?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this work, a rapid, convenient and sensitive Au nanoparticle-based immunochromatographic sensor (AuNPs-ICS) for simultaneous detection of various tadalafil adulterants in health food was established for the first time, based on one high affinity and broad-spectrum antibody through skeleton-specific hapten design according to the common structure of tadalafil drugs. This immunochromatographic sensor was developed by the polyclonal antibody conjugated with AuNPs and the IC50 was 10.04 ng mL−1 for tadalafil and 9.55, 11.46, 17.01 ng mL−1 for amino tadalafil, acetamino tadalafil and nortadalafil, respectively. The limit of detection results could be obtained visually by naked eyes for and computationally by the hand-held immunochromatography reader. The sensor could specially detect Tadalafil and its analogs within 10 min in health food samples, and the close correlation with UPLC-MS/MS method showed the sensor was accurate and reliable, which could apply to on site detection Tadalafil and its analogs rapidly and accurately.
Introduction
The pursuit of optimum human nutrition and health is producing huge market demand and economic benefits (Martí et al., Citation2010). However, this popularity has led to increasing illicit adulteration of health food, and synthetic drugs are frequently illegally added to herbal products and supplements to enhance their performance (Singh et al., Citation2009; Sugita & Miyakawa, Citation2010; Venhuis & de Kaste, Citation2012). Insufficient regulation and high accessibility put consumers at risk (Low et al., Citation2009).
Phosphodiesterase type 5 (PDE-5) inhibitors, namely tadalafil, sildenafil, and their analogs, are widely used for the treatment of erectile dysfunction (Abdel-Aziz, Asiri, El-Azab, Al-Omar, & Kunieda, Citation2011). Numerous reports have demonstrated PDE-5 inhibitors have significant clinical side effects, such as physical aches and pains, and visual or hearing impairment (Shindel, Citation2009). Moreover, PDE-5 inhibitors can drastically decrease blood pressure when taken in conjunction with certain nitrate containing drugs, resulting serious side effects, unconsciousness, and even death (Chrysant, Citation2013; Gur et al., Citation2013; Poon, Lam, Lai, Chan, & Mak, Citation2007).To date, over 50 analogs of prescription PDE-5 inhibitors have been synthesized, and at least 46 of these have been detected as adulterants in health supplements, functional foods, and energy drinks (Alp, Coşkun, & Göker, Citation2013; Huang et al. Citation2016a ; Lee et al., Citation2013; Li, Low, Ge, Bloodworth, & Koh, Citation2013). Furthermore, new PDE-5 inhibitors are continuously being developed and used illegally in health food, which creates food safety risks and difficulties with routine detection (Low et al., Citation2009). Therefore, accurate and reliable analytical methods for these inhibitors and their analogs are urgently required.
In recent years, trace instrumental analysis methods, such as chromatographic methods either alone or in combination with mass spectrometry, have proved to be powerful techniques for detection of PDE-5 inhibitor residues (Balayssac et al., Citation2009; Hoff & Kist, Citation2009; Kern, Nickum, Flurer, Toomey, & Litzau, Citation2015; Lee et al., Citation2015; Trefi, Gilard, Balayssac, Malet-Martino, & Martino, Citation2009; Ulloa et al., Citation2015). Although these methods are accurate and can provide structural information of known adulterants and related compounds, they have limited scope for the identification of new analogs with unique structural modifications. In addition, they are expensive, complex, and require trained operators, which means they may not be suitable for rapid primary on-site detection. Thus, further methodology improvements are needed.
The ideal screening method is rapid, specific, sensitive, accurate, and inexpensive, and can be used for simultaneous determination of various PDE-5 inhibitors and their analogs with minimal sample preparation (Patel et al., Citation2014; Xie et al., Citation2009). Taking these requirements into consideration, techniques based on immunological principles are highly appropriate because of the specificity of the antigen-antibody reaction (Singh, Sharma, & Nara, Citation2015). To date, numerous sensitive Au nanoparticles-based immunochromatographic sensors have been developed for diagnostic purposes and environmental monitoring (Chai, Wang, Wang, Li, & Su, Citation2010; Nara, Tripathi, Singh, & Shrivastav, Citation2010). Compared with classic enzyme linked immunosorbent assay (ELISA), Au immunochromatographic sensors are not restricted by incubation and washing steps, and give results in 5–15 min, which means they provide an attractive screening method for small analytes in the food safety area (Guo et al. Citation2017; Huang et al. Citation2016b; Liang et al., Citation2016; Peng et al., Citation2017; Yang et al., Citation2015; Zhao et al., Citation2008; Peng et al. Citation2017; Guo et al. Citation2017).
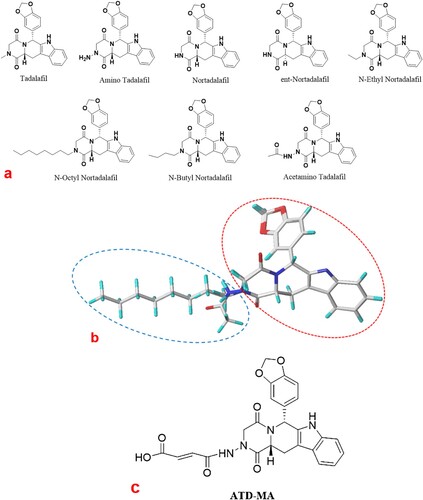
A series of immunoassay methods have been investigated for first-generation PDE-5 inhibitors such as sildenafil and vardenafil, where the similarities in their structures and binding sites allow for production of antibodies with high specificity (Guo, Xu, Huang, He, & Liu, Citation2010; Song, Wang, Zhang, & Wang, Citation2012). As a second-generation PDE-5 inhibitor, tadalafil differs markedly from sildenafil and vardenafil in its chemical structure and has improved PDE selectivity, superior therapeutic benefits, and a broad application range (Manallack, Hughes, & Thompson, Citation2005). Therefore, in the long-term, tadalafil may have greater side effects than other PDE-5 inhibitors. The identified tadalafil analogs all have the same characteristic skeleton (a), which makes a tadalafil class-specific immunoassay possible and preferable to instrumental analysis. To date, no one has reported an immunoassay for tadalafil and its analogs.
Figure 1. (a) Chemical structures of tadalafil and analogs. (b) Molecular alignment of all molecules based on their common skeleton. (c) Structure of the hapten ATD-MA.
In this study, we developed an immunochromatographic sensor (ICS) using Au nanoparticles (AuNPs) and a one analyte-specific polyclonal antibody based according to the competitive principle. This sensor could detect tadalafil and its analogs simultaneously in health food.
Material and methods
Reagents and instruments
Tadalafil (99%), ATD (99%), and triethylamine were obtained from Aladdin Chemical Technology Co., Ltd. (Shanghai, China). Complete and incomplete Freund's adjuvants, keyhole limpet hemocyanin (KLH), bovine serum albumin (BSA), dicyclohexylcarbodiimide (DCC), N-Hydroxysuccinimide (NHS), HAuCl4(99%), horseradish peroxidase, and peroxidase labelled goat anti-rabbit IgG (secondary antibody) were purchased from Sigma-Aldrich (St. Louis, MO, USA). N,N-Dimethylformamide (DMF), acetonitrile, Tween-20, ethanol, methanol, ethyl acetate, tetrahydrofuran, and chloroform were brought from Damao Chemical Reagent Co., Ltd. (Tianjin, China). All other reagents were of analytical reagent grade or higher purity.
The buffers used in this study were: phosphate-buffered saline (PBS; 0.1 g of KH2PO4, 0.1 g of KCl, 2.9 g of Na2HPO4·12 H2O, and 8.5 g of NaCl in 1 L of distilled water; pH 7.4; stored at 4°C); and PBS with Tween 20 (PBST) (0.05% volume fraction of Tween 20 in PBS; pH 7.4; stored at 4°C) for washing. The blocking solution was 5% (mass fraction) skim milk powder in PBS. The coating buffer for ELISA was 0.1 mol L−1 carbonate buffer (1.5 g of Na2CO3 and 2.695 g NaHCO3 in 1 L of distilled water; pH 9.6). The stopping reagent was 2 mol L−1 H2SO4.The TMB substrate solution was prepared using 10 mL of the substrate buffer, 150 mL of 15 mg L−1 TMB-DMF, and 2.5 mL of 6% (mass fraction) aqueous H2O2.
We used a hand-held nanogold immunochromatography strip reader (Nanjing Microdetection Bio-Tech Co. Ltd., Nanjing, China) and UPLC-MS/MS (1290–6495; Agilent Technologies, Santa Clara, CA, USA) to realize quantitative detection.
Molecular alignment
To compare the various space configurations, molecular alignment was conducted with the Sybyl 7.3 software package (Tripos, Inc., St. Louis, MO, USA). The tadalafil structures were optimized to low-energy conformations using the standard Tripos force field with an 8 Å cutoff for non-bonded interactions in conjunction with Gasteiger-Hückel charges, and a 0.005 kcal mol−1 Å−1 termination gradient. The optimum structures obtained were put into a database. The database included all previously optimized structures of tadalafil and its analogs. The common multi-ring structure of the tadalafil analogs was chosen as the basis for the alignment.
Preparation of antigen
The hapten ATD-MA was synthesized using ATD (0.2 mmol) and MA (0.3 mmol) in DMF (5 mL). The mixture was stirred at 60°C under reflux for 3 h. The solvent was evaporated under vacuum and the residue was recrystallized in ethyl acetate/hexane. The solution was filtered and dried to obtain a white solid.
Small molecules conjugated with carrier proteins should have immunogenicity (Houk, Leach, Kim, & Zhang, Citation2003). Therefore, ATD-MA-KLH (immunogen) and ATD-MA-BSA (coating antigen) conjugates were prepared by an active ester method (Byzova et al., Citation2014). ATD (1 equiv), NHS (1.5 equiv), and DCC (1.5 equiv) were dissolved in 200 μL of DMF. The mixture was stirred gently at 4°C overnight, and then centrifuged at 2500×g for 10 min. The supernatant (about 200 μL) was added dropwise to KLH (10 mg) and BSA (10 mg) in 5 mL of PBS (pH 7.4). The conjugate mixture was stirred at 4°C for 12 h, and then purified on Sephadex G-25 using 0.01 mol/L NaHCO3 as the eluent. The eluted conjugates were purified by dialysis against PBS (pH 7.4) for 3 days at 4°C. This removed the uncoupled free hapten and non-reacted reactants. A full wavelength (200–800 nm) UV-Vis scan was used to confirm the conjugation.
Preparation and production of polyclonal antibodies
Two New Zealand white rabbits (10-weeks-old, 1.5–2.0 kg) were raised at the South China Agricultural University Experimental Animal Centre under strictly controlled conditions. These two rabbits were immunized with ATD-MA-KLH conjugates by intradermal and subcutaneous injection. One microliter of an emulsion (1:1) containing 1 mg mL−1 of an immunogen in PBS and an equivalent amount of complete Freund's adjuvant were prepared for the first dose. Three booster immunizations were given at intervals of 3 weeks with the same dose of immunogen emulsified in Freund's incomplete adjuvant. Antisera were collected 1 week after the fourth immunization and were analyzed for anti-tadalafil activity by ic-ELISA. Three days after the fifth injection, blood was drawn from heart after anesthetizing the animals and left to coagulate for 1 h at 37°C. Antisera were separated by centrifugation at 4°C and 6000×g for 20 min. All the experiments were carried out in accordance with the guidelines issued by the Ethical Committee of South China Agricultural University.
To obtain purified polyclonal antibodies, the antisera were collected and purified by the caprylic acid–zinc sulfate method (Wang et al., Citation2017). Caprylic acid (33 μg mL−1) was added dropwise to a mixture of 5 mL of antiserum and 10 mL of sodium acetate buffer (0.06 mol L−1, pH 4.5) with stirring. The mixture was stirred for another 30 min and then centrifuged at 10,000×g and 4°C for 30 min to remove the precipitate. The supernatant was filtered through a 0.45-μm filtration membrane, mixed with PBS, and the pH adjusted to 7.4 with 1 mol L−1 NaOH solution. Ammonium sulfate was added to the cooled supernatant and the mixture was stirred for 30 min, and then centrifuged at 10,000×g and 4°C for 30 min. The supernatant was removed, and the precipitate was dissolved in PBS and dialyzed overnight. The resulting solution was dividing into aliquots and stored at −20°C until required for analysis.
ic-ELISA
The ic-ELISA was performed to characterize the sensitivity of the antibody. The inhibition rates against tadalafil and IC50 were evaluated by standard curves. The cross-reactivity (CR%) values of the antibody with tadalafil analogs at a concentration of 1 mg mL−1 were calculated using the following equation to determine the specificity:(1)
(1)
Preparation of AuNPs
The AuNPs were produced by the sodium citrate method (Wang et al., Citation2017). Briefly, 100 mL of 0.01% HAuCl4 solution was kept boiling for 1 min under constant stirring. Then, 1.5 mL of 1% sodium citrate was quickly added as the solution turned from yellow to red. The solution was heated and stirred for another 10 min until the red colour was stable. The obtained AuNPs solution was cooled to room temperature and stored at 4°C. Transmission electron microscopy and UV-visible spectrometry were used to characterize the AuNPs.
Formation of AuNPs-labelled conjugated probe
The experimental pH, antibody dose, and concentration were optimized according to established methods (Di Nardo, Baggiani, Giovannoli, Spano, & Anfossi, Citation2017; Kong et al., Citation2016). Tubes were prepared containing the AuNPs solution with the pH adjusted to between 5.0 and 9.0 with 0.1 mol L−1 HCl or K2CO3. The antibodies were added to the tubes, and the mixture was reacted for 10 min at RT. This was followed by addition of aqueous NaCl (10% volume fraction) and vortex mixing. The colour stability of the AuNPs solution was evaluated by measuring the absorbance at 521 nm. Then, various doses of antibody (1, 1.6, 2, 2.4, 3, 4 μL) were added along with 1 mL AuNPs solution for labelling, which was adjusted to the optimum pH. The mixture was incubated for 30 min at room temperature and then 10% BSA was added to block excess binding sites on the AuNPs for 5 min. After centrifuging at 10,000×g and 4°C for 15 min, the supernatant was removed, and the precipitate was dissolved in phosphoric acid buffer (0.01 mol L−1) and stored at 4°C. The stability of the AuNPs-antibody conjugation was evaluated by measuring the absorbance at 521 nm.
Assembly and principles of the AuNPs immunochromatographic sensor
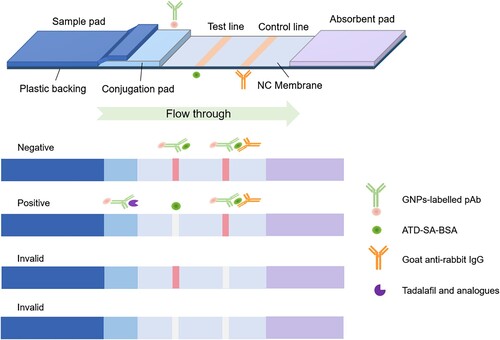
The sensor was composed of a sample pad (glass fibre, 15 × 60 mm, Owenscorning, Toledo, OH, USA), conjugate pad (glass fibre, 4 × 60 mm, Millipore, Billerica, MA, USA), nitrocellulose membrane (5 × 75 mm, GE, Marlborough, MA, USA), and absorbent pad (10 × 60 mm, Gene, Hong Kong, China). The conjugate pad was painted with the AuNPs-labelled antibody solution and dried for 1 h at 37°C. The sample pad was saturated with PBST buffer (pH 7.4, 0.01 mol L−1) and dried at room temperature. All four parts were pasted on a PVC baking card. The T line and control line (C line) were placed in the middle of the nitrocellulose membrane and situated 6 mm apart from each other. The coating antigen (ATD-MA-BSA, 1 mg mL−1) was immobilized on the T line near the sample pad, and goat anti-rabbit antibody was sprayed onto the C line. Then, the assembled card was dried for 2 h at 37°C and stored under dry conditions at room temperature in a desiccator. If the C line turns colourless during analysis, the strip is considered ineffective and invalid. The packing and testing operations were carried out at room temperature and the humidity was maintained below 30% with no air movement. The principle of the sensor is founded on the theory of competitive reaction. The standard solution or sample solution was dipped in a well on the sensor placed flat to start the capillary migration process from the sample pad to the absorbent pad. The results were judged within 10 min both by eye and using an immunochromatographic reader. All the procedures for optimization of the conditions are shown in Figure S2.
The T line of each strip was evaluated using a hand-held immunochromatography strip reader. The test sensor sensitivity was determined by applying it to a series of diluted standard sample extracts containing 0–100 ng of tadalafil. These tests were performed in triplicate. A standard curve was constructed by plotting the logarithm of the tadalafil concentration on the x-axis and the T/C, where T is the reading from the T line and C represents the value of a negative sample, on the y-axis using Origin 9.1(Originlab Corp., Northampton, MA, USA). To evaluate the specificity of the test strip sensor, other PDE-5 inhibitors and structural analogs of tadalafil were also tested at a concentration of 1 µg mL−1.
Recovery of spiked samples
In recovery experiments, health food spiked with tadalafil and its analogs were used to evaluate the sensitivity of the sensor. First, a 1 mg mL−1 solution of each drug was prepared in methanol, and these solutions were then diluted with PBST to a range final concentration. Because high methanol contents in liquid health care products and high carbohydrate contents in solid health care products may affect the colour intensities on the T and C lines, which could cause errors in the reading of results, we treated the health food samples to remove the matrix. After spiking with tadalafil and its analogs, the liquid health food were diluted ten-fold by PBST to eliminate matrix effects. The solid health care products were extracted with methanol after adding tadalafil and its analogs. For each extracted sample, the precipitate was removed by centrifugation at 8000×g and 4°C for 10 min, and the supernatant was dried under a stream of nitrogen gas. Methanol (100 μL) was added to the supernatant, and it was then diluted to 1 mL with PBST.
Verification testing was performed using a UPLC-MS/MS (1290–6495, Agilent Technologies). A Poroshell 120 SB-C18 column (2.1 × 100 mm, 2.7 μm) was used to separate the analytes. The column temperature was set at 35°C. The mobile phase was acetonitrile containing 0.1% formic acid with a flow rate of 0.4 mL/min. The sample volume was 2 μL. All solutions were sonicated for 5 min before use. Further details for the UPLC-MS/MS are given in Table S2.
The IC50 and cLOD were acquired from the standard curve. Intra-assay precision was estimated using one batch of the test strips. For inter-assay precision, three batches of the test strips were used. The coefficient of variation (%) and recovery was also calculated.
Analysis of tadalafil and its analogs in health food
9 liquids and 13 solid of health food were randomly purchased from markets and pharmacies in (Guangzhou, China). These samples were prepared for detection of tadalafil and its analogs using our sensor. Each sample was analyzed in triplicate. UPLC-MS/MS was used to confirm the accuracy of the sensor results. Statistical analysis was analyzed using SPSS version 15.0 (SPSS, Inc., Chicago, IL, USA).
Result and discussion
Hapten design and antigen characterization
Hapten design plays a vital role in the preparation of antibodies. An optimum hapten should preserve distinctive functional groups for good exposure to elicit antibody production (Harrison, Goodrow, Gee, & Hammock, Citation1991). The overall surface complementarity, specific interactions, and hydrogen bonding are also critical determinants in antigen recognition (Janeway, Travers, Walport, & Shlomchik, Citation2001). Tadalafil and its analogs have similar chemical structures ( a) and can be aligned based on their common structural features (b). These molecules share a multi-ring skeleton (shown by the red dotted line in b) and have similar electron distributions. The only difference among them is the side chain (shown by the blue dotted line in b), which mainly differs in the heteroatom and length of the carbon chain. We selected amino tadalafil (ATD) as the optimum substrate to design the hapten as it has not only had the common skeleton but also an amino group on side chain for linker arm derivatization, which makes it possible to produce a broad-spectrum and class-specific antibody for tadalafil and its analogs.
However, small molecule substances do not generally have sufficient immunogenicity to stimulate a host to produce antibodies (Isanga et al., Citation2017). Thus, the production of hapten-specific antibodies is required by linking small molecules to carrier proteins via an appropriate linker arm (Barnych et al., Citation2017). As a part of our ongoing research dedicated to the development of an immunoassay method based on rational hapten design, we reported a strategy for improving the specific immunoactivity of a hapten using an unsaturated linker arm (Liu et al., Citation2018; Shen et al., Citation2007). In the present study, we extended this strategy to hapten design for tadalafil and its analogs. Maleic anhydride (MA) was linked to the amino group of ATD to synthesize the hapten ATD-MA (Scheme 1 in Supporting Information). The structure of ATD-MA (c) was characterized by MS and NMR. The results were: HRMS (ESI) m/z: [M + H]+ calcd for C25H20N4O7, 489.13; found, 489.00. Anal. calcd for C25H20N4O7: C 62.47, H 3.41, N 6.78; found: C 61.47, H 4.13, N 11.47, O 22.93. 1H NMR (600 MHz, DMSO-d6, δ): 12.87 (s, 1H), 11.13 (s, 1H), 10.77 (s, 1H), 7.56 (d, J = 7.8 Hz, 1H), 7.32 (d, J = 8.1 Hz, 1H), 7.07 (ddd, J = 8.2, 7.0, 1.2 Hz, 1H), 7.01 (td, J = 7.5, 1.0 Hz, 1H), 6.88 (d, J = 1.6 Hz, 1H), 6.84–6.77 (m, 2H), 6.44 (d, J = 12.0 Hz, 1H), 6.33 (d, J = 12.0 Hz, 1H), 6.22 (s, 1H), 5.94 (s, 2H), 4.63 (dd, J = 11.6, 4.7 Hz, 1H), 4.54 (dd, J = 16.4, 1.2 Hz, 1H), 3.97 (d, J = 16.4 Hz, 1H), 3.51 (dd, J = 15.7, 4.9 Hz, 1H), 2.98 (dd, J = 15.6, 11.7 Hz, 1H), 2.89 (s, 1H), 2.73 (s, 1H). 13C NMR (150 MHz, DMSO-d6, δ): δ 166.98, 166.93, 166.61, 163.96, 147.62, 146.62, 136.95, 136.56, 134.30, 131.21, 130.39, 121.76, 119.60, 119.39, 118.62, 111.81, 108.48, 107.20, 104.77, 101.41, 55.53, 55.34, 53.25, 40.52, 39.62, 36.24, 22.91.
Furthermore, ATD-MA was covalently coupled via its carboxyl group to the free amino group of a carrier protein (KLH and BSA) by the active ester method, which generated an immunogen (ATD-MA-KLH) and coating antigen (ATD-MA-BSA). Their UV-visible absorption spectra were recorded (Figure S1). The hapten ATD-MA had a strong absorbance peak at 305 nm, and the carrier protein (KLH and BSA) peak was at 280 nm. The maximum absorbance peak of ATD-MA-BSA was at 295 nm, which was obviously offset from the BSA peak. Furthermore, the absorbance peak of ATD-MA-KLH had shifted compared with that of KLH. These obvious peak shifts indicated the antigen syntheses were successful. According to the TNBS method, the molar ratio of immunogen and coating antigen were calculated as 21:1 and 18:1.
Assessment of polyclonal antibody
The polyclonal antibody was assessed using indirect competitive-ELISA (ic-ELISA), which showed the antibody had a high titer of 1:32000 and a half maximal inhibitory concentration (IC50) of 0.90 ng mL−1 with an inhibition rate of 95% towards tadalafil (Table S1). The results of a specificity evaluation with cross-reactivity () demonstrated that the antibody could recognize tadalafil analogs to different degrees, and that recognition of other PDE-5 inhibitors (e.g. sildenafil and vardenafil) and other functional compounds (e.g. tryptophan) was negligible (<0.05%). These results might be attributed to the structural characteristic of tadalafil-class drugs (i.e. common skeleton but differences in the side chain) providing a basis for broad-spectrum class-specific recognition. However, compared with tadalafil, the other PDE-5 inhibitors and functional compounds have quite different heterocyclic structures and heteroatoms, which led to poor identification by the antibody. Our results suggested that the antibody had high specificity to the analyte, which could reduce the false positive rate considerably. Therefore, this antibody is reliable and effective for detection of tadalafil and its analogs.
Table 1. Cross-reactivity of the sensor with tadalafil analogues (n = 3).
Characterization of AuNPs and labelling with the antibody
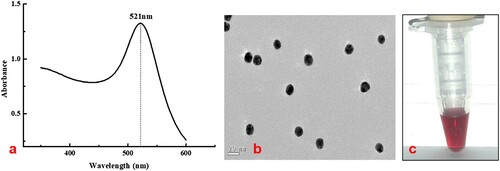
A literature search suggested that Au nanoparticles with particle diameters of approximately 20 nm were optimal for most diagnostic applications because of the balance between required visibility and steric hindrance (Guo, Liu, Gui, & Zhu, Citation2009). The prepared AuNPs had an absorption peak at 521 nm with a strong signal under UV irradiation (a), and their particle diameter was 16 nm according to the transmission electron microscopy (TEM) results (b). The AuNPs were red (c) with uniform particle appearances and proper sizes (b). These results indicated that preparation of the Au nanoparticles was successful. The sensor was illustrated in . Generally, if tadalafil is absent from the sample, both the C line and T line will be visible. This means the AuNPs-labelled antibody and the antigen on the T line conjugate to form the AuNPs-antibody-antigen, and redundant AuNPs-antibody and goat anti-rabbit IgG on the C line combine. All the detection reagent is trapped and forms red T and C lines, which is a negative result. By contrast, a positive result shows only a red C line. In this case, tadalafil will compete with the immobilized coating antigen for the limited amount of AuNPs-antibody. The colour of the T line is inversely proportional to the concentration of tadalafil in the sample. A higher concentration of tadalafil in a sample means that less AuNPs-antibody reacts with the antigen, giving a lighter colour on the T line. If there is sufficient tadalafil, it will completely block the reaction with the capture reagent, leaving no visible T line on the nitrocellulose membrane and showing a strong positive result. A lightly coloured T line is a weak positive result.
Figure 2. Characterization of Au nanoparticles: (a) UV-Vis spectra, (b) transmission electron microscopy image, and (c) photograph of Au nanoparticle solution.
According to the theory, the combination of AuNPs and an antibody can work effectively only under suitable conditions, such as the pH and dose. AuNPs and an antibody combine mainly through van der Waals forces and hydrogen bonds. Their stability is principally determined by the charge distribution on the surface of the AuNPs and antibody, and pH plays an important role (Paek, Lee, Cho, & Kim, Citation2000; Xiulan, Xiaolian, Tang, Zhou, & Chu, Citation2005). Conjugation is only stable when the pH of the system is equal to or 0.5 above the isoelectric point of the antibody (Chen et al. Citation2008). In this work, using a pH gradient, we found the maximum absorbance occurred at pH 8.5 and the isoelectric point of the antibody was around 8.0. Therefore, to obtain the maximum absorption of the AuNPs-labelled antibody and achieve the best results, the pH should be adjusted to 8.5 by 0.1 M K2CO3 in this study.
In addition, the dose of AuNPs-labelled antibody and the amount of coating antigen are key factors affecting the stability of the test line (T line) colour on the sensor (Xiulan et al., Citation2005). A lighter coloured T line suggests the presence of fewer labelled-antibodies and coating antigen than a darker T line, which could lead to misreading of a negative result as a false positive. By contrast, an excess of labelled-antibody and a high concentration of coating antigen could make the T line too dark, which could be interpreted as a false negative. Previous studies have suggested the most suitable antibody dose for labelling is the minimum dose that can maintain the red colour of the AuNP solution, or 20%–25% more than this dose to ensure stability in practice (Zhang et al., Citation2008). In this work, the absorbance peak and stable colour appeared when the dose of AuNP-labelled antibody was 3 μL (Figure S2 in Supporting Information).
Specificity and sensitivity
The specificity of the sensor was evaluated by testing the cross-reactivities of 30 tadalafil analogs under the optimized experimental conditions. Results were obtained rapidly (within 10 min). The results demonstrated the antibody had cross-reactivities of 40.0%–105.2% with tadalafil analogs, and extremely low cross-reactivities (<0.01%) with other PDE-5 inhibitors and functional compounds. Besides, compared with ic-ELISA, the sensor not only exhibited consistent accuracy, but also showed higher cross-reactivities with tadalafil and its analogs, except for nortadalafil. These results show that the prepared sensor has high specificity towards tadalafil and its analogs.
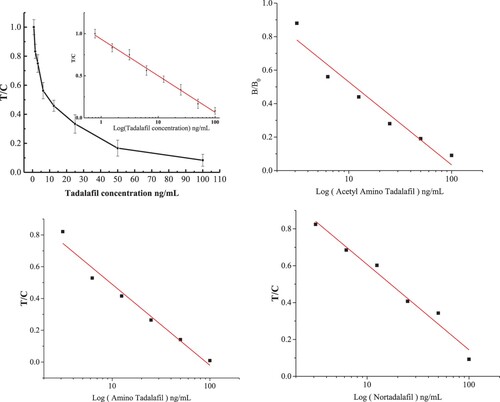
The sensitivity of the sensor was further confirmed by measuring its response to tadalafil standard samples. The responses of standard tadalafil and other analogs to the sensor were plotted in a sigmoidal curve (). For tadalafil, the regression equation was y = 0.93533 − 0.43458 lgx (R2 = 0.9954) with an IC50 of 10.04 ng mL−1 and a wide detection range (0.71–141.99 ng mL−1), which could meet the demands for detection of health food in practice. For the tadalafil analogs acetamino tadalafil, ATD and nortadalafil, the IC50 were 9.55, 11.46, and 17.01 ng mL−1, and the linear detection ranges were 1.12–115.43, 0.66–91.27, and 0.96–204.33 ng mL−1, respectively. The sensor showed high sensitivity and could be applied to trace and broad-spectrum quantitative determination of tadalafil and its analogs.
Semi-quantitative determination by the naked eye was carried out to evaluate the sensitivity of the sensor. We recorded photographs of the sensor to show the visual detection results () and compared visual and calculated data () for the IC50, calculated limit of detection (cLOD), visual limit of detection (vLOD), and cutoff values for standards of tadalafil and its analogs. For visual interpretation, a lighter T line indicates a higher concentration of tadalafil than a darker T line. Generally, cLOD is defined as the concentration that leads to 20% inhibition (IC20) of B/B0 (the OD value of test and control samples) from the standard curves (), and vLOD is the lowest concentration that makes the T line lighter in colour than the control line (Guo et al. Citation2017). The cutoff value refers to the drug concentration at which the T line becomes colourless. The cLOD values of tadalafil, acetamino tadalafil, ATD, and nortadalafil were 0.71, 1.12, 9.55, and 0.95 ng mL−1, respectively. For these compounds, the corresponding vLOD values were 5, 5, 12.5, and 6.25 ng mL−1 in the liquid health care products and 12.5, 12.5, 25, and 12.5 ng mL−1 in the solid health care products ( and ). The cutoff values were 50, 50, 100, and 50 ng mL−1 in the liquid health care products and 100, 150, 200, and 100 ng mL−1 in the solid health care products. Theoretically, there was a negative correlation between the antibody sensitivity and the cutoff value, and a low concentration of tadalafil could induce a colourless T line, indicating strong inhibition from the antibody. For the present study, this phenomenon is illustrated in for tadalafil and ATD. The cutoff and vLOD differences between the solid and liquid health care products might be caused by matrix interference and extraction losses. Overall, the sensor gave satisfactory semi-quantitative detection by the naked eye.
Figure 5. Images of sensor testing various liquid and solid health food spiked with tadalafil and its analogs (ng mL−1 levels). (A1) Tadalafil–liquid, (A2) tadalafil–solid, (B1) acetamino tadalafil–liquid, (B2) acetamino tadalafil–solid, (C1) nortadalafil–liquid, (C2) nortadalafil–solid, (D1) amino tadalafil–liquid, and (D2) amino tadalafil–solid.
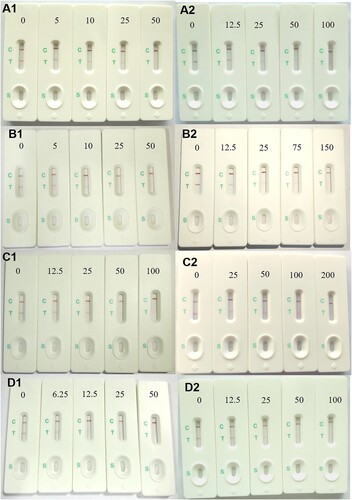
Table 2. The calculated and visual data of tadalafil and analogues (ng mL−1).
In summary, the immunochromatographic sensor exhibited high specificity, sensitivity, and efficiency in the detection of tadalafil and its analogs in liquid and solid health food.
Spiking and recovery
Spiking and recovery tests with tadalafil and its analogs were performed by the sensor and ultra-performance liquid chromatography-tandem mass spectrometer (UPLC-MS/MS) (). The recoveries obtained with the sensor ranged from 81.6% to 128.7% for the different amounts of tadalafil and its analogs spiked into different health food. These results were basically the same as the UPLC-MS/MS results, showing that the sensor has high accuracy and reliability. The mass spectra of tadalafil and its analogs are illustrated in Figure S3 and calibration curves for the concentration calculations are presented in Figure S4. The recovery results from these two methods were analyzed by linear regression and the two sets of data showed a positive correlation with a coefficient of determination (R2) of 0.9598 (Figure S5), revealing good reliability and accuracy for this sensor.
Table 3. Recoveries for health care products spiked with tadalafil [ng g−1(Ml), n = 3]a.
Blind test of commercial health food
We applied the sensor to common health food purchased in a market ( and ), including nine liquid samples and thirteen solid samples. The detection results for the health food showed that three of the nine liquid samples and four of the thirteen solid samples showed positive reactions with a detectable amount above microgram levels. The tadalafil detection rate and its analogs in these samples was 31.8%. To evaluate the reliability of these results, UPLC-MS/MS was used to verify the positive samples, and the results agreed with the sensor results. Thus, the sensor method could meet the high reliability and efficiency demands of detection. These findings also suggest that adulteration of health food with tadalafil and its analogs has become a considerable problem.
Figure 6. Photographs of the results for screening of seven commercial health care products using the sensor.

Table 4. Detected positive sample was by AuNPs-ICS and UPLC-MS/MS in Random sample detection [μg g−1(mL), n = 3].
Conclusion
In summary, an immunochromatographic sensor for detection of tadalafil and its analogs was developed by designing a specific hapten to obtain a broad-spectrum antibody labelled with AuNPs. The cross-reactivity, quantitative and semi-quantitative detection, recovery and comparison with UPLC-MS/MS results were used to evaluate the specificity, sensitivity, and accuracy of the sensor. Our results indicate that the sensor is reliable for detection of tadalafil and its analogs. The analysis of commercial health care products was conducted using the sensor, which verified the sensor could deliver results efficiently and directly either by the naked eye or immunochromatographic reader. The developed immunochromatographic sensor has broad potential for simultaneous on-site detection on tadalafil and its analogs in health food.
Ethical approval
This article does not contain any studies with human subjects. All animal experiments that described in the present study were performed in the animal centre of South China Agricultural University, following all institutional and national guidelines for the care and use of laboratory animals.
Informed Consent: Not applicable.
Supplemental Material
Download ()Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31871887), the National Key Research and Development of China (2018YFC1602904), the Science and Technology Planning Project of Guangzhou (201704020082), Science and Technology Planning Project of Guangxi (AB18126048) and Guangdong Provincial Natural Science Foundation (2018B030314005). We thank Gabrielle David, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abdel-Aziz, M., Asiri, A., El-Azab, S., Al-Omar, A., & Kunieda, T. (2011). Chapter 8–Tadalafil. Profiles of Drug Substances Excipients & Related Methodology, 36, 287–329.
- Alp, M., Coşkun, M., & Göker, H. (2013). Isolation and identification of a new sildenafil analogue adulterated in energy drink: Propoxyphenyl sildenafil. Journal of Pharmaceutical and Biomedical Analysis, 72(2), 155–158. doi: 10.1016/j.jpba.2012.09.017
- Balayssac, S., Trefi, S., Gilard, V., Malet-Martino, M., Martino, R., & Delsuc, M. A. (2009). 2D and 3D DOSY 1H NMR, a useful tool for analysis of complex mixtures: Application to herbal drugs or dietary supplements for erectile dysfunction. Journal of Pharmaceutical and Biomedical Analysis, 50(4), 602–612. doi: 10.1016/j.jpba.2008.10.034
- Barnych, B., Vasylieva, N., Joseph, T., Hulsizer, S., Nguyen, H. M., Cajka, T., … Hammock, B. D. (2017). Development of Tetramethylenedisulf otetramine (TETS) hapten library: Synthesis, electrophysiological studies, and immune response in rabbits. Chemistry - A European Journal, 23(35), 8466–8472. doi: 10.1002/chem.201700783
- Byzova, N., Smirnova, N., Zherdev, A., Eremin, S., Shanin, I., Lei, H., … Dzantiev, B. (2014). Rapid immunochromatographic assay for ofloxacin in animal original foodstuffs using native antisera labeled by colloidal gold. Talanta, 119, 125–132. doi: 10.1016/j.talanta.2013.10.054
- Chai, F., Wang, C., Wang, T., Li, L., & Su, Z. (2010). Colorimetric detection of Pb2+ using glutathione functionalized gold nanoparticles. ACS Applied Materials & Interfaces, 2(5), 1466–1470. doi: 10.1021/am100107k
- Chen , Y, Wang , Z, & Wang , Z. (2008). Rapid Enzyme-Linked Immunosorbent Assay and Colloidal Gold Immunoassay for Kanamycin and Tobramycin in Swine Tissues. Journal of Agricultural and Food Chemistry, 56(9), 2944–2952. http://doi.org/10.1021/jf703602b
- Chrysant, S. (2013). Effectiveness and safety of phosphodiesterase 5 inhibitors in patients with cardiovascular disease and hypertension. Current Hypertension Reports, 15(5), 475–483. doi: 10.1007/s11906-013-0377-9
- Di Nardo, F., Baggiani, C., Giovannoli, C., Spano, G., & Anfossi, L. (2017). Multicolor immunochromatographic strip test based on gold nanoparticles for the determination of aflatoxin B1 and fumonisins. Microchimica Acta, 184(5), 1295–1304. doi: 10.1007/s00604-017-2121-7
- Guo, J., Liu, W., Chen, H., Zhang, M., & Lan, X. (2017). Development and evaluation of a broad-specific immunochromatographic assay for screening of both tadalafil and its analogues in functional foods. Food and Agricultural Immunology, 28(4), 652–667. doi: 10.1080/09540105.2017.1309360
- Guo, Y., Liu, S., Gui, W., & Zhu, G. (2009). Gold immunochromatographic assay for simultaneous detection of carbofuran and triazophos in water samples. Analytical Biochemistry, 389(1), 32–39. doi: 10.1016/j.ab.2009.03.020
- Guo, L., Wu, X., Liu, L., Kuang, H., & Xu, C. (2017). Gold nanoparticle-based paper sensor for simultaneous detection of 11 benzimidazoles by one monoclonal antibody. Small, 14(6), 1701782. doi: 10.1002/smll.201701782
- Guo, J., Xu, Y., Huang, Z., He, Q., & Liu, S. (2010). Development of an immunoassay for rapid screening of vardenafil and its potential analogues in herbal products based on a group specific monoclonal antibody. Analytica Chimica Acta, 658(2), 197–203. doi: 10.1016/j.aca.2009.11.021
- Gur, S., Kadowitz, J., Gokce, A., Sikka, C., Lokman, U., & Hellstrom, J. (2013). Update on drug interactions with phosphodiesterase-5 inhibitors prescribed as first-line therapy for patients with erectile dysfunction or pulmonary hypertension. Current Drug Metabolism, 14(2), 265–269. doi: 10.2174/138920013804870600
- Harrison, R., Goodrow, M., Gee, S., & Hammock, B. (1991). Hapten synthesis for pesticide immunoassay development. Immunoassays or Trace Chemical Analysis, Chapter 2. ACS Symposium Series - American Chemical Society, 451, 14–27. doi: 10.1021/bk-1990-0451.ch002
- Hoff, R., & Kist, T. (2009). Analysis of sulfonamides by capillary electrophoresis. Journal of Separation Science, 32(5–6), 854. doi: 10.1002/jssc.200800738
- Houk, K., Leach, A., Kim, S., & Zhang, X. (2003). Binding affinities of host-guest, protein-ligand, and protein-transition-state complexes. Angewandte Chemie International Edition, 42(40), 4872–4897. doi: 10.1002/anie.200200565
- Huang, X., Aguilar, Z., Xu, H., Lai, W., & Xiong, Y. (2016a). Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: A review. Biosensors and Bioelectronics, 75, 166–180. doi: 10.1016/j.bios.2015.08.032
- Huang, Y., Lee, H., Lin, Y., Tsai, C., & Cheng, H. (2016b). Identification of a new tadalafil analogue, dipropylaminopretadalafil, in a dietary supplement. Food Additives & Contaminants: Part A, 33(6), 953–958. doi: 10.1080/19440049.2016.1184530
- Isanga, J., Mukunzi, D., Chen, Y., Suryoprabowo, S., Liu, L., Kuang, H., & Xu, C. (2017). Development of a monoclonal antibody assay and a lateral flow strip test for the detection of paromomycin residues in food matrices. Food and Agricultural Immunology, 28(3), 355–373. doi: 10.1080/09540105.2016.1272551
- Janeway, C. A., Travers, P., Walport, M., & Shlomchik, M. (2001). Immunobiology: The immune systems in health and disease. Immuno Biology, 5, 892. doi: 10.1111/j.1467-2494.1995.tb00120.x
- Kern, S., Nickum, E., Flurer, R., Toomey, V., & Litzau, J. (2015). Isolation and structural characterization of a new tadalafil analog (2-hydroxyethylnortadalafil) found in a dietary supplement. Journal of Pharmaceutical and Biomedical Analysis, 103, 99–103. doi: 10.1016/j.jpba.2014.10.021
- Kong, D., Liu, L., Song, S., Suryoprabowo, S., Li, A., Kuang, H., … Xu, C. (2016). A gold nanoparticle-based semi-quantitative and quantitative ultrasensitive paper sensor for the detection of twenty mycotoxins. Nanoscale, 8(9), 5245–5253. doi: 10.1039/c5nr09171c
- Lee, J., Kim, N., Han, K., Kim, S., Cho, S., & Kim, W. (2013). Monitoring by LC-MS/MS of 48 compounds of sildenafil, tadalafil, vardenafil and their analogues in illicit health food products in the Korean market advertised as enhancing male sexual performance. Food Additives & Contaminants: Part A, 30(11), 1849–1857. doi: 10.1080/19440049.2013.826878
- Lee, J., Kim, H., Noh, E., Kim, J., Cho, S., Do, J., … Kim, W. (2015). Identification and screening of a tadalafil analogue found in adulterated herbal products. Journal of Pharmaceutical and Biomedical Analysis, 103, 80–84. doi: 10.1016/j.jpba.2014.11.006
- Li, L., Low, M., Ge, X., Bloodworth, B., & Koh, H. (2013). Isolation and structural elucidation of a new sildenafil analogue from a functional coffee. Analytical and Bioanalytical Chemistry, 405(13), 4443–4450. doi: 10.1007/s00216-012-6236-8
- Liang, X., Fang, X., Yao, M., Yang, Y., Li, J., Liu, H., & Wang, L. (2016). Direct competitive chemiluminescence immunoassays based on gold-coated magnetic particles for detection of chloramphenicol. Luminescence, 31(1), 168–172. doi: 10.1002/bio.2940
- Liu, F., Chen, Z., Shen, Y., Sun, Y., Yang, J., Wang, H., … Xu, Z. (2018). Hapten synthesis and production of specific antibody against 3-amino-5-morpholinomethyl-2-oxazolidone for immunoassay without derivatisation. Food and Agricultural Immunology, 29(1), 332–345. doi:10.1080/09540105.2017.1376038
- Low, Y., Zeng, Y., Li, L., Ge X, W., Lee, R., Bloodworth, C., & Koh, L. (2009). Safety and quality assessment of 175 illegal sexual enhancement products seized in red-light districts in Singapore. Drug Safety, 32(12), 1141–1146. doi: 10.2165/11316690-000000000-00000
- Manallack, D., Hughes, R., & Thompson, P. (2005). The next generation of phosphodiesterase inhibitors: Structural clues to ligand and substrate selectivity of phosphodiesterases. Journal of Medicinal Chemistry, 48(10), 3449–3462. doi: 10.1021/jm040217u
- Martí, M., Ortiz, X., Gasser, M., Martí, R., Montaña, J., & Díaz-Ferrero, J. (2010). Persistent organic pollutants (PCDD/Fs, dioxin-like PCBs, marker PCBs, and PBDEs) in health supplements on the Spanish market. Chemosphere, 78(10), 1256–1262. doi: 10.1016/j.chemosphere.2009.12.038
- Nara, S., Tripathi, V., Singh, H., & Shrivastav, T. (2010). Colloidal gold probe based rapid immunochromatographic strip assay for cortisol. Analytica Chimica Acta, 682(1), 66–71. doi: 10.1016/j.aca.2010.09.041
- Paek, S., Lee, S., Cho, J., & Kim, Y. (2000). Development of rapid one-step immunochromatographic assay. Methods, 22(1), 53–60. doi: 10.1006/meth.2000.1036
- Patel, D., Li, L., Kee, C., Ge, X., Low, M., & Koh, H. (2014). Screening of synthetic PDE-5 inhibitors and their analogues as adulterants: Analytical techniques and challenges. Journal of Pharmaceutical and Biomedical Analysis, 87(1434), 176–190. doi: 10.1016/j.jpba.2013.04.037
- Peng, J., Liu, L., Xu, L., Song, S., Kuang, H., Cui, G., & Xu, C. (2017). Gold nanoparticle-based paper sensor for ultrasensitive and multiple detection of 32 (fluoro)quinolones by one monoclonal antibody. Nano Research, 10(1), 108–120. doi: 10.1007/s12274-016-1270-z
- Poon, W., Lam, Y., Lai, C., Chan, A., & Mak, T. (2007). Analogues of erectile dysfunction drugs: An under-recognised threat. Hong Kong Medical Journal, 13(5), 359–363. doi: 10.5465/amr.2013.0458
- Shen, Y., Wang, Y., Zhang, S., Xiao, Z., Sun, Y. M., Bu, X. Z., & Gu, L. (2007). Design and efficient synthesis of novel haptens and complete antigens for the AOZ, a toxic metabolite of furazolidone. Chinese Chemical Letters, 18(12), 1490–1492. doi: 10.1016/j.cclet.2007.10.030
- Shindel, A. W. (2009). Continuing medical education: 2009 update on phosphodiesterase type 5 inhibitor therapy part 1: Recent studies on routine dosing for penile rehabilitation, lower urinary tract symptoms, and other indications (CME). The Journal of Sexual Medicine, 6(7), 1794–1808. doi: 10.1111/j.1743-6109.2009.01347.x
- Singh, S., Prasad, B., Savaliya, A., Shah, P., Gohil, M., & Kaur, A. (2009). Strategies for characterizing sildenafil, vardenafil, tadalafil and their analogues in herbal dietary supplements, and detecting counterfeit products containing these drugs. TrAC Trends in Analytical Chemistry, 28(1), 13–28. doi: 10.1016/j.trac.2008.09.004
- Singh, J., Sharma, S., & Nara, S. (2015). Evaluation of gold nanoparticle based lateral flow assays for diagnosis of enterobacteriaceae members in food and water. Food Chemistry, 170, 470–483. doi: 10.1016/j.foodchem.2014.08.092
- Song, Y., Wang, Y. Y., Zhang, Y., & Wang, S. (2012). Development of enzyme-linked immunosorbent assay for rapid determination of sildenafil in adulterated functional foods. Food and Agricultural Immunology, 23(4), 338–351. doi: 10.1080/09540105.2011.630066
- Sugita, M., & Miyakawa, M. (2010). Economic analysis of use of counterfeit drugs: Health impairment risk of counterfeit phosphodiesterase type 5 inhibitor taken as an example. Environmental Health and Preventive Medicine, 15(4), 244–251. doi: 10.1007/s12199-010-0134-5
- Trefi, S., Gilard, V., Balayssac, S., Malet-Martino, M., & Martino, R. (2009). The usefulness of 2D DOSY and 3D DOSY-COSY 1H NMR for mixture analysis: Application to genuine and fake formulations of sildenafil (Viagra). Magnetic Resonance in Chemistry, 47(S1), S163–S173. doi: 10.1002/mrc.2490
- Ulloa, J., Sambrotta, L., Redko, F., Mazza, O. N., Garrido, G., Becher, E. F., & Muschietti, L. (2015). Detection of a tadalafil analogue as an adulterant in a dietary supplement for erectile dysfunction. The Journal of Sexual Medicine, 12(1), 152–157. doi: 10.1111/jsm.12759
- Venhuis, J., & de Kaste, D. (2012). Towards a decade of detecting new analogues of sildenafil, tadalafil and vardenafil in food supplements: A history, analytical aspects and health risks. Journal of Pharmaceutical and Biomedical Analysis, 69(8), 196–208. doi: 10.1016/j.jpba.2012.02.014
- Wang, W., Liu, L., Song, S., Xu, L., Kuang, H., Zhu, J., & Xu, C. (2017). Identification and quantification of eight Listeria monocytogene serotypes from Listeria spp. using a gold nanoparticle-based lateral flow assay. Microchimica Acta, 184(3), 1–10. doi: 10.1007/s00604-016-2028-8
- Xie, H., Ma, W., Liu, L., Chen, W., Peng, C., Xu, C., & Wang, L. (2009). Development and validation of an immunochromatographic assay for rapid multi-residues detection of cephems in milk. Analytica Chimica Acta, 634(1), 129–133. doi: 10.1016/j.aca.2008.12.004
- Xiulan, S., Xiaolian, Z., Tang, J., Zhou, J., & Chu, F. (2005). Preparation of gold-labeled antibody probe and its use in immunochromatography assay for detection of aflatoxin B1. International Journal of Food Microbiology, 99(2), 185–194. doi: 10.1016/j.ijfoodmicro.2004.07.021
- Yang, X., Wang, F., Song, C., Wu, S., Zhang, G., & Zeng, X. (2015). Establishment of a lateral flow colloidal gold immunoassay strip for the rapid detection of estradiol in milk samples. LWT - Food Science and Technology, 64(1), 88–94. doi: 10.1016/j.lwt.2015.04.022
- Zhang, G., Wang, X., Zhi, A., Bao, Y., Yang, Y., Qu, M., … Liu, Q. (2008). Development of a lateral flow immunoassay strip for screening of sulfamonomethoxine residues. Food Additives & Contaminants: Part A, 25(4), 413–423. doi: 10.1080/02652030701561452
- Zhao, Y., Zhang, G., Liu, Q., Teng, M., Yang, J., & Wang, J. (2008). Development of a lateral flow colloidal gold immunoassay strip for the rapid detection of enrofloxacin residues. Journal of Agricultural and Food Chemistry, 56(24), 12138–12142. doi: 10.1021/jf802648z