ABSTRACT
Bayberries are important sources of phytochemicals. In this paper, the active substances of bayberry extract (BE) were investigated, and based on this, the inhibitory effects of BE on the six tested food-borne pathogens were futher evaluated. Finally, the main antibacterial components in the extracts were identified by UV-Vis absorption spectroscopy, UPLC-UV spectrum and UPLC-ESI-MS. The results showed that the content of total phenolic was the greatest, and BE has a good inhibitory effect on the growth curve of six foodborne pathogens, and average inhibition zone diameter (IZD) reached 19.5 mm. Furthermore, the four main antimicrobial components, cyanidin 3-O-glucoside, flavonoid deoxy hexacoside, quercetin 3-O-glucoside, and quercetin deoxidized hexacoside, were identified from the BE.
Practical applications: Bayberries are important sources of phytochemicals such as phenolic acids, anthocyanins, and flavonol glycosides. Recent findings from our laboratory revealed the BE had a significant bacteriostatic effect. Therefore, this paper investigated the antibacterial activity of BE to 6 common foodborne pathogens, and on this basis further confirmed the effective active components of BE. This study provides an important reference for BE application in food, pharmaceutical and chemical fields.
GRAPHICAL ABSTRACT

1. Introduction
Chinese bayberry (Myrica rubra Sieb. et Zucc.) is an economically important subtropical fruit crop native in Southern China and other Asian countries. The fruit is popular because of its appealing colour, delicious taste, essential micronutrients and bioactive constituents (Sun et al., Citation2012). All parts of the Chinese bayberry plant were used in Chinese traditional medicine for various medicinal purposes (Sun, Huang, Xu, Li, & Chen, Citation2013). Bayberries are important sources of phytochemicals such as phenolic acids, anthocyanins, and flavonol glycosides. Many studies have shown that bayberry extracts had anti-inflammatory (Farrell et al., Citation2015) and their antioxidant(Chen, Su, Xu, Bao, & Zheng, Citation2016; Chen, Zhou, & Zheng, Citation2015; Huang, Sun, Lou, Li, & Ye, Citation2014), anti-diabetic (Sun et al., Citation2012; Zhang, Zhou, & Tao, Citation2016), anti-cancer (Yang et al., Citation2011a), anti-obesity (Meireles et al., Citation2016), anti-hyperlipidemia (Jurgonski, Juskiewicz, & Zdunczyk, Citation2008) and other effects. It also had remarkable effect in anti-atherosclerosis (Alarcon et al., Citation2015; Chan et al., Citation2014), antidiarrheal and inhibiting lipase activity (Li, Han, Chen, & Ye, Citation2012).
At present, foodborne disease caused by bacterial contamination is still one of the biggest problems affecting human health and food safety. Report pointed out that foodborne pathogens left 14 million people sick with 1800 people dead every year (Le, Baron, & Gautier, Citation2003). So far, most of the commonly used food preservatives are synthetic such as benzoic acid, sorbic acid and nitrite, etc. In addition, antibiotic substances are often used in the preservation of livestock products (Ju, Wang, Qiao, Li, & Li, Citation2017). However, the potential harmfulness to human health cannot be ignored. Consumer demand for natural antibacterial and antiseptic substances has grown as abuse of toxic synthetic food preservatives and antibiotics has become more widespread (Ju et al., Citation2017). Recent findings from our laboratory revealed the BE had a significant bacteriostatic effect (Ju et al., Citation2018). In addition, in previous studies, we also found that BE has a good effect on the treatment of diarrhea (Yao et al., Citation2011). In view of these important functions of BE, it has further aroused our interest in research. Therefore, this paper investigated the antibacterial activity of BE to six common foodborne pathogens, and on this basis further confirmed the effective active components of BE. This study provides an important reference for BE application in food, pharmaceutical and chemical fields.
2. Materials and methods
2.1. Material
In order to ensure the objective accuracy of our experimental results, the same variety of bayberry fruit (cultivar Biqi) was purchased from a supermarket in Wuxi City, Jiangsu Province, China. Portions of about 100 g (4–6 granules of fruit) were packed in polyethylene bags and subsequently frozen at −18°C.
2.2. Preparation of the BE
For the extraction, the frozen bayberry fruit was homogenized together with ethanol (1:2.5 = w/v) in a mortar. The obtained mixture was shaken at 200 rpm and 50°C for 24 h in a water bath shaker and then centrifuged at 4000 × g for 10 min. The supernatant was lyophilized to dryness. The lyophilized powder of bayberry extract was weighed (the yield was 8.28 g of 100 g fruit) and termed “BE” in our research. The BE was dissolved in 5.0 mL of water before the determination of the bioactivity detection.
2.3. Preparation of bacteria
The strains of Staphylococcus aureus ATCC 6538 (+), Listeria innocua ATCC 33090 (+), β-hemolytic streptococus CMCC 32210 (+), Salmonella enteritidis ATCC 13076 (-), Salmonella typhi CMCC 50013 (−), Shigella dysenteriae CMCC 51573-10 (−) were provided by National Center for Medical Culture Collection, China. All of the bacteria were grown in nutrient agar except β-hemolytic streptococus CMCC 32210, which was cultured in cation-adjusted Mueller-Hinton broth (Oxoid, Wesel, Germany) plus 5% lysed horse blood. Single colonies of the selected strains of bacteria were preinoculated in 10 mL of sterile nutrient broth and incubated at 37°C or 8 h; 1 mL of the culture was then added to 100 mL of broth and incubated at 37°Cfor 24 h. The culture was diluted to a suitable concentration and used for the antibacterial tests.
2.4. Determination of total acidity
The total acidity determination was performed by titration with a 0.1 mol/L sodium hydroxide and phenolphthalein solution using 10 mL of sample and 50 mL of distilled water as recommended by the official compendium of food analysis (IAL, Citation1985). The results were expressed in grams of citric acid per 100 mL of sample. The determination of salt was performed considering the chloride content of brine, using a mixture of 10 mL of sample and 50 mL of distilled water, followed by titration with 0.1 mol L−1 AgNO3 and potassium chromate solution as indicator. Results were expressed as grams of sodium chloride per 100 mL of sample. All determinations were performed in triplicate.
2.5. Determination of total polyphenol and total flavonoid
The total phenolic contents were determined in accordance with previously described methods (Kaur & Kapoor, Citation2002). The absorbance levels were quantitatively determined with a UV spectrometer (Hitachi U-2900) at λ725 nm. The results obtained were expressed as gallic acid equivalents (mg/mL).
The total flavonoid contents of four different solvent extracts were measured by aluminum chloride colorimetric assay based on the formation of a flavonoid–aluminum complex, having maximum absorbance values at 510 nm (Jing, Song, Zeng, Chang, & Shao, Citation2015). Rutin was used for the calibration curve. Briefly, 1 mL of sample or rutin standard solution was added to a 10 mL volumetric flask, mixed with 5 mL of 30% ethanol and 0.3 mL of 5% NaNO2 for 5 min. Then, 0.3 ml of 10% AlCl3 was added for another 6 min. The reaction was stopped by 4 mL of 4% NaOH, and the volume was adjusted to 10 mL with 30% ethanol. The absorbance levels were quantitatively determined using an UV spectrometer (Hitachi U-2900) at λ510 nm after 15 min.
2.6. Quality of the samples with organic acid and anthocyanins
Organic acid was determined in accordance with previously described methods (Švecová, Bordovská, Kalvachová, & Hájek, Citation2015). The mobile phase was prepared by mixing 150 mL of sulphuric acid and 500 mL redistilled water, and was adjusted to pH 2 (incase of need, NaOH solution was used for accurate adjustment). The mobile phase was then filtered through a PTFE filter (0.45 μm) and degassed using helium. Elution was performed using isocratic delivery, and the flow rate 1 mL/min. Injected volume was 10 mL. Separations were carried out at ambient temperature. The detection wavelength was 210 nm for all acids.
The anthocyanin concent of the BE was determined using the method described by Chen, McClung, and Bergman (Citation2017). The sample extracts were diluted with acidified methanol prior to its 1:40 dilution with KCl buffers (pH1.0 and pH 4.5), so as to keep the final absorbance values of the pH 1.0 buffer diluted sample extract within the range of 0.2–0.4. Each properly diluted sample extract was diluted with pH 1.0 and pH 4.5 buffers separately, at a 1:40 ratio and mixed. Absorbance was measured at 512 nm and at 700 nm. Sample extract absorbance was calculated as Abs = [(A512nm − A700nm)pH1.0 − (A512nm − A700nm)pH4.5]. Kuromanin was used as the standard. Its molar extinction coefficient was determined from a series of concentrations ranging from 2.6 to 30.9 μm, the concentration after 1:40 dilution with pH 1.0 buffer. The total anthocyanin concentration was calculated using this molar extinction coefficient.
2.7. In vitro antibacterial test
The cylinder diffusion method was used to detect the inhibition zone diameter (IZD) (Yao et al., Citation2011). The six types of bacteria were diluted to obtain a concentration of 106 cfu/ml with culture medium and then cultured at 48°C. 15 mL of each bacterial suspension was respectively poured into petri dishs. Three evenly placed wells (6 mm in diameter) were put onto the surface of the solidified medium, and 200 µL of the bayberry extract was added to the wells (Finnpipette, France). The inhibition zone diameter was measured to an accuracy of 0.1 mm, and the mean value of the six tests was calculated for the effect. Since the pH of the BE was 3.1; therefore, citric acid buffer solution (pH 3.1) and water were used as positive and negative control, respectively.
2.8. Determination of growth curve
Viable count method was used to determine the growth curve (Geng, Zhang, Xu, Wang, & Yao, Citation2007). Three kinds of positive and three kinds of negative gram bacteria with larger IZD were selected as experiment strains. Three test tubes were prepared for each bacteria and marked as No.1 to No.3 in turn. No.1 tubes only contained 15 mL of beef-protein medium (Sinopharm Chemical Reagent Co., Ltd, Shanghai, China) and acted as control group. No. 2 and No. 3 tubes acted as experimental groups and were added artificial intestinal juice (200 µL) and “BE” (200 µL)(AIC + BE), and “BE” only, respectively, based on No.1 tube. They were cultured at 37°C. The concentration of bacterial in each tube was adjusted to 106 cfu/mL, and the concentration of the “BE” solution was the minimum inhibitory concentration. The count of live bacteria was counted by the method of inverted plates, and the results were recorded every 4 h. Each of the analyses were repeated thrice.
2.9. Identification of the main bacteriostatic components
2.9.1. UPLC-ESI-MS and UV Vis absorption spectra analysis
The UPLC-ESI-MS and UV Vis absorption spectra analysis were obtained from (Yao et al., Citation2011). Analysis was performed on flavonoids with antibacterial activity, UPLC-ESI-MS (Waters Corp., United States) equipped with a photodiode array detector (DAD) was used. Before analysis, the samples were scanned at 190–700 nm (Unico UV-4802, Unico, NJ) to ensure the measurement of the flavonoids. Data acquisition was performed with Waters MassLynx_V4_RC9 software. The column was a BEH C-18 (1.7 µm particles, 2.1 mm × 50 mm), and the mobile phase (A) consisted of 0.1% formicacid, 5% acetonitrile, and 95% H2O (V/V) and acetonitrile containing 0.1% formic acid (B). A stepwise linear gradient was programmed at 99:1 (A/B, V/V) for 2 min, changed to 98:2 over 5 min, and changed to 60:40 over 1 min, followed by 10:90 over 5 min to wash the column. The injection volume was 5 µL, and the detection wavelength was 254 nm (each fraction had an absorbance at this wavelength). The column temperature was 40°C.
The mass spectra were achieved by electrospray ionization in the positive mode. Used were the following ion optics: capillary, 3.50 kV; cone, 25 V; and extractor, 5 V. The source block temperature was 120°C, and the desolvation temperature was 300°C. The electrospray probe flow was adjusted to 70 mL/min. Continuous mass spectra were recorded over the range m/z 100–800 with a 1 s scan time and an interscan delay of 0.1 s.
2.10. Statistical analyses
Experiments were performed in triplicate. Data were analyzed with OriginLab-8s (OriginLab Corporation, Hampton, MA, USA). Means were compared by Duncans new multiple range test. Statistically significant differences were set at p < 0.05.
3. Results and discussion
3.1. The active ingredients of BE
The BE contains rich active substances, including total phenolic, total flavonoid and anthocyanin. As shown in , the content of total phenolic was the greatest, which was 27.10 mg/mL. While the content of total flavonoid and anthocyanins were 5.21 and 4.31 mg/mL, respectively. This was similar to the results of Yao et al (Citation2011). In addition, BE also contains organic acid which mainly were citric acid and lactic acid, the contents of which were 0.473 and 0.004 mg/mL. Yang et al. (2011) studied the total phenolic acid content, total flavonoid content and antioxidant activity of dandelion flowers. The results indicated that the contents of total phenolic acid and total flavonoids in dandelion flowers were 4.09% and 3.65%, respectively. Wang et al. (Citation2010) also studied the antioxidant activities and contents of total phenolic acid and total flavonoids in vicia villosa roth honey. Results indicated that the content of total phenolic acid in the samples theoretically equivalent to that of catechin varied from 9.323 to 21.982 mg/100 g honey, and the content of total flavonoids theoretically equivalent to that of rutin from 0.842 to 2.295 mg/100 g honey. These research showed that the contents of total phenolic and total flavonoid in BE were significant (p < 0.05) higher than that in dandelion flowers and vicia villosa roth honey. It was because of these rich active substances that BE showed a satisfactory antibacterial effect.
Table 1. The content of active components in bayberry extractive.
3.2. Inhibitory effect of BE on different strains
As shown in , BE had a satisfactory antibacterial activity on all of the tested food-borne pathogens, including both Gram-positive bacteria and Gram-negative bacteria. The inhibitory effect of BE on S. aureus was strongest and the inhibition zone diameter reached 22.9 mm. However, the antibacterial activity of citric acid buffer solution was weak, not only the inhibition zone diameter was smaller, but also had no inhibitory effect on S. typhi and S. dysenteriae. Therefore, it can be inferred that the antibacterial activity of BE is not only caused by the lower pH value, but also contains other active components with antibacterial and bactericidal function. Li, Wang, Bu, Hu, and Zhou (Citation2009) had studied the inhibitory effect of Hibiscus mutabili leaf extract on S. aureus, E. coli, P.vulgaris and Enterococcus faecalis, and the results showed that the maximum inhibition zone diameter of Hibiscus mutabili leaf extract was 10, and 15, and 10, and 12 mm, respectively. Hu et al. (Citation2015) had also studied the inhibitory effect of Camellia sinensis var. assamica on S. aureus, E. coli, P. aeruginosa and B. subtilis. And the results showed that the inhibitory effect of C. sinensis var. assamica on S. aureus and E. coli were the best, and the inhibition zone diameter was 19.58 and 14.58 mm, respectively. Here, the results demonstrated that BE had strong antibacterial activity, hence it has a great application prospect.
Table 2. Antibacterial activities of the bayberry extractive.
3.3. Effect of BE on growth curve of different strains
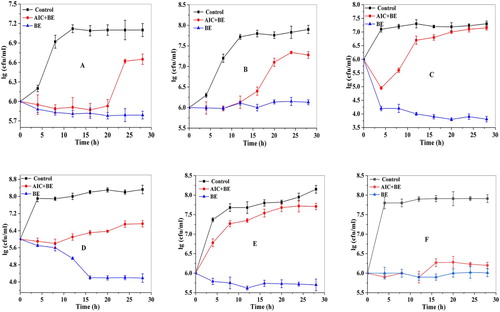
As presented in , the growth curves of the six kinds of bacteria in No. 2 (AIC + BE) and No.3 tube (BE) were significantly (p < 0.05) lower than that in No. 1 tube (Control) on the whole. And the growth curves of the six kinds of bacteria in No. 3 tube (BE) were maintained at a low level throughout the growth process. This result indicated that BE had a certain inhibitory effect on the six kinds of bacteria and this result was also consistent with the inhibition zone diameter (). Earlier, Geng et al. (Citation2007) has also proved that BE had a good antibacterial effect by researching the bacteriostatic circle and minimum inhibitory concentration of S. aureus, Salmonella and Streptococcus. showed that the growth of this five kinds of bacteria (A, B, C, D, F) in No. 2 tube (AIC + BE) were significantly (p < 0.05) inhibited in the early stage of growth, but with the extension of culture time, they growth rapidly and eventually grown to 6.65, 7.28, 7.15, 6.72, and 6.20 log (cfu/ml) at 28 h. This may be because BE decomposed under the action of trypsin and the sensitivity of these five kinds of bacteria to BE decreased with the extension of culture time, so the bacteria are inhibited at the initial stage of growth and then grow rapidly. However, S. typhimurium in tube 2 showed a strong growth activity, and was in a rising state throughout the culture period (E). But the number of S. typhimurium in No. 3 tube (BE) was always lower than that in No. 1 (Control) and No. 2 tubes (AIC + BE). This indicated that BE has higher antibacterial activity compared with the mixture of BE with artificial intestinal juice (AIC + BE).
Figure 1. Effect of bayberry extract on growth curve of different strains. Notes: A: S. aureus (+), B: L. innocua (+), C: β-hemolytic streptococus (+) D: Salmonella enteritidis (−), E: S. typhi (−), and F: S. dysenteriae (−), respectively.
In view of the antibacterial activity of BE, researchers in previous years had investigated the preservation effect of BE on surimi. And the results showed that the BE had a significant inhibitory effect on the Serratia marcescens and Pseudomonas aeruginosa, and the addition of BE could prolong the shelf life of the surimi products storage at room temperature (Li et al., Citation2012). In addition, researchers had also explored the preservation effect of bayberry leaf extract on the large yellow croaker, and the results also showed that the bayberry leaf extract could prolong the shelf life of the yellow croaker (Su, Chen, & Fu, Citation2014). It can be seen that BE not only has good antibacterial effect, but also has great application value.
3.4. UV-Vis and UPLC-UV absorption spectrum analysis
Flavonoid compounds have characteristic absorption peaks at wavelengths of 200–520 nm (Zhao, Wang, Xu, He, & Tian, Citation2013). The absorption peaks in the range of 300–380 nm was considered to be related to the structure of cinnamic acid and the absorption peak in the range of 240–280 nm was considered to be related to the structure of benzoyl (Hong & Wrolstad, Citation1990). The chromatograms of BE obtained by the treatment of ethanol for 24 h, and they had the largest absorption peaks in the range of 200–265, 342–343 and 490–510 nm, respectively (A). Therefore, it can be concluded that the BE may contain flavonols.
Figure 2. UV-Vis and UPLC-UV absorption spectrum analysis. Notes: 2a purple represents diluted once; blue represents diluted twice; red represents dilution three times.
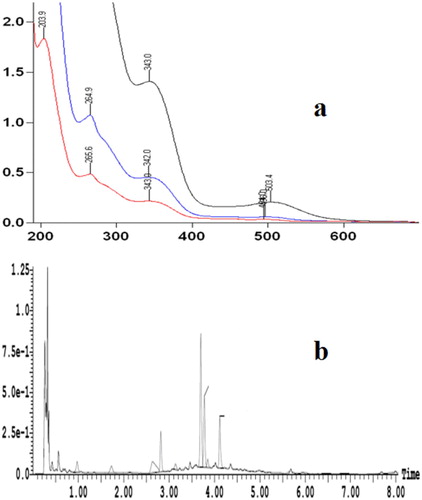
As presented in b, that the BE had four peaks on UPLC-UV spectrum by ethanol processed 24 h, and their retention times were 2.8, 3.7, 3.8 and 4.1 min, respectively. Thus, it can be seen that the number of peaks in the UV spectrum was the same as the number of peaks in b, and their retention time was consistent with the retention times of the peaks in . Therefore, we can initially determine the four substances was the effective inhibitory components of BE.
3.5. UPLC-ESI-MS spectrum analysis
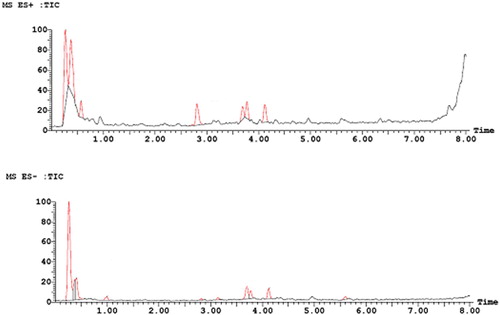
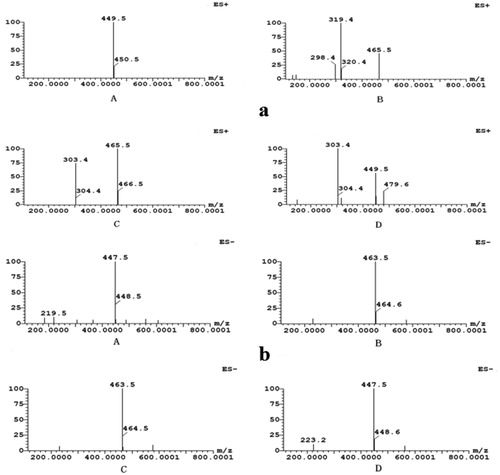
The UPLC-ESI-MS spectrum showed that there were four peaks in the positive ion mode (ES+) and negative ion mode (ES-), respectively (), which was consistent with the retention time of the peaks in the b. This result further proved the conclusion of . However, it can be found that the area of the four peaks in the positive ion mode was larger than that in the negative ion mode. This may due to the strong sensitivity of the substance in positive ion mode. This was consistent with the previous research conclusions (Fan, Citation2015).
showed positive-ion ESI-MS (a) and negative-ion ESI-MS (b) of single ion chromatograms (EIC). A comprehensive elution of BE and their derivatives was achieved within 10 min. The mass spectra showed that the m/z in positive ion mode and negative ion modes were 449.5 and 447.5, respectively (aA and bA). Therefore, it can be speculated that the molecular weight of BE was 448.5. This corresponded to the molecular weight of cyanidin 3-O-glucoside. This result was in accord with the ones described by Yao et al. (Citation2011). aB and bB showed that the m/z, fragment ion m/z and m/z loss of the substance in the positive ion mode were 465.5, 319.4 and 146.1, respectively, which was corresponded to the deoxyhexa carbonyl group. The m/z of the substance in the negative ion mode was 463.5, which further proved that its molecular weight was 464.5. Thus, the substance may be flavonoid deoxy hexacoside (Sun et al., Citation2013). aC and bC showed that the m/z, fragment ion m/z and m/z loss of the substance in the positive ion mode were 465.5, 319.4 and 146.1, respectively, which was corresponded to the deoxyhexa carbonyl group. The m/z of the substance in the negative ion mode was 463.5, which further proved that its molecular weight was 464.5. Thus, the substance may be quercetin 3-O-glucoside (Süntar et al., Citation2010). aD and bD showed that the m/z, fragment ion m/z and m/z loss of the substance in the positive ion mode was 449.5, 303.4 and 146.1, respectively, which was equivalent to the deoxidized hexa-sugar group attached to the quercetin ligand. The m/z of the substance in the negative ion mode was 447.5, which further proved that its molecular weight was 448.5. Thus, the substance may be quercetin deoxidized hexacoside (Shi et al., Citation2019).
Therefore, the above four substances were identified as cyanidin 3-O-glucoside, flavonoid deoxy hexacoside, quercetin 3-O-glucoside, and quercetin deoxidized hexacoside, respectively, by comparing their retention time, UV-vis spectroscopic data and the pseudomolecular ion [M+H]+ with the authenticated standards. This result was coincided with the report that flavonol myricetin, quercetin and kaempferol, were the main flavonols and they may exist in the form of indicans in fruit (Brasathe et al., Citation2016).
4. Conclusions
In this paper, the active substances and antibacterial effect of BE were studied and the results showed that the content of total phenolic was the greatest, and BE had significant (p < 0.05) inhibitory effect on the six kinds of common food-borne pathogens. Based on this, the four main antimicrobial components, cyanidin 3-O-glucoside, flavonoid deoxy hexacoside, quercetin 3-O-glucoside, and quercetin deoxidized hexacoside, were identified from BE by comparing their retention time, UV-vis spectroscopic data and the pseudomolecular ion [M + H]+ with the authenticated standards.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alarcon, M., Fuentes, E., Olate, N., Navarrete, S., Carrasco, G., & Palomo, I. (2015). Strawberry extract presents antiplatelet activity by inhibition of inflammatory mediator of atherosclerosis (sP-selectin, sCD40L, RANTES, and IL-1β) and thrombus formation. Platelets, 26(3), 224–229. doi: 10.3109/09537104.2014.898747
- Brasathe, J., Nimal, P. P. A., Dananjaya, K. A. J., Ranatunga, M. A. B., Abeysinghe, I. S. B., Kumudini, G. M. T., & B. M. R. Bandara. (2016). Genetic variation of flavonols quercetin, myricetin, and kaempferol in the sri lankan tea (\r, camellia sinensis\r, l.) and their health-promoting aspects. International Journal of Food Science, 2016, 1–9.
- Chan, K. C., Ho, H. H., Lin, M. C., Wu, C. H., Huang, C. N., Chang, W. C., & Wang, C. J. (2014). Mulberry water extracts inhibit rabbit atherosclerosis through stimulation of vascular smooth muscle cell apoptosis via activating p53 and regulating both intrinsic and extrinsic pathways. Journal of Agricultural and Food Chemistry, 62(22), 5092–5101. doi: 10.1021/jf501466t
- Chen, M. H., McClung, A. M., & Bergman, C. J. (2017). Phenolic content, anthocyanins and antiradical capacity of diverse purple bran rice genotypes as compared to other bran colors. Journal of Cereal Science, 77, 110–119. doi: 10.1016/j.jcs.2017.07.010
- Chen, W., Su, H., Xu, Y., Bao, T., & Zheng, X. (2016). Protective effect of wild raspberry (Rubus hirsutus Thunb.) extract against acrylamide-induced oxidative damage is potentiated after simulated gastrointestinal digestion. Food Chemistry, 196, 943–952. doi: 10.1016/j.foodchem.2015.10.024
- Chen, W., Zhou, S., & Zheng, X. (2015). A new function of Chinese bayberry extract: Protection against oxidative DNA damage. LWT - Food Science and Technology, 60(2), 1200–1205. doi: 10.1016/j.lwt.2014.09.011
- Fan, Y. H. (2015). Studies on migration and control of food contaminations in three-piece metal cans (Master’s thesis). Jiangnan University.
- Farrell, N. J., Norris, G. H., Ryan, J., Porter, C. M., Jiang, C., & Blesso, C. N. (2015). Black elderberry extract attenuates inflammation and metabolic dysfunction in diet-induced obese mice. British Journal of Nutrition, 114(8), 1123–1131. doi: 10.1017/S0007114515002962
- Geng, X. L., Zhang, B. X., Xu, L. L., Wang, N., & Yao, W. R. (2007). Study on Bacteriostasis of waxberry fruit extract. Food Technology, 3, 120–122.
- Hong, V., & Wrolstad, R. E. (1990). Use of HPLC separation/photodiode array detection for characterization of anthocyanins. Journal of Agricultural and Food Chemistry, 38, 708–715. doi: 10.1021/jf00093a026
- Hu, Y. J., Han, X. X., Xue, Q. L., & Yang, H. S. (2015). Study on antibacterial activity of different extracts of Camellia sinensis var. assamica. Modern Food Science and Technology, 29(8), 1770–1773.
- Huang, H., Sun, Y., Lou, S., Li, H., & Ye, X. (2014). In vitro digestion combined with cellular assay to determine the antioxidant activity in Chinese bayberry (Myrica rubra Sieb. et Zucc.) fruits: A comparison with traditional methods. Food Chemistry, 146, 363–370. doi: 10.1016/j.foodchem.2013.09.071
- IAL. (1985). Normas analíticas do Instituto Adolfo Lutz. Métodos químicos efísicos para análise de alimentos, (Ed.), 3 ed, São Paulo, pp. 25–26.
- Jing, E. L., Song, T. F., Zeng, H. Q., Chang, L., & Shao, P. N. (2015). Total flavonoids content, antioxidant and antimicrobial activities of extracts from Mosla chinensis Maxim. cv. Jiangxiangru. LWT - Food Science and Technology, 64, 1022–1027. doi: 10.1016/j.lwt.2015.07.033
- Ju, J., Wang, C., Qiao, Y., Li, D., & Li, W. (2017). Effects of tea polyphenol combined with nisin on the quality of weever (Lateolabrax japonicus) in the initial stage of fresh-frozen or chilled storage state. Journal of Aquatic Food Product Technology, 26(5), 543–552. doi: 10.1080/10498850.2016.1233472
- Ju, J., Xu, X., Xie, Y., Guo, Y., Cheng, Y., Qian, H., & Yao, W. (2018). Assessment of the antibacterial activity and the main bacteriostatic components from bayberry fruit extract. International Journal of Food Properties, 21, 190–201. doi: 10.1080/10942912.2018.1479861
- Jurgonski, A., Juskiewicz, J., & Zdunczyk, Z. (2008). Ingestion of black chokeberry fruit extract leads to intestinal and systemic changes in a rat model of prediabetes and hyperlipidemia. Plant Foods for Human Nutrition, 63(4), 176–182. doi: 10.1007/s11130-008-0087-7
- Kaur, C., & Kapoor, H. C. (2002). Anti-oxidant activity and total phenolic content of some Asian vegetables. International Journal of Food Science & Technology, 37(2), 153–161. doi: 10.1046/j.1365-2621.2002.00552.x
- Le, L. Y., Baron, F., & Gautier, M. (2003). Staphylococcus aureus and food poisoning. Genetics and Molecular Research, 2(1), 63–76.
- Li, J., Han, Q., Chen, W., & Ye, L. (2012). Antimicrobial activity of Chinese bayberry extract for the preservation of surimi. Journal of the Science of Food and Agriculture, 92(11), 2358–2365. doi: 10.1002/jsfa.5641
- Li, X. Y., Wang, J. Q., Bu, D. P., Hu, H., & Zhou, L. Y. (2009). Advanced research of effect of polyunsaturated fatty acids on cell membrane function. Biotechnology Bulletin, 12(1), 22–26.
- Meireles, M., Rodriguez-Alcala, L. M., Marques, C., Norberto, S., Freitas, J., Fernandes, I., & Calhau, C. (2016). Effect of chronic consumption of blackberry extract on high-fat induced obesity in rats and its correlation with metabolic and brain outcomes. Food & Function, 7(1), 127–139. doi: 10.1039/C5FO00925A
- Shi, G. J., Li, Y., Cao, Q. H., Wu, H. X., Tang, X. Y., Gao, X. H., & Yang, Y. (2019). In vitro and in vivo evidence that quercetin protects against diabetes and its complications: A systematic review of the literature. Biomedicine & Pharmacotherapy, 109, 1085–1099. doi: 10.1016/j.biopha.2018.10.130
- Su, H., Chen, W., & Fu, S. (2014). Antimicrobial effect of bayberry leaf extract for the preservation of large yellow croaker (Pseudosciaena crocea). Journal of the Science of Food and Agriculture, 94(5), 935–942. doi: 10.1002/jsfa.6338
- Sun, C. D., Huang, H. Z., Xu, C. J., Li, X., & Chen, K. S. (2013). Biological activities of extracts from Chinese bayberry (Myrica rubra Sieb. et Zucc.): A review. Plant Foods for Human Nutrition, 68(2), 97–106. doi: 10.1007/s11130-013-0349-x
- Sun, C. D., Zheng, Y. X., Chen, Q. J., Tang, X. L., Jiang, M., Zhang, J. K. … Chen, K. S. (2012). Purification and anti-tumour activity of cyanidin-3-O-glucoside from Chinese bayberry fruit. Food Chemistry, 131(4), 1287–1294. doi: 10.1016/j.foodchem.2011.09.121
- Süntar, I. P., Akkol, E. K., Yalçın, F. N., Koca, U., Keleş, H., & Yesilada, E. (2010). Wound healing potential of Sambucus ebulus L. Leaves and isolation of an active component, quercetin 3-O-glucoside. Journal of Ethnopharmacology, 129(1), 106–114. doi: 10.1016/j.jep.2010.01.051
- Švecová, B., Bordovská, M., Kalvachová, D., & Hájek, T. (2015). Analysis of Czech meads: Sugar content, organic acids content and selected phenolic compounds content. Journal of Food Composition and Analysis, 38, 80–88. doi: 10.1016/j.jfca.2014.11.002
- Wang, M. M., Yu, H. X., Dong, Rui., Li, Wei., Cong, H. D., & Chen, Y. N. (2010). Antioxidant activities and contents of total phenolic acid and total flavonoids in vicia villosa roth honey. Food Science, 1, 54–57.
- Yang, L., Li, H. F., Diao, H. F., Tang, X. Y., Peng, T. F., & Sun, T. J. (2011a). Total phenolic acid content, total flavonoid content and antioxidant activity of dandelion flowers. Food Science, 32(17), 160–163.
- Yao, W. R., Wang, H. Y., Wang, S. T., Sun, S. L., Zhou, J., & Luan, Y. Y. (2011). Assessment of the antibacterial activity and the antidiarrheal function of flavonoids from bayberry fruit. Journal of Agricultural and Food Chemistry, 59(10), 5312–5317. doi: 10.1021/jf200211m
- Zhang, Y., Zhou, X., & Tao, W. (2016). Antioxidant and antiproliferative activities of proanthocyanidins from Chinese bayberry (Myrica rubra Sieb. et Zucc.) leaves. Journal of Functional Foods, 27, 645–654. doi: 10.1016/j.jff.2016.10.004
- Zhao, X. L., Wang, F. R., Xu, L. Y., He, H. P., & Tian, Y. C. (2013). Determination of anthocyanin in different fruit of peach by HPLC method. Food Science, 34(8), 208–211.