?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Layer-by-layer (LBL) technique is a well-established method for the formation of nanocapsules, which could encapsulate nanoparticles by polyelectrolyte and has received great interest in some areas of research. In this study, colloidal gold nanoparticles (AuNPs) were used as a vehicle for loading horseradish peroxidase (HRP), and AuNPs-HRP was encapsulated by using LBL technique to form a signal amplification nanocapsule probe. Based on the probe, an immunoassay was developed for the detection of imidacloprid in agricultural food samples (rice, pear and cabbage). The IC50 value of the assay was 0.11 ng mL−1 which was 8-9954 times lower than those of traditional ELISAs and well below the maximum residue limits (MRLs) permitted by the European Union (EU). The recoveries of imidacloprid from three different samples were 80.34–120.84% that had a good correlation (R2 = 0.9739) with those obtained by the commercial kit. The developed assay could provide a sensitive method for detection of imidacloprid.
1. Introduction
Imidacloprid (1-(6-chloro-3-pyridylmethyl)-N-nitroimidazolidin-2- ylideneamine) is a systemic and chloronicotinyl neonicotinoid insecticide (Gang, Citation2006; Wanatabe et al., Citation2001), which has been widely used in agricultural production for control of sucking and chewing insects including aphids, thrips, plant hoppers, diamondback moths and whiteflies (Li et al., Citation2007). Some studies showed that imidacloprid could induce histological alterations in bone (Mzid, Badraoui, Khedir, Sahnoun, & Rebai, Citation2017), liver (Toor, Sangha, & Khera, Citation2013) and adversely affect brain development (Kimura-Kuroda, Komuta, Kuroda, Hayashi, & Kawano, Citation2012) through their residues in agricultural products.
In the past decade, many efforts have been made to develop efficient methods to determine imidacloprid in agricultural food samples, including gas chromatography-mass spectrometry (GC–MS) (Liang et al., Citation2017), high-performance liquid chromatography (HPLC) (Seccia, Fidente, Montesano, & Morrica, Citation2008; Vichapong, Burakham, & Srijaranai, Citation2015), liquid chromatography combined mass spectrometry (HPLC–MS) (Di Muccio et al., Citation2006; Jovanov et al., Citation2013), gold immunochromatographic assay (GICA) (Fang et al., Citation2015), surface plasmon resonance (SPR) sensing (Lee et al., Citation2016) and selection of phage-displayed peptides based assay (Liu et al., Citation2015). Although these methods have high selectivity, specificity and accuracy, they either involve complicated sample pretreatment, sophisticated equipment and skilled analysts or just suit for laboratory detection and have relatively low sensitivity.
At present, enzyme-linked immunosorbent assays (ELISAs) have been reported for imidacloprid (). Compared with the above techniques, ELISAs have simple sample pretreatment and operation steps. However, the sensitivities of some ELISAs were not high enough to meet the demand of MRLs permitted by the EU. It is important to develop reliable signal amplification probe for the detection of imidacloprid in agricultural products.
Table 1. Comparison of the proposed assay with reported methods for imidacloprid detection.
Colloidal gold nanoparticles (AuNPs) have been proved to be an ideal candidate in the biotechnological platform because of their unique chemical and physical properties, such as easy preparation, high surface-to-volume ratio and good biocompatibility (Gao, Xu, Hou, Chen, & Tang, Citation2013; Lin et al., Citation2015; Tang, Tang, Niessner, Chen, & Knopp, Citation2011; Wu et al., Citation2014; Zhou, Lai, Zhuang, Tang, & Tang, Citation2013). AuNPs can facilitate variety surface reactions and load more biomolecules such as enzyme (Shu et al., Citation2016), antibody (Zhang et al., Citation2012), DNA (Howes, Chandrawati, & Stevens, Citation2014; Orza, Olenic, Pruneanu, Pogacean, & Biris, Citation2010) and other proteins for synthesis of probe to enhance the signal amplification.
Layer-by-layer (LBL) technique is a well-established method for the formation of microcapsules or nanocapsules, which could encapsulate almost all types of nanoparticles, such as lipid nanoparticles (Cui & Mumper, Citation2002), inorganic (Aqil et al., Citation2008; Neumann, Kovtun, Dietzel, Epple, & Heumann, Citation2009) and polymeric nanoparticles (Sheng et al., Citation2009). The capsule was produced by LBL deposition of polyelectrolyte around the surface of nanoparticulate templates where the composition, charge and thickness of the shell were dependent on the material of layer deposited (Pereira, Barros-Timmons, & Trindade, Citation2014). A primary property of this technique was the preparation of nanoparticles with core-independent (Del et al., Citation2014; Labouta & Schneider, Citation2010). This technique has attracted intensive attention in various technological fields such as drug (Ariga, Lvov, Kawakami, Ji, & Hill, Citation2011; Chen et al., Citation2017; Del et al., Citation2014; Luo, Venkatraman, & Neu, Citation2013) and vaccine delivery (Chong et al., Citation2009), biosensor (Kreft, Javier, Sukhorukov, & Parak, Citation2007; Xuan, Jia, Jiang, Abdel-Halim, & Zhu, Citation2012) and bioreactor (Matsusaki, Amemori, Kadowaki, & Akashi, Citation2011) mostly because they can protect the encapsulated bioactive agents from the surrounding environmental hazards (Balabushevich, Sukhorukov, & Larionova, Citation2005; Lvov & Caruso, Citation2001) and encapsulate nanoparticles to load more molecules to enhance the signal amplification of immunoassays (Harshala, Parab, & Ru-Shi Liu, Citation2009; Oaew, Charlermroj, Pattarakankul, & Karoonuthaisiri, Citation2012; Trau & Renneberg, Citation2003).
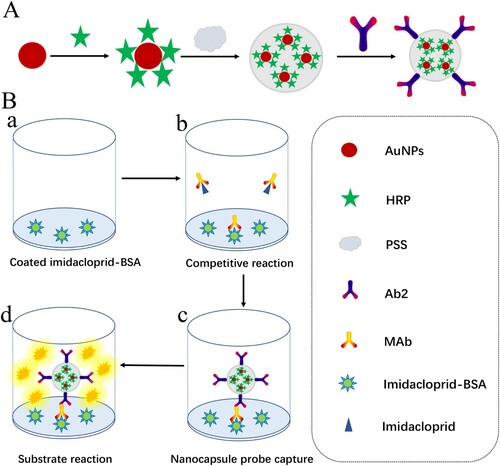
Take into account the advantages of the encapsulation ability of LBL technique and the surface functionalization ability of AuNPs, a signal amplified nanocapsule probe was synthesized for sensitive determination of imidacloprid (A). Firstly, 20 nm AuNPs were employed to synthesize high surface-to-volume ratio protein vehicle to label with a large amount of horseradish peroxidase (HRP) on their surface. Then AuNPs-HRP were encapsulated into nanocapsule by poly (styrenesulfonic acid) sodium salt (PSS). Finally, goat anti-mouse IgG (Ab2) was absorbed on the nanocapsule surface by electrostatic interaction. Small molecule like 3, 3′, 5, 5′-tetramethylbenzidine (TMB) could across the polymer and interact with HRP to produce an amplified signal. Based on the synthetic nanocapsule probe, an immunoassay was developed for sensitive detection of imidacloprid in agricultural food samples (B).
Figure 1. Schematic of preparation of nanocapsule probe and the probe-based assay procedure. (A) Synthesis illustration of nanocapsule probe and (B) protocol of the enhanced assay. (a) Coating antigen (0.25 μg mL−1, 100 µL) was added to 96-well plate and incubated 2 h at 37°C. (b) Imidacloprid (100 µL) and mAb (0.125 μg mL−1, 100 µL) were added and incubated 1 h at 37°C. (c) The probe (100 µL) was added and incubated 1 h at 37°C. (d) Substrate solution (100 µL) was added and incubated 15 min at 37°C.
2. Materials and methods
2.1. Chemicals and reagents
Imidacloprid, thiacloprid, acetamiprid, clothianidin, imidaclothiz, thiamethoxam, nitenpyram and dinotefuran were purchased from Jiangsu Pesticide Research Institute (Jiangsu, China). Anti-imidacloprid monoclonal antibody (mAb) and coating antigen imidacloprid-BSA were purchased from Thpower Biotechnologies Co., Ltd. (Shenzhen, China). Horseradish peroxidase (HRP), 3, 3′, 5, 5′-tetramethylbenzidine (TMB), Poly (styrene sulfonic acid) sodium salt (MW = 70,000) were purchased from Sigma Chemicals Co. (St. Louis, MO, USA). Chloroauric acid (HAuCl4) and trisodium citrate were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Bovine serum albumin (BSA) was purchased from Sangon Biotech Co., Ltd. (Shanghai, China). Polyethylene glycol (MW = 20,000) was purchased from Alfa Aesar Chemical Co., Ltd. (Shanghai, China). Goat anti-mouse IgG was purchased from Bioss Biotech Co., Ltd. (Beijing, China). Tween-20 was obtained from Amresco (USA). Commercial ELISA kit was purchased from Glory science Co., Ltd. (Hangzhou, China). The 96-well polystyrene plate was purchased from Costar Group, Inc. (USA). All other chemicals were of analytical grade and obtained from Beijing Chemical Reagent Co. (Beijing, China). Distilled water was used throughout all the experiments.
2.2. Buffers and solutions
PB solution: 10 mM sodium phosphate buffer (pH 6.0 and 6.3). PBS solution: 10 mM sodium phosphate buffer (pH 7.4) containing 140 mM NaCl. Coating buffer, carbonate-bicarbonate buffer (50 mM, pH 9.6). Washing buffer: PBST (0.05% (v/v) Tween in 0.01 M PBS) and PBT (0.05% (v/v) Tween in 0.01 M PB [pH 6.3]). Blocking buffer: 0.01 M PBS buffer containing 2% (w/v) BSA. Probe dilution buffer: 0.01 M PB (pH 6.3) buffer containing 2% (w/v) BSA. Sample solution: 0.01 M PBS buffer containing 5% (v/v) methanol. TMB solution: citrate buffer (pH 5.0) containing 0.01% (w/v) TMB and 0.005% (v/v) urea hydrogen peroxide. Stop solution: 2 M H2SO4.
2.3. Preparation of the nanocapsule probe.
AuNPs 20 nm in diameter were prepared according to the reported method with minor modifications (Zhou et al., Citation2009). Briefly, a stirred aqueous solution of HAuCl4 (10%, 0.1 mL) in 100 mL of Milli-Q purified water was heated to reflux, and trisodium citrate solution (1%, 2.6 mL) was added. The solution was heated under reflux with constant stirring for another 3 min, during which its colour changed from yellow to wine red. Finally, the colloidal gold solution was cooled to room temperature with a continuous and slow stiring. The solution was stored at 4°C. The dispersity of AuNPs was checked by a transmission electron microscope (TEM, H-7650).
The nanocapsule probe was synthesized using a modified method (Oaew et al., Citation2012). Briefly, 10 mL AuNPs was centrifuged at 11,360 × g for 30 min and resuspended into 1 mL of Milli-Q purified water. Then 20 μL HRP (5 mg mL−1) was added to 1 mL AuNPs solution, and the mixture was further incubated for 1 h at room temperature under gentle stirring. Afterward, the mixture was centrifuged at 11,360 × g for 30 min at 4°C to remove the excess HRP. The obtained AuNPs-HRP was resuspended in 10 mL of PB (PH 6.0). In order to encapsulate AuNPs-HRP into capsules, 10 mL of AuNPs-HRP was added into PSS (30 mg mL−1, 10 mL) in 1 mM NaCl, and the mixture was further incubated for 40 min at room temperature, the mixture was centrifuged at 9680 × g for 30 min to remove the excess PSS. Finally, Ab2 was added into AuNPs-HRP-PSS solution at a final concentration of 200 μg mL−1 and incubated at room temperature for 2 h under gentle stirring. Following that, polyethylene glycol (20 mg mL−1, 50 μL) was added into the mixture and incubated for 30 min, which was centrifuged at 9680 × g for 30 min and washed three times to remove unconjugated Ab2 and excess polyethylene glycol. The obtained AuNPs-HRP-PSS-Ab2 was resuspended in 1 mL of PB (PH 6.0) and stored at 4°C. The synthesis of AuNPs-HRP-PSS-Ab2 was confirmed by TEM and UV-spectra.
2.4. Procedure of the nanocapsule probe-based assay for imidacloprid detection
The 96-well polystyrene plates were coated with imidacloprid-BSA (0.25 μg mL−1, 100 µL) for 2 h at 37°C. After washing the plates with PBST (200 μL), the plates were blocked with blocking buffer (100 µL) for 1 h at 37°C. After a washing step, imidacloprid (100 µg mL−1, 100 µL) with the serial sample solution and mAb (0.125 μg mL−1, 100 µL) diluted by PBS were added and incubated at 37°C for 1 h. After another washing step, the probe (100 µL) diluted 5-fold with probe dilution buffer was added and incubated at 37°C for 1 h. After a final washing step with PBT (200 μL), TMB substrate solution (100 µL) was added to each well and incubated for 15 min at 37°C. The reaction was terminated by stop solution (50 µL) and the OD values were measured with Epoch Microplate Spectrophotometer at 450 nm. The IC50 value (50% inhibition) for each compound was measured by the inhibition formula (Meng et al., Citation2015): Inhibition (%) = [1 − B/B0] × 100. B was the corresponding OD450 values at different concentrations of imidacloprid, B0 was the OD450 value without imidacloprid.
2.5. Selectivity of the nanocapsule probe
The selectivity was evaluated by using the standard solution of imidacloprid and some analogues including thiacloprid, acetamiprid, clothianidin, imidaclothiz, thiamethoxam, nitenpyram and dinotefuran.
2.6. Samples analysis
Three different agricultural samples (rice, pear and cabbage) were chosen to evaluate the performance of the assay. All samples were collected from the local supermarket of Changchun (China). The samples were spiked with different concentrations of imidacloprid (2, 10 and 20 μg Kg−1) and stirred for 10 min. Subsequently, all samples were extracted by ultrasonic extraction in 5% (v/v) methanol in PBS for 10 min and centrifuged for 10 min at 4000 × g. The supernatant was collected and the solutions were comparatively analyzed by the developed assay and commercial kit. The recoveries were calculated according to the following equation (Meng et al., Citation2015):where CD was the detected concentration of the spiked sample, CN was the concentration of the unspiked sample, CS was the spiked concentration of imidacloprid. Analyses were always made in triplicate.
3. Results and discussion
3.1. Synthesis and confirmation of nanocapsule probe
In this study, AuNPs were chosen as a carrier for loading HRP, LBL technique was used to encapsulate AuNPs-HRP to enhance the signal amplification. The IgG had a net positive charge at pH 6.0 and the PSS-encapsulated AuNPs showed a negative charge on the surface due to the presence of anionic SO3 groups of PSS (Harshala et al., Citation2009; Wang, Chen, Wang, Ma, & Su, Citation2007). Therefore, in the presence of PB solution (pH 6.0), IgG could be spontaneously immobilized onto the surface of AuNPs-HRP-PSS without using any cross-linking reagents mainly due to the electrostatic interaction between positively charged IgG and the negatively charged PSS.
The modification of nanocapsule was analyzed by TEM and UV–Vis absorption spectra. TEM image in A (b) showed the AuNPs were encapsulated in PSS shell forming a polymer capsule. Fifty nanocapsules were counted, the average number of AuNPs in one nanocapsule were twenty-three. The UV–Vis absorption spectra of AuNPs, AuNPs-HRP, AuNPs-HRP-PSS and AuNPs-HRP-PSS-Ab2 were shown in B. The AuNPs had surface plasmon resonance (λSPR) at 519 nm, the effect of immobilization caused λSPR of AuNPs-HRP redshift from 519 to 527 nm. After the LBL deposition process, AuNPs-HRP-PSS had a broader absorption peak than AuNPs-HRP, displaying a successful aggregation of AuNPs-HRP. The λSPR redshift of AuNPs-HRP-PSS-Ab2 was from 527 to 530 nm, indicating successful link interaction between IgG and AuNPs-HRP-PSS. There was no significant colour change in the synthesis process of the probe.
Figure 2. Synthesis confirmation of nanocapsule probe. (A) TEM images of the AuNPs (a) and the synthesized nanocapsule probe (b). (B) UV-spectra analysis of synthesis process of nanocapsule: AuNPs (a); AuNPs-HRP (b); AuNPs-HRP-PSS (c); AuNPs-HRP-PSS-Ab2 (d). (C) The relationship between the amount of Ab2 and the absorbance value. P/N value is the positive OD value/blank OD value.
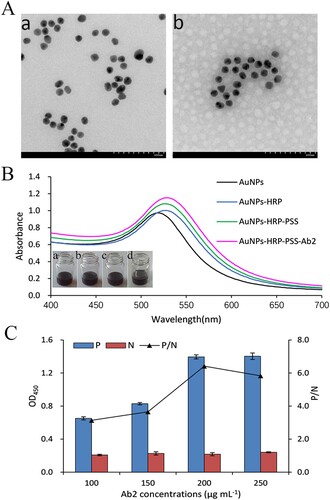
In the probe, the amount of Ab2 adsorbed on the surface of nanocapsule is one of the most important components, which can affect the sensitivity of the probe. As shown in C, the absorbance and P/N values increased gradually along with the amount of IgG adsorbed on nanocapsule, and became almost constant at 200 μg mL−1. Thus, 200 μg mL−1 was chosen as the optimum amount.
3.2. Calibration curve and specificity
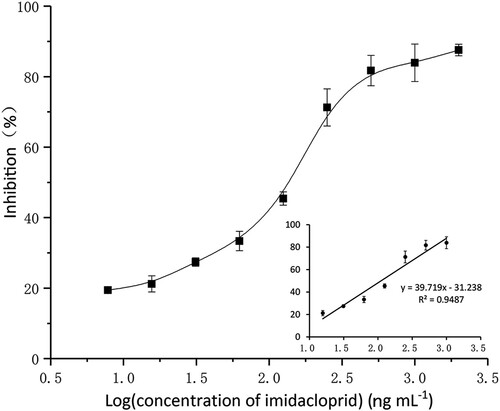
A calibration curve was obtained by plotting the values of inhibition and the logarithm of the concentrations of imidacloprid (). The linear regression equation was y = 39.719 × −31.238, with a correlation coefficient (R2) of 0.9487. The linear range (IC15-IC85) was 0.02 to 0.84 ng mL−1, the limit of detection (LOD, IC10) and the IC50 value was 0.01 and 0.11 ng mL−1. The sensitivity of the developed method was higher than traditional ELISA, commercial kit and other reported methods ().
Figure 3. Standard curve of nanocapsule probe-based assay for imidacloprid. The curve was obtained by using the relationship between the values of inhibition and the logarithm of the concentrations of imidacloprid. Results are mean ± SD (n = 3).
The specificity of the probe was evaluated by cross-reaction (CR) values with imidacloprid and seven other structurally similar neonicotinoid insecticides. As shown in , there was almost no CR with other analogues except thiacloprid, acetamiprid and clothianidin (CR < 10%). The results confirmed the good specificity of the probe for the detection of imidacloprid.
Table 2. Cross-reactivity (CR) of the probe with imidacloprid and its analogues.
3.3. Spiked sample detection
In order to reduce matrix interference and correspond with the detection linear range of commercial kit, 50-fold matrix dilution was used for this analysis. As shown in , the recoveries of imidacloprid from rice, pear and cabbage were 88.05–117.02%, 89.53–109.50% and 80.34–120.84%, respectively, with the coefficient of variations (CVs) within 13%. The recoveries of imidacloprid had a good correlation (R2 = 0.9739) between the developed method and commercial kit and were in accordance with the AOAC international standard which the recoveries should be in the range between 50% and 150% (Koerner et al., Citation2013). These date suggested that the developed method can be used as a quantitative tool for the determination of imidacloprid in agricultural food samples.
Table 3. Recovery of spiked samples.
3.4. Stability of the probe
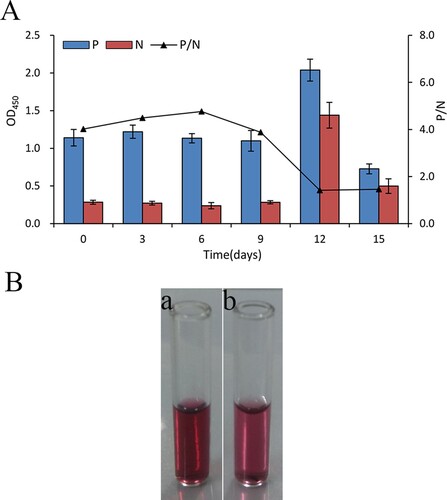
The stability of the probe was tested by using the same procedures described in section 2.4 except for no adding of the standard solution of imidacloprid. As shown in A, The OD450 values only change from 1.09 to 1.22 and the P/N values were almost constant in the first nine days, suggesting that the AuNPs-HRP-PSS-Ab2 conjugate has almost no major difference in stability after storage nine days. The AuNPs-HRP-PSS-Ab2 was clumped and became light in colour from day twelve (B). These date indicated that the probe can be used for repeated measurements after 9 days storage at 4°C.
4. Conclusion
A signal amplified nanocapsule probe was synthesized using the LBL technique and an immunoassay was developed based on the probe for sensitive detection of imidacloprid in agricultural samples. The sensitivity of the developed assay was higher than traditional ELISA, commercial kit and well below the MRLs permitted by the EU. The values of recoveries and CVs had a good correlation with those from a commercial kit, indicated the accuracy and precision of the developed method. The synthesized nanocapsule probe could be used for sensitive and selective detection of imidacloprid in agricultural samples.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aqil, A., Qiu, H., Greisch, J., Jérôme, R., De Pauw, E., & Jérôme, C. (2008). Coating of gold nanoparticles by thermosensitive poly(N-isopropylacrylamide) end-capped by biotin. Polymer, 49(5), 1145–1153. doi: 10.1016/j.polymer.2007.12.033
- Ariga, K., Lvov, Y. M., Kawakami, K., Ji, Q., & Hill, J. P. (2011). Layer-by-layer self-assembled shells for drug delivery. Advanced Drug Delivery Reviews, 63(9), 762–771. doi: 10.1016/j.addr.2011.03.016
- Balabushevich, N. G., Sukhorukov, G. B., & Larionova, N. I. (2005). Polyelectrolyte multilayer microspheres as carriers for bienzyme system: Preparation and characterization. Macromolecular Rapid Communications, 26(14), 1168–1172. doi: 10.1002/marc.200500141
- Chen, J., Ratnayaka, S., Alford, A., Kozlovskaya, V., Liu, F., Xue, B., … Kharlampieva, E. (2017). Theranostic multilayer capsules for ultrasound imaging and guided drug delivery. ACS Nano, 11(3), 3135–3146. doi: 10.1021/acsnano.7b00151
- Chong, S., Sexton, A., De Rose, R., Kent, S. J., Zelikin, A. N., & Caruso, F. (2009). A paradigm for peptide vaccine delivery using viral epitopes encapsulated in degradable polymer hydrogel capsules. Biomaterials, 30(28), 5178–5186. doi: 10.1016/j.biomaterials.2009.05.078
- Cui, Z., & Mumper, R. J. (2002). Coating of cationized protein on engineered nanoparticles results in enhanced immune responses. International Journal of Pharmaceutics, 238(1–2), 229–239. doi: 10.1016/s0378-5173(02)00079-0
- Del, M. L., Ferraro, M. M., Baldassarre, F., Mancarella, S., Greco, V., Rinaldi, R., & Leporatti, S. (2014). Biological applications of LbL multilayer capsules: From drug delivery to sensing. Advances in Colloid and Interface Science, 207, 139–154. doi: 10.1016/j.cis.2014.02.014
- Di Muccio, A., Fidente, P., Barbini, D. A., Dommarco, R., Seccia, S., & Morrica, P. (2006). Application of solid-phase extraction and liquid chromatography–mass spectrometry to the determination of neonicotinoid pesticide residues in fruit and vegetables. Journal of Chromatography A, 1108(1), 1–6. doi: 10.1016/j.chroma.2005.12.111
- Fang, Q., Wang, L., Cheng, Q., Cai, J., Wang, Y., Yang, M., … Liu, F. (2015). A bare-eye based one-step signal amplified semiquantitative immunochromatographic assay for the detection of imidacloprid in Chinese cabbage samples. Analytica Chimica Acta, 881, 82–89. doi: 10.1016/j.aca.2015.04.047
- Gang, Z. G. G. W. (2006). Synthesis and Identification of artificial antigen for imidacloprid. Agricultural Sciences in China, 5(4), 307–312. doi: 10.1016/S1671-2927(06)60054-0
- Gao, Z., Xu, M., Hou, L., Chen, G., & Tang, D. (2013). Magnetic bead-based reverse colorimetric immunoassay strategy for sensing biomolecules. Analytical Chemistry, 85(14), 6945–6952. doi: 10.1021/ac401433p
- Girotti, S., Maiolini, E., Ghini, S., Eremin, S., & Ma Es, J. (2010). Quantification of imidacloprid in honeybees: Development of a chemiluminescent ELISA. Analytical Letters, 43(3), 466–475. doi: 10.1080/00032710903402309
- Harshala, J., Parab, H. M. C. T., & Ru-Shi Liu, M. H. C. C. (2009). Biosensing, cytotoxicity, and cellular uptake studies of surface-modified gold nanorods. Journal of Physical Chemistry C, 18(113), 7574–7578. doi: 10.1021/jp9000169
- Howes, P. D., Chandrawati, R., & Stevens, M. M. (2014). Colloidal nanoparticles as advanced biological sensors. Science, 346(6205), 1247390. doi: 10.1126/science.1247390
- Jovanov, P., Guzsvány, V., Franko, M., Lazić, S., Sakač, M., Šarić, B., & Banjac, V. (2013). Multi-residue method for determination of selected neonicotinoid insecticides in honey using optimized dispersive liquid–liquid microextraction combined with liquid chromatography-tandem mass spectrometry. Talanta, 111, 125–133. doi: 10.1016/j.talanta.2013.02.059
- Kimura-Kuroda, J., Komuta, Y., Kuroda, Y., Hayashi, M., & Kawano, H. (2012). Nicotine-like effects of the neonicotinoid insecticides acetamiprid and imidacloprid on cerebellar neurons from neonatal rats. PLoS One, 7(2), e32432. doi: 10.1371/journal.pone.0032432
- Koerner, T. B., Abbott, M., Godefroy, S. B., Popping, B., Yeung, J. M., Diaz-Amigo, C., … Koehler, P. (2013). Validation procedures for quantitative gluten ELISA methods: AOAC allergen community guidance and best practices. Journal of AOAC International, 96(5), 1033–1040. doi: 10.5740/jaoacint.13-043
- Kreft, O., Javier, A. M., Sukhorukov, G. B., & Parak, W. J. (2007). Polymer microcapsules as mobile local pH-sensors. Journal of Materials Chemistry, 17(42), 4471. doi: 10.1039/b705419j
- Labouta, H. I., & Schneider, M. (2010). Tailor-made biofunctionalized nanoparticles using layer-by-layer technology. International Journal of Pharmaceutics, 395(1-2), 236–242. doi: 10.1016/j.ijpharm.2010.05.019
- Lee, J. K., Ahn, K. C., Park, O. S., Kang, S. Y., & Hammock, B. D. (2001). Development of an ELISA for the detection of the residues of the insecticide imidacloprid in agricultural and environmental samples. Journal of Agricultural and Food Chemistry, 49(5), 2159–2167. doi: 10.1021/jf001140v
- Lee, K. L., You, M. L., Tsai, C. H., Lin, E. H., Hsieh, S. Y., Ho, M. H., … Wei, P. K. (2016). Nanoplasmonic biochips for rapid label-free detection of imidacloprid pesticides with a smartphone. Biosensors and Bioelectronics, 75, 88–95. doi: 10.1016/j.bios.2015.08.010
- Li, K., & Li, Q. X. (2000). Development of an enzyme-linked immunosorbent assay for the insecticide imidacloprid. Journal of Agricultural and Food Chemistry, 48(8), 3378–3382. doi: 10.1021/jf991257n
- Li, Z., Liu, Y., Sun, Y., Wu, Q., Lei, H., Wang, H., & Xiao, Z. (2007). Development of polyclonal antibody based enzyme-linked immunosorbent assay for the analysis of the agricultural insecticide imidacloprid: Food quality and safety. Asia Pacific Journal of Clinical Nutrition, 16(Suppl. 1), 102–105. doi: 10.6133/apjcn.2007.16.s1.19
- Liang, W., Wang, J., Zang, X., Dong, W., Wang, C., & Wang, Z. (2017). Barley husk carbon as the fiber coating for the solid-phase microextraction of twelve pesticides in vegetables prior to gas chromatography–mass spectrometric detection. Journal of Chromatography A, 1491, 9–15. doi: 10.1016/j.chroma.2017.02.034
- Lin, Y., Zhou, Q., Lin, Y., Tang, D., Niessner, R., & Knopp, D. (2015). Enzymatic hydrolysate-induced displacement reaction with multifunctional silica beads doped with horseradish peroxidase–thionine conjugate for ultrasensitive electrochemical immunoassay. Analytical Chemistry, 87(16), 8531–8540. doi: 10.1021/acs.analchem.5b02253
- Liu, Z., Liu, J., Wang, K., Li, W., Shelver, W. L., Li, Q. X., … Xu, T. (2015). Selection of phage-displayed peptides for the detection of imidacloprid in water and soil. Analytical Biochemistry, 485, 28–33. doi: 10.1016/j.ab.2015.05.014
- Luo, R., Venkatraman, S. S., & Neu, B. (2013). Layer-by-layer polyelectrolyte–polyester hybrid microcapsules for encapsulation and delivery of hydrophobic drugs. Biomacromolecules, 14(7), 2262–2271. doi: 10.1021/bm4003915
- Lvov, Y., & Caruso, F. (2001). Biocolloids with ordered urease multilayer shells as enzymatic reactors. Analytical Chemistry, 73(17), 4212–4217. doi: 10.1021/ac010118d
- Ma, H., Xu, Y., Li, Q. X., Xu, T., Wang, X., & Li, J. (2009). Application of enzyme-linked immunosorbent assay for quantification of the insecticides imidacloprid and thiamethoxam in honey samples. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 26(5), 713–718. doi: 10.1080/02652030802672638
- Matsusaki, M., Amemori, S., Kadowaki, K., & Akashi, M. (2011). Quantitative 3D analysis of nitric oxide diffusion in a 3D artery model using sensor particles. Angewandte Chemie International Edition, 50(33), 7557–7561. doi: 10.1002/anie.201008204
- Meng, X. Y., Li, Y. S., Zhou, Y., Zhang, Y. Y., Yang, L., Qiao, B., … Wang, X. R. (2015). An enzyme-linked immunosorbent assay for detection of pyrene and related polycyclic aromatic hydrocarbons. Analytical Biochemistry, 473, 1–6. doi: 10.1016/j.ab.2014.12.002
- Mzid, M., Badraoui, R., Khedir, S. B., Sahnoun, Z., & Rebai, T. (2017). Protective effect of ethanolic extract of Urtica urens L. against the toxicity of imidacloprid on bone remodeling in rats and antioxidant activities. Biomedicine & Pharmacotherapy, 91, 1022–1041. doi: 10.1016/j.biopha.2017.05.023
- Neumann, S., Kovtun, A., Dietzel, I. D., Epple, M., & Heumann, R. (2009). The use of size-defined DNA-functionalized calcium phosphate nanoparticles to minimise intracellular calcium disturbance during transfection. Biomaterials, 30(35), 6794–6802. doi: 10.1016/j.biomaterials.2009.08.043
- Oaew, S., Charlermroj, R., Pattarakankul, T., & Karoonuthaisiri, N. (2012). Gold nanoparticles/horseradish peroxidase encapsulated polyelectrolyte nanocapsule for signal amplification in Listeria monocytogenes detection. Biosensors and Bioelectronics, 34(1), 238–243. doi: 10.1016/j.bios.2012.02.011
- Orza, A., Olenic, L., Pruneanu, S., Pogacean, F., & Biris, A. S. (2010). Morphological and electrical characteristics of amino acid–AuNP nanostructured two-dimensional ensembles. Chemical Physics, 373(3), 295–299. doi: 10.1016/j.chemphys.2010.06.001
- Pereira, S. O., Barros-Timmons, A., & Trindade, T. (2014). Biofunctionalisation of colloidal gold nanoparticles via polyelectrolytes assemblies. Colloid and Polymer Science, 292(1), 33–50. doi: 10.1007/s00396-013-3037-3
- Seccia, S., Fidente, P., Montesano, D., & Morrica, P. (2008). Determination of neonicotinoid insecticides residues in bovine milk samples by solid-phase extraction clean-up and liquid chromatography with diode-array detection. Journal of Chromatography A, 1214(1–2), 115–120. doi: 10.1016/j.chroma.2008.10.088
- Sheng, Y., Liu, C., Yuan, Y., Tao, X., Yang, F., Shan, X., … Xu, F. (2009). Long-circulating polymeric nanoparticles bearing a combinatorial coating of PEG and water-soluble chitosan. Biomaterials, 30(12), 2340–2348. doi: 10.1016/j.biomaterials.2008.12.070
- Shu, J., Qiu, Z., Zhou, Q., Lin, Y., Lu, M., & Tang, D. (2016). Enzymatic oxydate-triggered self-illuminated photoelectrochemical sensing platform for portable immunoassay using digital multimeter. Analytical Chemistry, 88(5), 2958–2966. doi: 10.1021/acs.analchem.6b00262
- Tang, J., Tang, D., Niessner, R., Chen, G., & Knopp, D. (2011). Magneto-controlled graphene immunosensing platform for simultaneous multiplexed electrochemical immunoassay using distinguishable signal tags. Analytical Chemistry, 83(13), 5407–5414. doi: 10.1021/ac200969w
- Toor, H. K., Sangha, G. K., & Khera, K. S. (2013). Imidacloprid induced histological and biochemical alterations in liver of female albino rats. Pesticide Biochemistry and Physiology, 105(1), 1–4. doi: 10.1016/j.pestbp.2012.10.001
- Trau, D., & Renneberg, R. (2003). Encapsulation of glucose oxidase microparticles within a nanoscale layer-by-layer film: Immobilization and biosensor applications. Biosensors and Bioelectronics, 18(12), 1491–1499. doi: 10.1016/s0956-5663(03)00119-2
- Vichapong, J., Burakham, R., & Srijaranai, S. (2015). In-coupled syringe assisted octanol–water partition microextraction coupled with high-performance liquid chromatography for simultaneous determination of neonicotinoid insecticide residues in honey. Talanta, 139, 21–26. doi: 10.1016/j.talanta.2015.02.033
- Wanatabe, S., Ito, S., Kamata, Y., Omoda, N., Yamazaki, T., Munakata, H., … Yuasa, Y. (2001). Development of competitive enzyme-linked immunosorbent assays (ELISAs) based on monoclonal antibodies for chloronicotinoid insecticides imidacloprid and acetamiprid. Analytica Chimica Acta, 427(2), 211–219. doi: 10.1016/S0003-2670(00)01126-0
- Wang, C., Chen, Y., Wang, T., Ma, Z., & Su, Z. (2007). Biorecognition-driven self-assembly of gold nanorods: A rapid and sensitive approach toward antibody sensing. Chemistry of Materials, 19(24), 5809–5811. doi: 10.1021/cm0700899
- Wang, R., Wang, Z., Yang, H., Wang, Y., & Deng, A. (2012). Highly sensitive and specific detection of neonicotinoid insecticide imidacloprid in environmental and food samples by a polyclonal antibody-based enzyme-linked immunosorbent assay. Journal of The Science of Food and Agriculture, 92(6), 1253–1260. doi: 10.1002/jsfa.4691
- Watanabe, E., Eun, H., Baba, K., Arao, T., Ishii, Y., Endo, S., & Ueji, M. (2004). Evaluation and validation of a commercially available enzyme-linked immunosorbent assay for the neonicotinoid insecticide imidacloprid in agricultural samples. Journal of Agricultural and Food Chemistry, 52(10), 2756–2762. doi: 10.1021/jf0498867
- Wu, W., Li, J., Pan, D., Li, J., Song, S., Rong, M., … Lu, J. (2014). Gold nanoparticle-based enzyme-linked antibody-aptamer sandwich assay for detection of salmonella typhimurium. ACS Applied Materials and Interfaces, 6(19), 16974–16981. doi: 10.1021/am5045828
- Xuan, J., Jia, X. D., Jiang, L. P., Abdel-Halim, E. S., & Zhu, J. J. (2012). Gold nanoparticle-assembled capsules and their application as hydrogen peroxide biosensor based on hemoglobin. Bioelectrochemistry, 84, 32–37. doi: 10.1016/j.bioelechem.2011.10.007
- Zhang, B., Liu, B., Tang, D., Niessner, R., Chen, G., & Knopp, D. (2012). DNA-based hybridization chain reaction for amplified bioelectronic signal and ultrasensitive detection of proteins. Analytical Chemistry, 84(12), 5392–5399. doi: 10.1021/ac3009065
- Zhou, J., Lai, W., Zhuang, J., Tang, J., & Tang, D. (2013). Nanogold-functionalized DNAzyme concatamers with redox-active intercalators for quadruple signal amplification of electrochemical immunoassay. ACS Applied Materials and Interfaces, 5(7), 2773–2781. doi: 10.1021/am400652g
- Zhou, Y., Pan, F., Li, Y., Zhang, Y., Zhang, J., Lu, S., … Liu, Z. (2009). Colloidal gold probe-based immunochromatographic assay for the rapid detection of brevetoxins in fishery product samples. Biosensors and Bioelectronics, 24(8), 2744–2747. doi: 10.1016/j.bios.2009.01.034