transmission in the central nervous system may lead to pain by down regulating the descending spinal pain modulatory pathways (Millan, Citation2002). The inhibition of NE and 5-HT reuptake is the mechanism of a number of effective analgesics, such as amitriptyline, imipramine, and clomipramine (Sindrup, Otto, Finnerup, & Jensen, Citation2005). Via modulation of the serotonergic system, lappaconitine shows analgesic properties (Ono & Satoh, Citation1992). Tramadol relieves pain by suppressing the reuptake of 5-HT and NE (Corona-Ramos et al., Citation2016). Enhanced levels of 5-HT and NE have previously been linked with the suppression of endogenous analgesia, which was further confirmed in this study.
Our results show that the analgesic effect of AB is partially related to the Ca2+ channel, indicated by the enhanced analgesic abilities of AB after co-administration with nimodipine for 14 days. By inactivating the K+ and Cl− channels, which regulate the levels of transporter associated with algesthesis, the Ca2+ channel influences both central and peripheral analgesia (Gemes et al., Citation2013; Huang et al., Citation2013; Kitayama, Morita, Motoyama, & Dohi, Citation2016; Li et al., Citation2016). During this process, the extracellular Ca2+ influx, the release of neurotransmitters and the algetic impulse of nerve cells are all promoted (Chen, Yu et al., Citation2018). Interestingly, 5-HT can open the receptor-gated ion channels and further regulate intracellular Ca2+ levels (Tran & Keele, Citation2016). Meanwhile, the Ca2+/CaMKII-dependent pathway is involved in the vomiting caused by serotonin (Zhong, Hutchinson, Chebolu, & Darmani, Citation2014). As we all known regulator of calcium signalling, CaMKII serves as a promising downstream target for pain sensitization in naive mice (Choi et al., Citation2006). In this study, AB strongly reduced the phosphorylation of CaMKII and JNK, which may be related to changes of the Ca2+ channel and intracellular Ca2+ influx (Shuang et al., Citation2015). As reported, nNOS can activate the Ca2+ channel via directly influencing NO levels (Endo, Yanagawa, & Komatsu, Citation2016). Altogether, the Ca2+ channel partially contributes to the analgesic properties of AB in mice.
Altogether, AB shows analgesia in BALB/c mice, partially by modulation of the Ca2+ channels, which provides the first experimental evidence to support the further evaluation of AB as an agent for pain treatment.
Availability of data and materials
All data generated and analyzed during the present study are included in this published article.
Ethical approval
The experimental animal protocol was approved by the Animal Ethics Committee of Jilin University.
Acknowledgements
This work was supported by the Special Projects of Cooperation among Jilin University and Jilin Province in P. R. China (SXGJSF2017-1) and Science Foundation in Jilin Province of P. R. China (Grant No. 20180101098JC).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Allegaert, K., De Hoon, J., Debeer, A., & Gewillig, M. (2010). Renal side effects of non-steroidal anti-inflammatory drugs in neonates. Pharmaceuticals (Basel), 3(2), 393–405. doi: 10.3390/ph3020393
- Chen, S., Yu, C., Rong, L., Li, C. H., Qin, X., Ryu, H., & Park, H. (2018). Altered synaptic vesicle release and Ca(2+) influx at single presynaptic terminals of cortical neurons in a knock-in mouse model of Huntington’s disease. Frontiers in Molecular Neuroscience, 11, 478. doi: 10.3389/fnmol.2018.00478
- Chen, F., Zhu, X., Sun, Z., & Ma, Y. (2018). Astilbin inhibits high glucose-induced inflammation and extracellular matrix accumulation by suppressing the TLR4/MyD88/NF-kappaB pathway in rat glomerular mesangial cells. Frontiers in Pharmacology, 9, 1187. doi: 10.3389/fphar.2018.01187
- Choi, E., & Koo, S. (2005). Anti-nociceptive and anti-inflammatory effects of the ethanolic extract of potato (Solanum tuberlosum). Food & Agricultural Immunology, 16(1), 29–39. doi: 10.1080/09540100500064320
- Choi, S. S., Seo, Y. J., Shim, E. J., Kwon, M. S., Lee, J. Y., Ham, Y. O., & Suh, H. W. (2006). Involvement of phosphorylated Ca2+/calmodulin-dependent protein kinase II and phosphorylated extracellular signal-regulated protein in the mouse formalin pain model. Brain Research, 1108(1), 28–38. doi: 10.1016/j.brainres.2006.06.048
- Corona-Ramos, J. N., De la, O. A. M., Deciga-Campos, M., Medina-Lopez, J. R., Dominguez-Ramirez, A. M., Jaramillo-Morales, O. A., … Lopez-Munoz, F. J. (2016). The antinociceptive effects of tramadol and/or gabapentin on rat neuropathic pain induced by a chronic constriction injury. Drug Development Research, 77(5), 217–226. doi: 10.1002/ddr.21313
- Donnerer, J., & Liebmann, I. (2013). The pain pathway in the rat following noxious thermal stimulation: Effect of morphine on pERK1/2 and TRPV1 at the dorsal horn level, and on hyperalgesia. Pharmacology, 92(1-2), 32–38. doi: 10.1159/000353141
- Endo, T., Yanagawa, Y., & Komatsu, Y. (2016). Substance P activates Ca2+-permeable nonselective cation channels through a phosphatidylcholine-specific phospholipase C signaling pathway in nNOS-expressing GABAergic neurons in visual cortex. Cerebral Cortex, 26(2), 669–682. doi: 10.1093/cercor/bhu233
- Farhanchi, A., Karkhanei, B., Amani, N., Aghajanloo, M., Khanlarzadeh, E., & Emami, Z. (2018). Association of serum serotonin and pain in patients with chronic low back pain before and after spinal surgery. Pain Research and Treatment, 2018, 4901242. doi: 10.1155/2018/4901242
- Gemes, G., Koopmeiners, A., Rigaud, M., Lirk, P., Sapunar, D., Bangaru, M. L., … Hogan, Q. H. (2013). Failure of action potential propagation in sensory neurons: Mechanisms and loss of afferent filtering in C-type units after painful nerve injury. The Journal of Physiology, 591(4), 1111–1131. doi: 10.1113/jphysiol.2012.242750
- Gozum, S., Tezel, A., & Koc, M. (2003). Complementary alternative treatments used by patients with cancer in eastern Turkey. Cancer Nursing, 26(3), 230–236. doi: 10.1097/00002820-200306000-00010
- Huang, F., Wang, X., Ostertag, E. M., Nuwal, T., Huang, B., Jan, Y. N., … Jan, L. Y. (2013). TMEM16C facilitates Na(+)-activated K+ currents in rat sensory neurons and regulates pain processing. Nature Neuroscience, 16(9), 1284–1290. doi: 10.1038/nn.3468
- Islam, S., Shajib, M. S., & Ahmed, T. (2016). Antinociceptive effect of methanol extract of Celosia cristata Linn. In mice. BMC Complementary and Alternative Medicine, 16(1), 400. doi: 10.1186/s12906-016-1393-5
- Khan, S., Mehmood, M. H., Ali, A. N., Ahmed, F. S., Dar, A., & Gilani, A. H. (2011). Studies on anti-inflammatory and analgesic activities of betel nut in rodents. Journal of Ethnopharmacology, 135(3), 654–661. doi: 10.1016/j.jep.2011.03.064
- Kim, J. M., Jeong, S. W., Yang, J., Lee, S. H., Kim, W. M., Jeong, S., … Choi, J. I. (2015). Spinal 5-HT1A, not the 5-HT1B or 5-HT3 receptors, mediates descending serotonergic inhibition for late-phase mechanical allodynia of carrageenan-induced peripheral inflammation. Neuroscience Letters, 600, 91–97. doi: 10.1016/j.neulet.2015.05.058
- Kitayama, T., Morita, K., Motoyama, N., & Dohi, T. (2016). Down-regulation of zinc transporter-1 in astrocytes induces neuropathic pain via the brain-derived neurotrophic factor - K(+)-Cl(-) co-transporter-2 signaling pathway in the mouse spinal cord. Neurochemistry International, 101, 120–131. doi: 10.1016/j.neuint.2016.11.001
- Lapeyre-Mestre, M., Grolleau, S., & Montastruc, J. L. (2013). Adverse drug reactions associated with the use of NSAIDs: A case/noncase analysis of spontaneous reports from the French pharmacovigilance database 2002-2006. Fundamental & Clinical Pharmacology, 27(2), 223–230. doi: 10.1111/j.1472-8206.2011.00991.x
- Lee, W. H., Xu, Z., Ashpole, N. M., Hudmon, A., Kulkarni, P. M., Thakur, G. A., … Hohmann, A. G. (2015). Small molecule inhibitors of PSD95-nNOS protein-protein interactions as novel analgesics. Neuropharmacology, 97, 464–475. doi: 10.1016/j.neuropharm.2015.05.038
- Li, L., Chen, S. R., Chen, H., Wen, L., Hittelman, W. N., Xie, J. D., & Pan, H. L. (2016). Chloride homeostasis critically regulates synaptic NMDA receptor activity in neuropathic pain. Cell Reports, 15(7), 1376–1383. doi: 10.1016/j.celrep.2016.04.039
- Lu, C. L., Zhu, Y. F., Hu, M. M., Wang, D. M., Xu, X. J., Lu, C. J., & Zhu, W. (2015). Optimization of astilbin extraction from the rhizome of Smilax glabra, and evaluation of its anti-inflammatory effect and probable underlying mechanism in lipopolysaccharide-induced RAW264.7 macrophages. Molecules, 20(1), 625–644. doi: 10.3390/molecules20010625
- Lv, Q. Q., Wu, W. J., Guo, X. L., Liu, R. L., Yang, Y. P., Zhou, D. S., … Liu, J. Y. (2014). Antidepressant activity of astilbin: Involvement of monoaminergic neurotransmitters and BDNF signal pathway. Biological and Pharmaceutical Bulletin, 37(6), 987–995. doi: 10.1248/bpb.b13-00968
- Malmberg, A. B., & Yaksh, T. L. (1994). Voltage-sensitive calcium channels in spinal nociceptive processing: Blockade of N- and P-type channels inhibits formalin-induced nociception. The Journal of Neuroscience, 14(8), 4882–4890. doi: 10.1523/JNEUROSCI.14-08-04882.1994
- McCarberg, B. H. (2011). Pain management in primary care: Strategies to mitigate opioid misuse, abuse, and diversion. Postgraduate Medicine, 123(2), 119–130. doi: 10.3810/pgm.2011.03.2270
- Meng, Q. F., Zhang, Z., Wang, Y. J., Chen, W., Li, F. F., Yue, L. T., … Li, Y. B. (2016). Astilbin ameliorates experimental autoimmune myasthenia gravis by decreased Th17 cytokines and up-regulated T regulatory cells. Journal of Neuroimmunology, 298, 138–145. doi: 10.1016/j.jneuroim.2016.07.016
- Millan, M. J. (2002). Descending control of pain. Progress in Neurobiology, 66(6), 355–474. doi: 10.1016/S0301-0082(02)00009-6
- Nielsen, A. N., Mathiesen, C., & Blackburn-Munro, G. (2004). Pharmacological characterisation of acid-induced muscle allodynia in rats. European Journal of Pharmacology, 487(1-3), 93–103. doi: 10.1016/j.ejphar.2004.01.017
- Ono, M., & Satoh, T. (1992). Pharmacological studies on lappaconitine: Possible interaction with endogenous noradrenergic and serotonergic pathways to induce antinociception. The Japanese Journal of Pharmacology, 58(3), 251–257. doi: 10.1254/jjp.58.251
- Pertovaara, A. (2006). Noradrenergic pain modulation. Progress in Neurobiology, 80(2), 53–83. doi: 10.1016/j.pneurobio.2006.08.001
- Ren, C., Liu, J., Zhou, J., Liang, H., Wang, Y., Sun, Y., … Yin, Y. (2018). Low levels of serum serotonin and amino acids identified in migraine patients. Biochemical and Biophysical Research Communications, 496(2), 267–273. doi: 10.1016/j.bbrc.2017.11.203
- Shajib, M. S., Akter, S., Ahmed, T., & Imam, M. Z. (2015). Antinociceptive and neuropharmacological activities of methanol extract of Phoenix sylvestris fruit pulp. Frontiers in Pharmacology, 6, 212. doi: 10.3389/fphar.2015.00212
- Shuang, G., Xin, Y., Fang, B., Huang, Y., Xu, L., & Jing, L. (2015). The toxicity of 3-monochloro-1,2-propanediol (+) to activated T cells in mice. Food & Agricultural Immunology, 28(4), 1–13.
- Sindrup, S. H., Otto, M., Finnerup, N. B., & Jensen, T. S. (2005). Antidepressants in the treatment of neuropathic pain. Basic & Clinical Pharmacology & Toxicology, 96(6), 399–409. doi: 10.1111/j.1742-7843.2005.pto_96696601.x
- Sommer, C. (2004). Serotonin in pain and analgesia: Actions in the periphery. Molecular Neurobiology, 30(2), 117–125. doi: 10.1385/mn:30:2:117
- Srinivasan, K., Muruganandan, S., Lal, J., Chandra, S., Tandan, S. K., Raviprakash, V., & Kumar, D. (2003). Antinociceptive and antipyretic activities of Pongamia pinnata leaves. Phytotherapy Research, 17(3), 259–264. doi: 10.1002/ptr.1126
- Sudo, R. T., Neto, M. L., Monteiro, C. E., Amaral, R. V., Resende, A. C., Souza, P. J., … Moura, R. S. (2015). Antinociceptive effects of hydroalcoholic extract from Euterpe oleracea Mart. (Acai) in a rodent model of acute and neuropathic pain. BMC Complementary and Alternative Medicine., 15, 208. doi: 10.1186/s12906-015-0724-2
- Tian, Y., Teng, L. R., Song, J. J., Meng, Q. F., Lu, J. H., Zhang, W. W., … Teng, L. S. (2014). Studies on the analgesic activities of Jia-Yuan-Qing pill and its safety evaluation in mice. Protoplasma, 251(5), 1245–1253. doi: 10.1007/s00709-014-0637-9
- Tran, L., & Keele, N. B. (2016). CaMKIIalpha knockdown decreases anxiety in the open field and low serotonin-induced upregulation of GluA1 in the basolateral amygdala. Behavioural Brain Research, 303, 152–159. doi: 10.1016/j.bbr.2016.01.053
- Trang, T., Al-Hasani, R., Salvemini, D., Salter, M. W., Gutstein, H., & Cahill, C. M. (2015). Pain and poppies: The good, the bad, and the ugly of opioid analgesics. Journal of Neuroscience, 35(41), 13879–13888. doi: 10.1523/jneurosci.2711-15.2015
- Van den Beuken-van Everdingen, M. H., de Rijke, J. M., Kessels, A. G., Schouten, H. C., van Kleef, M., & Patijn, J. (2007). Prevalence of pain in patients with cancer: A systematic review of the past 40 years. Annals of Oncology, 18(9), 1437–1449. doi: 10.1093/annonc/mdm056
- Woolf, C. J., & Mannion, R. J. (1999). Neuropathic pain: Aetiology, symptoms, mechanisms, and management. Lancet, 353(9168), 1959–1964. doi: 10.1016/s0140-6736(99)01307-0
- Zhang, Y., Sun, D., Meng, Q., Guo, W., Chen, Q., & Zhang, Y. (2016). Calcium channels contribute to albiflorin-mediated antinociceptive effects in mouse model. Neuroscience Letters, 628, 105–109. doi: 10.1016/j.neulet.2016.03.054
- Zhang, Q.-F., Zhang, Z.-R., & Cheung, H.-Y. (2009). Antioxidant activity of Rhizoma Smilacis Glabrae extracts and its key constituent-astilbin. Food Chemistry, 115(1), 297–303. doi: 10.1016/j.foodchem.2008.11.053
- Zhong, W., Hutchinson, T. E., Chebolu, S., & Darmani, N. A. (2014). Serotonin 5-HT3 receptor-mediated vomiting occurs via the activation of Ca2+/CaMKII-dependent ERK1/2 signaling in the least shrew (Cryptotis parva). PLoS One, 9(8), e104718. doi: 10.1371/journal.pone.0104718
