ABSTRACT
Osteoarthritis (OA) commonly affects the synovial joint and is characterized by degradation of articular cartilage, which is largely mobilized by increased matrix metalloproteinase (MMP) activity. Soya-cerebroside, an extract of Cordyceps militaris, inhibits inflammatory cytokine production and monocyte migration in human synovial fibroblasts, although its effects are uncertain on MMP expression in chondrocytes and cartilage degradation. In this study, soya-cerebroside antagonized interleukin 1 beta (IL-1β)-induced MMP-1 production in chondrocytes, without cytotoxic effects. Functional genomic data confirm high levels of MMP-1 expression in OA tissue compared with normal tissue. Soya-cerebroside reduced MMP-1 expression via the focal adhesion kinase (FAK), mitogen-activated protein kinase (MEK), extracellular signal-regulated kinase (ERK) and AP-1 signalling pathways. In addition, soya-cerebroside suppressed IL-1β-promoted MMP-1 expression and cartilage degradation in animal models. Our report is the first to reveal that soya-cerebroside reduces MMP-1 production in chondrocytes and protects cartilage degradation through the FAK, MEK, ERK, and AP-1 signalling cascade.
1. Introduction
Osteoarthritis (OA) is the most common form of arthritis and a critical determinant of disability in older adults (Chou, Su, Huang, & Tang, Citation2014). No specific cause has been identified for OA, although aging, gender, obesity, genetic factors and injury all increase the risk of OA (Marshall, Watt, Vincent, & Dziedzic, Citation2018; Wu, Goh, Wang, & Ma, Citation2018; Wu, Lin, & Hsiung, Citation2015). Inflammation is believed to be involved in the early stage of OA disease, its progression and development (Wang & He, Citation2018). Of the several cytokines that are involved in intra-articular inflammation in OA, interleukin 1 beta (IL-1β) is a critical mediator of the inflammatory response which is a member of the IL-1 family (Barksby et al., Citation2006; Chou et al., Citation2014) and the initiation of the OA process (Chien et al., Citation2016).
Under normal physiological conditions, chondrocytes ensure the structural and functional integrity of articular cartilage by maintaining the anabolic-catabolic balance (Zhuo, Yang, Chen, & Wang, Citation2012). Moreover, chondrocytes mediate the matrix turnover that remodels cartilage, by expressing proteolytic enzymes, including aggrecanases and matrix metalloproteinases (MMPs) (Pelletier, Martel-Pelletier, & Abramson, Citation2001). Loss of balance in chondrocyte function increases the production of proteolytic enzymes and stimulates the destruction of OA cartilage (Huang et al., Citation2013). Targeting MMPs offers a promising strategy for the treatment of OA.
Cordyceps militaris, an entomopathogenic fungus, has long been used to treat inflammatory diseases in humans (Brent et al., Citation2016). We have previously shown that the C. militaris extract, soya-cerebroside, reduces lipopolysaccharide (LPS)-induced production of pro-inflammatory cytokines, inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in macrophages (Chiu et al., Citation2016), and also inhibits IL-1β-promoted monocyte chemoattractant protein-1 (MCP-1) expression and monocyte migration in OA synovial fibroblasts (Liu et al., Citation2017). However, the effects of soya-cerebroside on MMP production in chondrocytes are uncertain. This study demonstrates that soya-cerebroside reverses IL-1β-induced MMP-1 production in chondrocytes. It also shows that soya-cerebroside is involved in the focal adhesion kinase (FAK), mitogen-activated protein kinase (MEK), extracellular signal-regulated kinase (ERK) and AP-1 signalling pathways. In addition, the ability of soya-cerebroside to antagonize IL-1β-induced cartilage degradation and MMP-1 expression in rat and mouse inflammatory models further adds to the evidence in support of soya-cerebroside in the treatment of OA disease.
2. Materials and methods
2.1. Materials
Soya-cerebrosides were synthesized at the Kaohsiung Medical University, Kaohsiung, Taiwan, as described in our previous reports (Chiu et al., Citation2016; Liu et al., Citation2017). We purchased human IL-1β from PeproTech (Rocky Hill, NJ). Small interfering RNAs (siRNAs) against FAK, MEK, ERK, c-Jun and control were purchased from Dharmacon (Lafayette, CO, USA). We purchased FAK, MEK, ERK and c-Jun primary antibodies (Santa Cruz Biotechnology, CA, USA), as well as rabbit polyclonal antibodies specific for p-FAK, p-MEK, p-ERK and p–c-Jun (Cell Signaling Technology, MA, USA). Gibco-BRL life technologies (Grand Island, NY, USA) supplied fetal bovine serum (FBS), DMEM, Ham’s F12 medium, and all other cell culture reagents. All other chemicals or inhibitors were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Cell culture
The mouse chondrogenic cell line ATDC5 was kindly provided by Dr. Shyh-Ming Kuo (I-Shou University, Kaohsiung, Taiwan) and maintained in a 1:1 mixture of DMEM and Ham’s F12 medium containing 10% FBS (Wu, Fong, Lin, Huang, & Tang, Citation2018).
2.3. Analysis of the Gene Expression Omnibus (GEO) dataset
mRNA sequencing data for 5 normal healthy controls and 5 patients with OA were retrieved from the GEO dataset (accession code; GDS2126).
2.4. Cell viability assay
Cells were treated with or without soya-cerebroside for 24 or 48 h, then incubated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; 0.5 mg/mL) for 30 min. MTT was dissolved in dimethylsulfoxide and the absorbance was measured at 550 nm using a microplate reader (Bio-Tek, Winooski, VT, USA) (Lee et al., Citation2019; Liu, Chen, Chen, Chang, & Tang, Citation2016).
2.5. Quantitative real-time PCR (qPCR) of mRNA
Total RNA was extracted from chondrogenic ATDC5 cells using TRIzol reagent. qPCR analysis was performed according to an established protocol (Chen, Su et al., Citation2017; Chen, Tang et al., Citation2017).
2.6. Western blot analysis
Cell lysates underwent electrophoresis with SDS-PAGE and were transferred to polyvinylidene difluoride (PVDF) membranes, as described in our previous studies (Hu et al., Citation2017; Lee et al., Citation2018). After blocking the blots with 4% bovine serum albumin, we treated them with primary antibody then with peroxidase-conjugated secondary antibody. Blots were visualized using the chemiluminescence UVP BioSpectrum system (UVP, Upland, CA, USA).
2.7. Immunohistochemistry
Serial sections (5 μm thick) were cut from the medial side of knee joints and stained with Safranin-O/fast green and hematoxylin and eosin (H/E) for viewing under polarized light microscopy, as described in our previous studies (Liu et al., Citation2017). Tissue sections were incubated with anti-MMP-1 (1:200) primary antibody at 4°C overnight and then incubated with secondary antibody (1:200) for 1 h at room temperature. Finally, the sections were stained with diaminobenzidine.
2.8. Statistical analysis
Data are presented as the mean ± standard error of the mean. Statistical analysis of comparisons between 2 samples was performed using the Student’s t-test. Statistical comparisons of more than 2 groups were performed using one-way analysis of variance, followed by the Bonferroni post-hoc test. In all cases, p < 0.05 was considered to be statistically significant.
3. Results
3.1. Soya-cerebroside inhibits IL-1β-promoted MMP-1 expression in chondrocytes
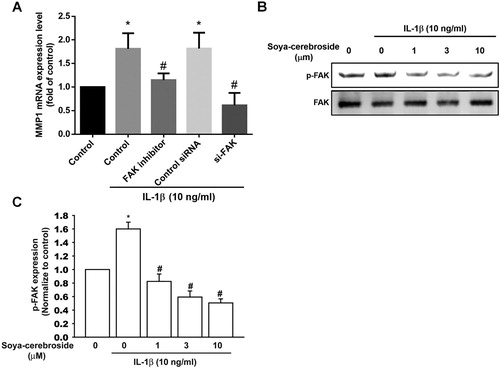
We have previously demonstrated anti-inflammatory effects of soya-cerebroside (Chiu et al., Citation2016; Liu et al., Citation2017). However, no data exist as to its role in the production of chondrocyte MMPs. Stimulation of ATDC5 cells with different concentrations of soya-cerebroside did not affect cell viability ((A)). Whereas MMP-1 and MMP-3 expression was increased in chondrocytes incubated with IL-1β, soya-cerebroside treatment blocked the effect of IL-1β on promoting MMP-1 levels, but had no effect on the IL-1β-induced increase in MMP-3 expression ((B-D)). Our analysis of mRNA expression profiles from the GEO database revealed higher levels of MMP-1 expression in OA tissue compared with those in normal tissue ((E,F)). These results indicate that soya-cerebroside reduces IL-1β-promoted MMP-1 expression in chondrocytes.
Figure 1. Soya-cerebroside reduces IL-1β-promoted MMP-1 production in chondrocytes. (A) ATDC5 cells were incubated with the indicated concentrations of soya-cerebroside for 24 or 48 h, then cell viability was determined using the MTT assay. (B-D) Cells were pretreated with soya-cerebroside (1–10 µM) for 30 min then stimulated with IL-1β for 24 h. MMP expression was examined by qPCR and Western blot. (E&F) Expression levels of MMP-1 in paired normal and OA tissues retrieved from GEO dataset records (GDS5403). Data represent the mean ± S.E.M. *, p < 0.05 compared with the control group; #, p < 0.05 compared with the IL-1β-treated group.
3.2. Soya-cerebroside antagonizes IL-1β-promoted MMP-1 production through the FAK, MEK, ERK and AP-1 signalling pathways
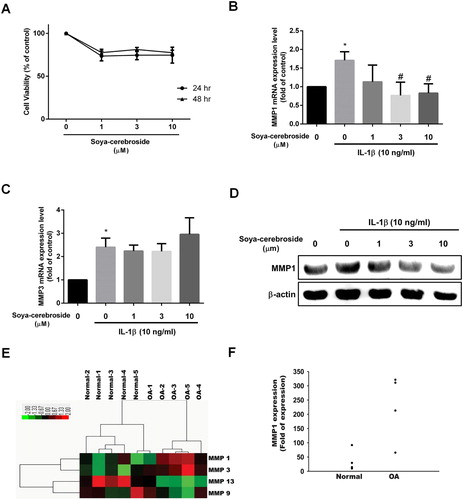
FAK activation appears to regulate MMP expression (Chuang et al., Citation2012; Wu et al., Citation2012). In this study, incubation of cells with a FAK inhibitor reversed IL-1β-induced increases in MMP-1 expression ((A)). Transfection with FAK siRNAs produced similar effects ((A)), suggesting that FAK plays a role in IL-1β-induced MMP-1 production. Interestingly, soya-cerebroside treatment of chondrocytes diminished IL-1β-enhanced phosphorylation of FAK ((B,C)), indicating that soya-cerebroside reduces IL-1β-promoted MMP-1 production through FAK activation.
Figure 2. The FAK pathway is involved in soya-cerebroside-induced reductions in MMP-1 production. (A) ATDC5 cells were pretreated with a FAK inhibitor for 30 min or transfected with a FAK siRNA for 24 h then stimulated with IL-1β for 24 h; MMP-1 expression was examined by qPCR. (B&C) ATDC5 cells were pretreated with soya-cerebroside for 30 min then stimulated with IL-1β. FAK phosphorylation was examined by Western blot. Data represent the mean ± S.E.M. *, p < 0.05 compared with the control group; #, p < 0.05 compared with the IL-1β-treated group.
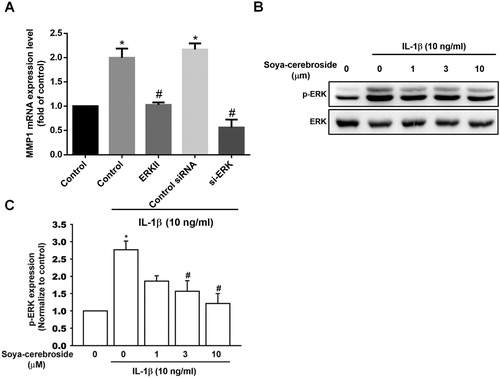
Activation of MEK/ERK signalling is a common event after FAK phosphorylation (Liu, Hsu, Fong, Chuang, & Tang, Citation2013; Tsai, Huang, Su, & Tang, Citation2014). We found that stimulation of chondrocytes with MEK inhibitors (U0126 and PD98059) or an ERK inhibitor (ERKII) diminished IL-1β-promoted MMP-1 production ((A) and (A)). Furthermore, the MEK and ERK siRNAs also inhibited IL-1β-promoted MMP-1 production ((A) and (A)). We also found that soya-cerebroside antagonized IL-1β-enhanced phosphorylation of MEK and ERK ( and (B,C)), suggesting that soya-cerebroside reduces MMP-1 production through the MEK and ERK pathways.
Figure 3. The MEK pathway is involved in soya-cerebroside-induced reductions in MMP-1 production. (A) ATDC5 cells were pretreated with MEK inhibitors (U0126 or PD98095) for 30 min or transfected with a MEK siRNA for 24 h then stimulated with IL-1β for 24 h; MMP-1 expression was examined by qPCR. (B&C) ATDC5 cells were pretreated with soya-cerebroside for 30 min then stimulated with IL-1β. MEK phosphorylation was examined by Western blot. Data represent the mean ± S.E.M. *, p < 0.05 compared with the control group; #, p < 0.05 compared with the IL-1β-treated group.
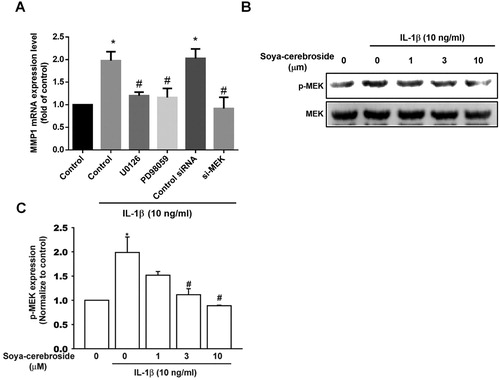
Figure 4. The ERK pathway is involved in soya-cerebroside-induced reductions in MMP-1 production. (A) ATDC5 cells were pretreated with a ERK inhibitor for 30 min or transfected with a ERK siRNA for 24 h then stimulated with IL-1β for 24 h; MMP-1 expression was examined by qPCR. (B&C) ATDC5 cells were pretreated with soya-cerebroside for 30 min then stimulated with IL-1β. ERK phosphorylation was examined by Western blot. Data represent the mean ± S.E.M. *, p < 0.05 compared with the control group; #, p < 0.05 compared with the IL-1β-treated group.
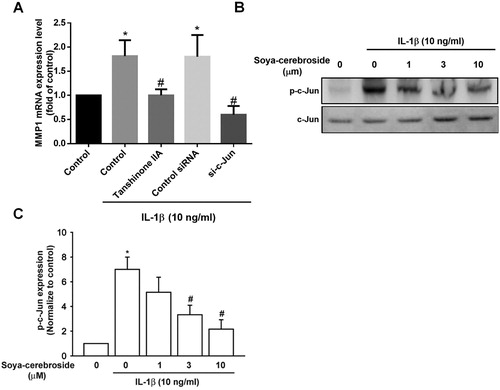
AP-1 is a critical transcription factor that controls MMP-1 production (Pang, Yen, Wu, & Huang, Citation2017; Wu, Lin, Tsai, Huang, & Tang, Citation2018). We examined whether AP-1 controls IL-1β-induced MMP-1 production. After treating chondrocytes with AP-1 inhibitor (tanshinone IIA) or transfecting them with c-Jun siRNA, exposure to IL-1β led to a reduction in MMP-1 production ((A)). Soya-cerebroside blocked IL-1β-induced increases in c-Jun phosphorylation ((B,C)), suggesting that soya-cerebroside reduces MMP-1 production via AP-1.
Figure 5. The AP-1 pathway is involved in soya-cerebroside-induced reductions in MMP-1 production. (A) ATDC5 cells were pretreated with AP-1 inhibitors (tanshinone IIA) for 30 min or transfected with a c-Jun siRNA for 24 h then stimulated with IL-1β for 24 h; MMP-1 expression was examined by qPCR. (B&C) ATDC5 cells were pretreated with soya-cerebroside for 30 min then stimulated with IL-1β. c-Jun phosphorylation was examined by Western blot. Data represent the mean ± S.E.M. *, p < 0.05 compared with the control group; #, p < 0.05 compared with the IL-1β-treated group.
3.3. Soya-cerebroside diminishes IL-1β-induced cartilage degradation and MMP-1 expression in vivo
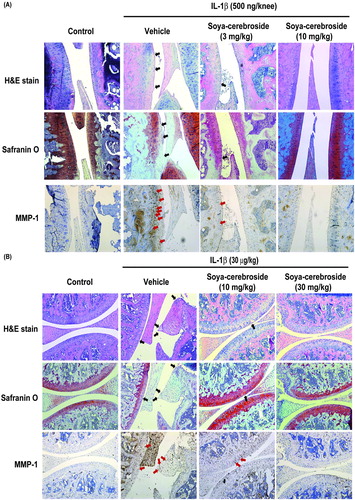
Our previous investigations found that soya-cerebroside reversed synovial inflammation, MCP-1 expression and monocyte infiltration in rat and mouse inflammatory models (Liu et al., Citation2017). In this study, intra-articular injections of IL-1β induced cartilage degradation in mouse and rat knee joints stained with H/E and Safranin-O (), while soya-cerebroside dose-dependently reversed cartilage degradation in both animal models (; black arrows). Soya-cerebroside also reversed IL-1β-induced MMP-1 upregulation in cartilage (; red arrows). These results demonstrate promising cartilage protective effects of soya-cerebroside in vivo.
Figure 6. Soya-cerebroside reverses IL-1β-promoted MMP-1 production and cartilage degradation in animal models. Rats (A) or mice (B) were injected intra-articularly with IL-1β or saline as a negative control and then administered intra-peritoneal injections of saline or soya-cerebroside daily for 35 (A) or 7 days (B) (Liu et al., Citation2017). Photomicrographs of knee joint sections were stained with H&E or Safranin-O (black arrows) and immunostained with MMP-1 (red arrows).
4. Discussion
OA pathogenesis is little understood. It is well accepted, however, that cartilage degradation is a critical step during OA development. Dysregulated MMP activity is obvious in several pathological conditions associated with loss of normal collagen architecture (Yang et al., Citation2017). Researchers have therefore proposed that therapeutic strategies should target MMPs in the treatment of arthritis (Troeberg & Nagase, Citation2012; Wang, Xu, Hunter, & Ding, Citation2015). We have previously indicated that soya-cerebroside, an extract of C. militaris, antagonizes MCP-1-dependent monocyte migration in human synovial fibroblasts (Liu et al., Citation2017). In addition, soya-cerebroside reverses synovial inflammation and monocyte infiltration in rat and mouse inflammatory models, suggesting that soya-cerebroside has an anti-inflammatory effect in arthritic disease (Liu et al., Citation2017). However, the role of soya-cerebroside on MMP production in chondrocytes has been unclear. Here, we report that soya-cerebroside diminished IL-1β-promoted MMP-1 production in chondrocytes, but had no effect on IL-1β-induced enhancement of MMP-3 expression. According to our evidence, the FAK, MEK, ERK and AP-1 signalling cascade is involved in the effects of soya-cerebroside. Furthermore, we found that soya-cerebroside diminished IL-1β-induced cartilage degradation and MMP-1 upregulation in two animal models, suggesting that soya-cerebroside is a potential therapeutic candidate for OA disease.
The FAK, MEK, ERK and AP-1 signalling cascade reportedly regulates MMP-1 expression in chondrocytes (Wu, Lin et al., Citation2018). In this study, the inhibitors of FAK, MEK (U0126 and PD98059), ERK and AP-1 (tanshinone IIA) inhibited IL-1β-enhanced MMP-1 production. Our use of siRNAs against FAK, MEK, ERK and c-Jun confirmed that this pathway is involved in IL-1β-promoted MMP-1 production. Notably, soya-cerebroside diminished IL-1β-augmented phosphorylation of FAK, MEK, ERK and c-Jun. These results suggest that FAK, MEK, ERK and AP-1 activation requires soya-cerebroside-induced inhibition of MMP-1 production in chondrocytes.
MMPs are expressed in several different types of cells and have important roles in diverse cellular processes (Araki & Mimura, Citation2017), including the degradation of different components of the extracellular matrix (Amar, Smith, & Fields, Citation2017). Among all MMPs, MMP-1 has been reported to be upregulated in OA patients (Amar et al., Citation2017; Hwang et al., Citation2018), implying that anti-MMP-1 antibody is a promising new strategy for treatment of OA. In the current study, the GEO database confirmed higher levels of MMP-1 expression in OA tissue compared with those in normal tissue. Otherwise, soya-cerebroside reduced IL-1β-increased MMP-1 mRNA and protein expression in chondrocytes, suggesting soya-cerebroside is a novel MMP-1 inhibitor for OA treatment.
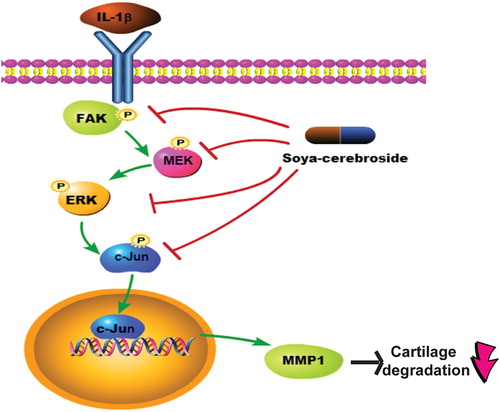
Soya-cerebroside, an extract of C. militaris, exhibits anti-inflammatory activity in human synovial fibroblasts (Liu et al., Citation2017). Soya-cerebroside also antagonizes synovial inflammation and monocyte infiltration in inflammatory models (Araki & Mimura, Citation2017). However, the role of soya-cerebroside in chondrocyte MMP expression has been unclear. We report for the first time that soya-cerebroside inhibits IL-1β-increased MMP-1 production in chondrocytes and that the FAK, MEK, ERK and AP-1 signalling pathways are involved in soya-cerebroside-induced reductions in MMP-1 production (). At nontoxic concentrations, soya-cerebroside diminished IL-1β-induced MMP-1 expression and cartilage degradation in vivo. Soya-cerebroside deserves further investigation for the treatment of OA.
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology of Taiwan (MOST 105-2320-B-039-015-MY3); China Medical University Hospital (DMR-105-179); “Chinese Medicine Research Center, China Medical University” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan (CMRC-CHM-3-1).
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Yuan-Man Hsu http://orcid.org/0000-0002-4575-7475
Additional information
Funding
References
- Amar, S., Smith, L., & Fields, G. B. (2017). Matrix metalloproteinase collagenolysis in health and disease. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 1864, 1940–1951. doi: 10.1016/j.bbamcr.2017.04.015
- Araki, Y., & Mimura, T. (2017). Matrix metalloproteinase gene activation resulting from disordred epigenetic mechanisms in rheumatoid arthritis. International Journal of Molecular Sciences, 18, 905. doi: 10.3390/ijms18050905
- Barksby, H. E., Hui, W., Wappler, I., Peters, H. H., Milner, J. M., Richards, C. D., … Rowan, A. D. (2006). Interleukin-1 in combination with oncostatin M up-regulates multiple genes in chondrocytes: Implications for cartilage destruction and repair. Arthritis & Rheumatism, 54, 540–550. doi: 10.1002/art.21574
- Brent, C. S., Miyasaki, K., Vuong, C., Miranda, B., Steele, B., Brent, K. G., & Nath, R. (2016). Regulatory roles of biogenic amines and juvenile hormone in the reproductive behavior of the western tarnished plant bug (Lygus hesperus). Journal of Comparative Physiology B, Biochemical, Systemic, and Environmental Physiology, 186, 169–179. doi: 10.1007/s00360-015-0953-1
- Chen, C. Y., Su, C. M., Hsu, C. J., Huang, C. C., Wang, S. W., Liu, S. C., … Tang, C.-H. (2017). CCN1 Promotes VEGF production in osteoblasts and induces endothelial progenitor cell angiogenesis by inhibiting miR-126 expression in rheumatoid arthritis. Journal of Bone and Mineral Research, The Official Journal of the American Society for Bone and Mineral Research, 32, 34–45. doi: 10.1002/jbmr.2926
- Chen, P. C., Tang, C. H., Lin, L. W., Tsai, C. H., Chu, C. Y., Lin, T. H., & Huang, Y. L. (2017). Thrombospondin-2 promotes prostate cancer bone metastasis by the up-regulation of matrix metalloproteinase-2 through down-regulating miR-376c expression. Journal of Hematology & Oncology, 10, 33. doi: 10.1186/s13045-017-0390-6
- Chien, S. Y., Huang, C. Y., Tsai, C. H., Wang, S. W., Lin, Y. M., & Tang, C. H. (2016). Interleukin-1beta induces fibroblast growth factor 2 expression and subsequently promotes endothelial progenitor cell angiogenesis in chondrocytes. Clinical Science, 130, 667–681. doi: 10.1042/CS20150622
- Chiu, C. P., Liu, S. C., Tang, C. H., Chan, Y., El-Shazly, M., Lee, C. L., … Wu, Y.-C. (2016). Anti-inflammatory cerebrosides from cultivated cordyceps militaris. Journal of Agricultural and Food Chemistry, 64, 1540–1548. doi: 10.1021/acs.jafc.5b05931
- Chou, P. Y., Su, C. M., Huang, C. Y., & Tang, C. H. (2014). The characteristics of thrombin in osteoarthritic pathogenesis and treatment. BioMed Research International, 2014, 407518.
- Chuang, J. Y., Yu, N. Y., Chiang, I. P., Lai, C. H., Lin, C. D., & Tang, C. H. (2012). Cyr61 increases matrix metalloproteinase-3 expression and cell motility in human oral squamous cell carcinoma cells. Journal of Cellular Biochemistry, 113, 1977–1986. doi: 10.1002/jcb.24066
- Hu, S. L., Chang, A. C., Huang, C. C., Tsai, C. H., Lin, C. C., & Tang, C. H. (2017). Myostatin promotes interleukin-1beta expression in rheumatoid arthritis synovial fibroblasts through inhibition of miR-21-5p. Frontiers in Immunology, 8, 1747. doi: 10.3389/fimmu.2017.01747
- Huang, C. Y., Lin, H. J., Chen, H. S., Cheng, S. Y., Hsu, H. C., & Tang, C. H. (2013). Thrombin promotes matrix metalloproteinase-13 expression through the PKCdelta c-Src/EGFR/PI3 K/Akt/AP-1 signaling pathway in human chondrocytes. Mediators of Inflammation, 2013, 326041. doi: 10.1155/2013/326041
- Hwang, I. Y., Youm, Y. S., Cho, S. D., Choi, S. W., Bae, M. H., Park, S. J., & Kim, H.-W. (2018). Synovial fluid levels of TWEAK and matrix metalloproteinase 1 in patients with osteoarthritis, and associations with disease severity. Journal of Orthopaedic Surgery, 26, 2309499018760112. doi: 10.1177/2309499018760112
- Lee, H.-P., Chen, P.-C., Wang, S.-W., Fong, Y.-C., Tsai, C.-H., Tsai, F.-J., … Tang, C.-H. (2019). Plumbagin suppresses endothelial progenitor cell-related angiogenesis in vitro and in vivo. Journal of Functional Foods, 52, 537–544. doi: 10.1016/j.jff.2018.11.040
- Lee, C. F., Chiang, N. N., Lu, Y. H., Huang, Y. S., Yang, J. S., Tsai, S. C., … Chen, F. A. (2018). Benzyl isothiocyanate (BITC) triggers mitochondria-mediated apoptotic machinery in human cisplatin-resistant oral cancer CAR cells. BioMedicine (Taipei), 8, 15. doi: 10.1051/bmdcn/2018080315
- Liu, J. F., Chen, C. Y., Chen, H. T., Chang, C. S., & Tang, C. H. (2016). BL-038, a benzofuran derivative, induces cell apoptosis in human chondrosarcoma cells through reactive oxygen species/mitochondrial dysfunction and the caspases dependent pathway. International Journal of Molecular Sciences, 17, 1491. doi: 10.3390/ijms17091491
- Liu, S. C., Chiu, C. P., Tsai, C. H., Hung, C. Y., Li, T. M., Wu, Y. C., & Tang, C. H. (2017). Soya-cerebroside, an extract of Cordyceps militaris, suppresses monocyte migration and prevents cartilage degradation in inflammatory animal models. Scientific Reports, 7, 43205. doi: 10.1038/srep43205
- Liu, S. C., Hsu, C. J., Fong, Y. C., Chuang, S. M., & Tang, C. H. (2013). CTGF induces monocyte chemoattractant protein-1 expression to enhance monocyte migration in human synovial fibroblasts. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 1833, 1114–1124. doi: 10.1016/j.bbamcr.2012.12.014
- Marshall, M., Watt, F. E., Vincent, T. L., & Dziedzic, K. (2018). Hand osteoarthritis: Clinical phenotypes, molecular mechanisms and disease management. Nature Reviews Rheumatology, 14, 641–656. doi: 10.1038/s41584-018-0095-4
- Pang, J. S., Yen, J. H., Wu, H. T., & Huang, S. T. (2017). Gallic acid inhibited matrix invasion and AP-1/ETS-1-mediated MMP-1 transcription in human nasopharyngeal carcinoma cells. International Journal of Molecular Sciences, 18, 1354. doi: 10.3390/ijms18071354
- Pelletier, J. P., Martel-Pelletier, J., & Abramson, S. B. (2001). Osteoarthritis, an inflammatory disease: Potential implication for the selection of new therapeutic targets. Arthritis and Rheumatism, 44, 1237–1247. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F
- Troeberg, L., & Nagase, H. (2012). Proteases involved in cartilage matrix degradation in osteoarthritis. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, 1824, 133–145. doi: 10.1016/j.bbapap.2011.06.020
- Tsai, H. C., Huang, C. Y., Su, H. L., & Tang, C. H. (2014). CCN2 enhances resistance to cisplatin-mediating cell apoptosis in human osteosarcoma. PloS one, 9, e90159. doi: 10.1371/journal.pone.0090159
- Wang, T., & He, C. (2018). Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine & Growth Factor Reviews, 44, 38–50. doi: 10.1016/j.cytogfr.2018.10.002
- Wang, K., Xu, J., Hunter, D. J., & Ding, C. (2015). Investigational drugs for the treatment of osteoarthritis. Expert Opinion on Investigational Drugs, 24, 1539–1556. doi: 10.1517/13543784.2015.1091880
- Wu, T. J., Fong, Y. C., Lin, C. Y., Huang, Y. L., & Tang, C. H. (2018). Glucose enhances aggrecan expression in chondrocytes via the PKCalpha/p38-miR141-3p signaling pathway. Journal of Cellular Physiology, 233, 6878–6887. doi: 10.1002/jcp.26451
- Wu, Y., Goh, E. L., Wang, D., & Ma, S. (2018). Novel treatments for osteoarthritis: An update. Open Access Rheumatology: Research and Reviews, 10, 135–140. doi: 10.2147/OARRR.S176666
- Wu, I. C., Lin, C. C., & Hsiung, C. A. (2015). Emerging roles of frailty and inflammaging in risk assessment of age-related chronic diseases in older adults: The intersection between aging biology and personalized medicine. BioMedicine (Taipei), 5, 1. doi: 10.7603/s40681-015-0001-1
- Wu, T. J., Lin, C. Y., Tsai, C. H., Huang, Y. L., & Tang, C. H. (2018). Glucose suppresses IL-1beta-induced MMP-1 expression through the FAK, MEK, ERK, and AP-1 signaling pathways. Environmental Toxicology, 33, 1061–1068. doi: 10.1002/tox.22618
- Wu, M. H., Lo, J. F., Kuo, C. H., Lin, J. A., Lin, Y. M., Chen, L. M., … Tang, C.-H. (2012). Endothelin-1 promotes MMP-13 production and migration in human chondrosarcoma cells through FAK/PI3K/Akt/mTOR pathways. Journal of Cellular Physiology, 227, 3016–3026. doi: 10.1002/jcp.23043
- Yang, M. D., Lin, K. C., Lu, M. C., Jeng, L. B., Hsiao, C. L., Yueh, T. C., … Tsai, F.-J. (2017). Contribution of matrix metalloproteinases-1 genotypes to gastric cancer susceptibility in Taiwan. BioMedicine (Taipei), 7, 10. doi: 10.1051/bmdcn/2017070203
- Zhuo, Q., Yang, W., Chen, J., & Wang, Y. (2012). Metabolic syndrome meets osteoarthritis. Nature Reviews Rheumatology, 8, 729–737. doi: 10.1038/nrrheum.2012.135