ABSTRACT
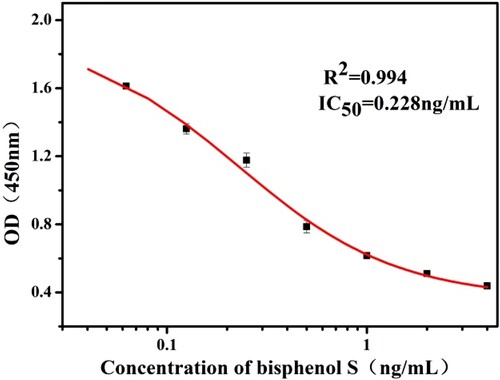
A highly sensitive and specific monoclonal antibody against bisphenol S (BPS) was prepared. The derived BPS was coupled to keyhole limpet hemocyanin as the immunogen and ovalbumin as the coating antigen. Based on monoclonal antibodies, an indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) was established, and the conditions of action were optimized. The half maximal inhibitory concentration (IC50) of BPS was 0.228 ng/mL and the linear range was 0.064–0.810 ng/mL. In the recovery test for the milk samples, the recovery rates were in the range of 89%–95% and coefficient of variation ranged from 1.0% to 6.0% respectively. In addition, the cut-off value of the immunochromatographic strip detection method in milk samples was 5 ng/mL. Therefore, both methods are suitable for use in milk samples. Furthermore, this immunochromatographic strip detection method is suitable for on-site testing and screening of very large samples.
Introduction
Bisphenol S (4,4'-Sulfonyldiphenol), (BPS) is an industrial chemical and a structural analogue of bisphenol A, which is mainly used as a raw material for the production of polycarbonate, epoxy resin, polyesters and phenolic resin, as well as polysulfone and polyethersulfone (Shan, Yao, Wen, & Shao, Citation2019). In addition, BPS is also a vital raw material for medical polymer materials and various plastics (Qiu et al., Citation2019), which are widely used as packaging in the food industry (Giacomo et al., Citation2018; Siracusa, Yin, Measel, Liang, & Yu, Citation2018). In recent years, BPS has been widely used as a substitute for bisphenol A (Simoneau, Valzacchi, Morkunas, & Eede, Citation2011). However, many studies have shown that BPS has endocrine disrupting activity along with bisphenol A (Aung, Ferguson, Cantonwine, McElrath, & Meeker, Citation2019), which can disrupt the reproductive system (Eladak et al., Citation2015; Ullah, Jahan, Ain, Shaheen, & Ahsan, Citation2016; Ullah, Pirzada et al., Citation2018). Japan and Korea have explicitly stipulated that the migration of BPS in food simulators should not exceed 0.05 mg/L. Furthermore, both The European Union Plastics Regulation (EU) No.10/2011 and The China National Standards (GB 9685-2008) have stipulated that the migration limit of BPS in food simulators should not exceed 0.05 mg/kg.
The reported detection methods for BPS include liquid chromatograph-mass spectrometry (LC-MS) (Loureiro, Quirós, & Sendón, Citation2018), liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Regueiro & Wenzl, Citation2015; Vázquez Loureiro, Rodríguez-Bernaldo De Quirós, & Sendón, Citation2018; Xie, Zhao, Zhao, Chen, & Cai, Citation2018), gas chromatography- mass spectrometry (GC-MS) (Viñas, Campillo, Martínez-Castillo, & Hernández-Córdoba, Citation2010; Wang, Zhu, et al., Citation2017), ultra-performance liquid chromatography-high resolution mass spectrometry (UPLC-HRMS) (Shengdong et al., Citation2017), high performance liquid chromatography (HPLC) (Xiong et al., Citation2018) and ultra-high performance liquid chromatography-tandem mass spectrometry (UPLC-LC-MS/MS) (Grandin et al., Citation2017). Although the instrumental detection method is precise, the sample pre-treatment process is complex, and the equipment is expensive, and time-consuming. Furthermore, professional technicians are required, as the instrumental methods are difficult to apply to large sample sizes. Compared with instrumental methods, immunoassays can be used for simultaneous detection of multiple samples with good reproducibility, which meets the market demand.
At present, there are few reports looking at the preparation of BSP monoclonal antibodies and the establishment of an immunoassay detection system such as ELISA kits. In this study, we have developed a high specificity and sensitive monoclonal antibody for the detection of BSP. From this an immunological assay for BPS in milk samples was designed.
Materials and methods
Chemicals
BPS was purchased from J&K Scientific Ltd. (Beijing, China), N-hydroxysuccinimide,1-ethyl-3-(3-dimethylaminopropyl) carbodi-imide, N,N-dimethylformamide, 2-morpholino-ethanesulfonic acid, Keyhole limpet hemocyanin (KLH), ovalbumin (OVA), Freund’s complete adjuvant, Freund’s incomplete adjuvant, enzyme immunoassay-grade horseradish peroxidase (HRP)-labelled goat anti-mouse immunoglobulin, gelatin, 3,3’,5,5’-tetramethyl-benzidine, Tween-20 and polyethylene glycol 1500 were all purchased from Sigma-Aldrich (St. Louis, MO, USA). RPMI-1640 cell culture medium, 100 × HT (hypoxanthine-thymidine supplement), 50 × HAT (hypoxanthine-aminopterin-thymidine supplement), and fetal calf serum were acquired from Gibco BRL (Paisley, UK). All other chemicals and reagents were obtained from the National Pharmaceutical Group Chemical Reagent Co., Ltd. (Beijing, China).
Apparatus
The instruments used in this study consisted of a UV/Vis scanner purchased from Bokin instruments (Tsushima, Japan), a Multiskan MKS microplate reader obtained from Thermo Labsystems Company (Beijing, China), a vortex machine purchased from Shanghai Huxi Analysis Instrument Factory Co. Ltd (Shanghai, China) and a membrane dispenser obtained from Xinqidian Gene-Technology Co. Ltd (Beijing, China).
Buffers and solutions
We prepared the following solutions: boracic acid buffer (BB, pH8.8), coating buffer (CB; 0.05 M carbonate bicarbonate, pH9.6), blocking buffer (0.2% gelatin in CB), washing buffer (PBST; 0.05% Tween-20 (v/v) in 0.01 M PBS), and stop solution (2 M sulfuric acid). The substrate buffer consisted of a mixture of solutions A (0.3% H2O2, v/v in phosphate buffer) and B (0.01% TMB, w/v in glycol) at a 5:1 (v/v) ratio.
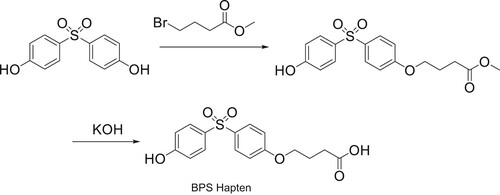
Hapten Synthesis
In this study, a new BPS hapten was synthesized using methyl 4-bromobutyrate. BPS contains two phenolic hydroxyl groups. This method involved the derivation of one hydroxyl group into a carboxyl group with linked arms, which was used to couple to the carrier protein (). The specific steps are described as follows: 1 g of 4,4'-dihydroxydiphenyl sulfone was added into a round-bottomed flask, then approximately 6 mL of pure water was added and heated to 94°C to dissolve the 4,4'-dihydroxydiphenyl sulfone. Next, 0.24 g of solid sodium hydroxide was added. After one hour, of heating and reflux, 4-methyl bromide was added. The mixture was left to react for 30 h, and then hydrochloric acid was added to regulate the pH to approximately pH3-4. The reaction was extracted three times with ether, and the organic phase was merged with saturated salt water. The saturated salt water was stewing after full oscillation, then the organic phase was separated into the water phase. The organic phase was dried with anhydrous sodium sulfate, and after a period of standing, the sodium sulfate was filtered out and the solvent was removed by vacuum evaporation. The crude product was purified by column chromatography, and the purified compound A was obtained.
The 0.55 g of compound A produced was dissolved in 10 mL of methanol. During stirring, the prepared KOH solution (n(KOH): ncompound A = 6:1) was added. After stirring for 48 h at room temperature, hydrochloric acid was added to adjust the pH value of the reaction system to approximately pH3.0, and a quenching reaction was carried out. The reaction system was extracted, dried, decompressed and distilled, and the purified compound B was obtained by column chromatography, which represented the BPS hapten, which was characterized using LC-MS.
Preparation and Identification of the complete antigen
The synthesized BPS was conjugated to bovine serum albumin (BSA) using the EDC coupling procedure, to form the complete antigen (BPS-KLH), as previously described (Kong, Liu, Song, Kuang, & Xu, Citation2017; Wang, Xie, et al., Citation2017). The BPS hapten (1.2 mg) was dissolved in 400 μL of dimethylformamide, then N-hydroxysuccinimide (2.7 mg) and 1-ethyl-3-(3-dimethylaminopropyl) carbodi-imide (2.2 mg) were added to the mixture. The mixture was stirred at room temperature for 8 h and then slowly added to 10 mg of KLH which was diluted with BB, and then stirred overnight at room temperature. The BPS-KLH conjugate was dialyzed against 0.01 M PBS for 3 days with six changes of dialysate and stored at −20°C until used. BPS was conjugated to OVA by the same method as used for the coating antigen, for the detection of serum titres and suppression of mice by ic-ELISA. The conjugates were identified by ultraviolet spectroscopy (Yamada, Satoh, Yamashita, Kambegawa, & Iwata, Citation1997).
Production of the BPS monoclonal antibody (mAb)
Female BALB/c mice (6–8 weeks of age) were immunized with complete antigen (BPS-KLH). Tail vein blood sampling was carried out after the third immunization and serum was analyzed using an ic-ELISA (Liu, Xu, Suryoprabowo, Song, & Kuang, Citation2018). The mouse with the highest inhibitory rate and titre was selected for cell fusion. The selected hybridoma cell lines were cultured and intraperitoneally injected into mice for ascites production (Kong, Liu, Song, Zheng, et al., Citation2017). After purification with octanoic acid-ammonium sulfate precipitation (Liang et al., Citation2014; Wang, Zheng, et al., Citation2017) and dialysis, monoclonal antibodies against BPS were obtained.
Development of the ic-ELISA method
The complete antigen (BPS-OVA) was diluted with CB (0.05 M, pH9.6) and added to 96-well plates (100 μL per well), incubated at 37°C for 2 h, washed three times, and blocked with blocking buffer (200 μL per well). After a 2 h incubation at 37°C, it was washed, dried, and stored at 4°C until used. Fifty micro litres of BPS standards and 50 μL of BPS mAb were added to each well and incubated at 37°C for 30 min. Then goat anti-mouse IgG-HRP was added to each well and incubated at 37°C for 30 min. After three washes, the substrate buffer was mixed with solutions A and B at a 5:1 (v/v) ratio and was added into each well and incubated at 37°C for 15 min. Following the addition of stop solution (2 M H2SO4, 50 μL per well), the absorbance was measured using a microplate reader at a wavelength of 450 nm. A standard curve of BPS mAb against BPS standard concentrations was then constructed.
Optimization of the ic-ELISA method
The methanol content, pH value and ionic strength (NaCl content) of the standard diluent were optimized under the same conditions. The ODmax and IC50 values of the standard curve were used to evaluate the advantages and disadvantages of each optimization condition. Methanol was added to the PBS to make a final concentration of 0%, 5%, 10% and 20% (v/v), respectively. The effect of pH value was estimated using buffer solutions with different pH values ranging from 4.1 to 9.0. To optimize the ionic strength, PBS containing different concentrations of NaCl (0%, 0.8%, 1.6%, 3.2%) were tested.
Sensitivity of the BPS mAb
The half maximal inhibitory concentration (IC50) means that the half maximum inhibitory concentration of the antagonist was measured. The optimum conditions for the action of the BPS mAb were determined by the above optimization process. Under these conditions, as shown in , an indirect competitive inhibition curve for the BPS mAb was drawn by using Origin software and the IC50 value was obtained.
Determination of cross-reactivity
The analogues and metabolites of the target drug were determined by cross-reaction. The IC50 of the antibody was determined according to the procedure used for the ic-ELISA. The cross-reaction rate was calculated using the following formula: CR = (IC50 of the original drug/IC50 of the drug to be tested) × 100% (Xu, Pan, Yu, Wei, & Wang, Citation2017). Generally, a cross-reaction rate of less than 10% can be regarded as having no cross-reaction.
Detection of BPS in milk using a recovery experiment
Milk samples were obtained from a local supermarket in Wuxi, and a 1 mL milk sample was diluted eight times with known BPS concentrations (0.5, 1 and 2.5 ng/mL). The ic-ELISA method was then used to detect BPS in the samples and establish a corresponding standard curve to calculate the added recovery rate (Wang, Wang et al. Citation2013).
Labelling of the mAb with colloidal gold
The BPS mAb was labelled with gold nanoparticles (Guo, Xu, Song, Liu, & Kuang, Citation2018; Liu, Peng et al., Citation2017). One millilitre of colloidal gold solution, 0.1 M K2CO3 solution and 8 μg of mAb were added to a centrifuge tube in turn, and then mixed. The labelling reaction was carried out at room temperature for 2 h. After labelling, 10% (w/v) of BSA solution dissolved in 100 μL of ultrapure water was added slowly and stirred at room temperature for 2 h to block the uncoupled antibody binding sites on the colloidal gold particles. Thereby, prevent the colloidal gold particles from adsorbing each other and keep the stability of the system. Then the reaction was centrifuged at 9,500 rcf for 20 mins, and the supernatant was discarded to remove excess antibody and BSA. The precipitation was re-diluted in 1 mL of labelled detergent, washed, and centrifuged repeatedly. The final supernatant was discarded, and the precipitate was resuspended with 100 μL of heavy suspension and stored at 4°C until use.
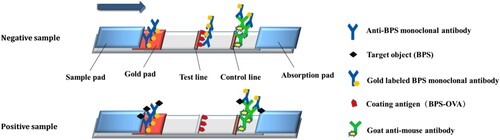
Preparation of the immunochromatographic strip
The structure of the colloidal gold strip is shown in . The colloidal gold strip consisted of a PVC backboard, sample pad, gold pad, water absorbent pad and cellulose acetate film (NC film). The NC membrane contains both a detection line (T line) and control line (C line). The coating antigen (0.25 mg/mL) was sprayed onto the T line and the goat anti-mouse antibody (1 mg/mL) was sprayed onto the C line. The sample pad is located at the end, near the T line, and the upper part of the back plate, which was treated with 0.01 M PBS containing 1% BSA, 1% sucrose, and 0.2% Tween 20, and dried at room temperature for 4 h. The gold pad with gold labelled monoclonal antibody was above the NC film and below the sample pad. A Water absorbent pad was at the end near the C line. The sample pad was immersed in the sample pad treatment solution, dried and stored for later use.
Principle of the immunochromatographic strip assay
The immunochromatographic strip assay is based on an antibody–antigen reaction and competitive immunoreactions (Kong et al., Citation2019; Wang, Gu et al., Citation2018). As shown in , for sample determination, the sample solution was first mixed with colloidal gold labelled mAb and reacted for 5 min. The mixture was then added to the sample pad, where it migrated towards the absorbent pad by capillary action. The results could be read by eye after 5 min.
For a positive sample, BPS will compete with the coating antigen adsorbed onto the T-line and combine with the gold labelled mAb. The higher the concentration of BPS in the positive sample, the weaker the T-line colour, until the colour of T-line disappears. The gold labelled mAb will bind with the goat anti-mouse antibody on the C line to make the C line become red whether BPS is present or absent within the sample. For a negative sample, the gold labelled mAb will definitely bind to the coating antigen at the T-line, making the T-line become red. If the C-line is colourless however, it means that the strip is invalid.
Sample analysis
Liquid milk selected for sample analysis, was purchased from a local supermarket in Wuxi. According to the linear detection range and IC50 value of the antibody, a suitable known concentration of BPS was added to 1 mL of milk and diluted eight times and tested.
Results and discussion
Hapten
As a small molecule, BPS must be coupled with a macromolecular carrier protein to produce immunogenicity. The BPS molecule contains two active characteristic hydroxyl groups, which may be shielded by the process of its insertion into the protein vector, and thereby resulting in a loss of antibody specificity to the shielded groups. In order to fully expose the characteristic groups in small molecule compounds, one phenolic hydroxyl group is retained, and a carboxyl group is derived by introducing a straight chain spacer arm at the opposite end to the hydroxyl group. This maintains the original molecular structure of the hapten so that the artificial antigen is expose to the surface and can be recognized by immune active cells of the animal, thereby stimulating the body to produce antibodies with high affinity and specificity for the assay.
Complete antigen and coating antigen
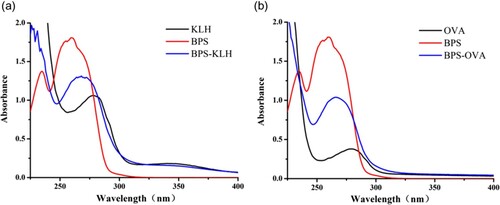
As a small molecule with a relative molecular mass of 250.27, BPS is unable to induce an immune response. To induce the organism to produce antibodies, BPS must be coupled to a carrier protein. The carboxyl group of the BPS hapten was activated by the carbodiimide method and coupled to both KLH and OVA to synthesize the immunogen (BPS-KLH) and coating antigen (BPS-OVA). BPS-KLH and BPS-OVA were measured by absorbance spectra analysis within the wavelength range of 200–500 nm. The ultraviolet absorption curves are shown in . As shown in the figure, KLH has a characteristic absorption peak at 278 nm, whereas the BPS hapten has a characteristic absorption peak at 262 nm. The peak for BPS-KLH is between 262 and 278 nm. The maximum characteristic absorption peak shift of the BPS hapten occurs due to its coupling to KLH.
Optimization of assay conditions
The methanol content, pH value and ionic strength (NaCl content) in standard diluent were optimized. The best condition was obtained by comparing the Amax value and IC50 value under various conditions. Amax reflects the binding properties of the coating antigen and antibody in the absence of standards, whereas IC50 is a measure of antibody sensitivity. A highly sensitive mAb possesses lower IC50 values.
Firstly, the effect of methanol content on the assay is shown in (a). The IC50 value of the standard diluent containing 20% methanol is approximately two times higher when compared with |diluent containing no methanol. The results showed that the presence of methanol could affect the sensitivity of the BPS monoclonal antibody. In addition, the Amax value decreased with an increase in methanol content. In view of the adverse effects of methanol on the sensitivity and Amax value of the BPS monoclonal antibody, a methanol-free PBS buffer was selected as the standard diluent.
Figure 5. Influence of methanol content (a), pH (b), and NaCl content (c) on IC50 value and maximum absorbance (Amax) for the ic-ELISA of bisphenol S.
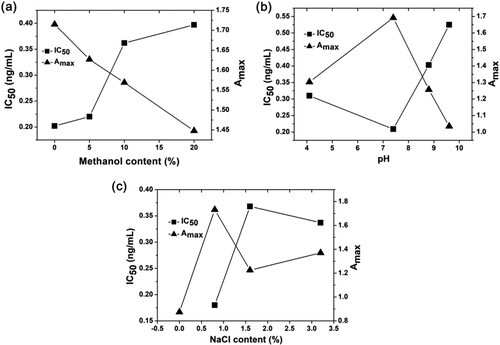
Secondly, the influence of pH value on the BPS monoclonal antibody is shown in (b). It can be seen from the curves in (b), that Amax values generally decreased even if the standard diluent was acidic or alkaline, while the IC50 value almost changed. Therefore, the most suitable standard diluent pH value was 7.4. Thirdly, the effect of ionic strength on the detection method is shown in (c). The appropriate ionic strength is necessary for the formation of antigen–antibody complexes. In the absence of ions the antibody curve was linear, and its titre was low. However, high ionic strength resulted in a decrease in Amax value, whereas the IC50 value was not significantly affected. Therefore, a 0.8% NaCl content for the BPS standard diluent was selected.
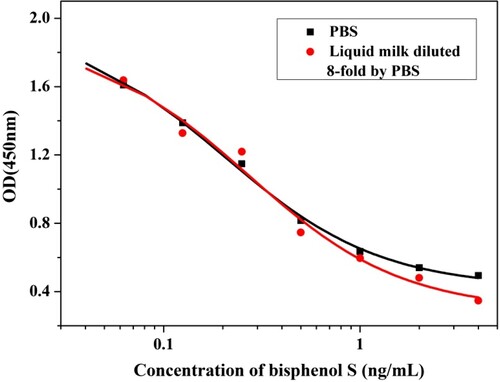
As shown in , under optimal conditions for the standard diluent (PBS buffer, pH 7.4), the indirect competitive inhibition curve is drawn with a logarithmic value of BPS standard concentration as abscissa, and absorbance value at 450 nm as ordinate. The curve had a linear range of 0.064–0.810 ng/mL, and the IC50 value of the curve was 0.228 ng/mL.
Cross-Reactivity
The cross-reaction rate between drugs and their structural analogues can be used to describe the specificity of monoclonal antibodies and was used here also. Moreover, the lower the cross-reaction rate, the higher the specificity of the monoclonal antibody for its target. Data displayed in showed that the rate of cross-reaction of the BPS mAb’s with other structural analogues was below 0.0228%, whereas with BPS, it was 100%. It is suggested therefore, that the BPS mAb is a monoclonal antibody with high specificity. The main reason for this is that the monoclonal antibody recognized the structure of diphenyl sulfone of BPS and a hydroxyl group on one side of the benzene ring. The structures of other analogues were quite different from that of BPS, which explained the reason for the antibody being unable to recognize these structures, and the cross-reaction rates, therefore, were low.
Table 1. Cross-reactivity results of bisphenol S monoclonal antibody.
Milk sample analysis
Matrix effect
In the analysis of the samples, matrix interference may affect the accuracy of detection. Therefore, the samples needed to be pre-treated before analysis (Wang, Xu, Zhang, & He, Citation2008; Zhao et al., Citation2017). Liquid milk contains a certain amount of fat, protein and lactose in addition to water, which may affect the recognition of antigens and antibodies, resulting in the precision and accuracy of the results being affected. In order to assess the effect of the matrix on the performance of the BPS mAb, indirect competitive inhibition curves of antibodies were established with PBS diluted eight times in milk. As shown in , the curves were almost overlapping, indicating that the matrix effect was not significant. Therefore, milk samples can be diluted eight times with PBS and analyzed by the ic-ELISA procedure.
Recovery Experiments
The results of recovery rate and coefficient of variation are shown in . The recovery rate of BPS in milk ranged from 89% to 95%, which was within the allowable range. In addition, the coefficient of variation was lower than 15%. The results demonstrated that the ic-ELISA method using the BPS mAb is feasible for sample detection.
Table 2. Recovery rates and coefficient of variation of bisphenol S spiked in milk samples.
Sample analysis using the immunochromatographic strip assay
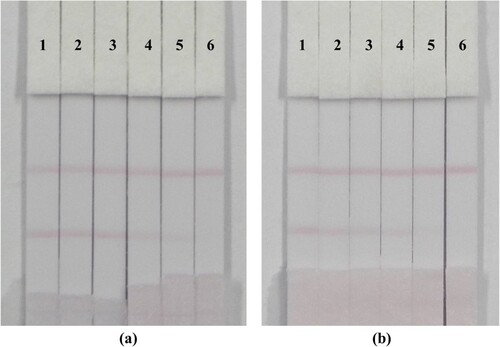
Milk is the best source of calcium and protein for the human body. Therefore, the supervision and management of the quality and safety of dairy products are particularly important, to ensured that people drink high-quality milk. In addition, milk is suitable for people of all ages, and milk products available on the market are abundant and easy to purchase. To verify the feasibility of the immunochromatographic strip assay method, common liquid milk samples from a market were selected. As seen in (a), the milk was spiked with BPS (0, 0.1, 0.25, 0.5, 1 and 2.5 ng/mL). As the concentration of BPS standard increased, the colour of the T line became gradually lighter. The standard concentration corresponding to the disappearance of the C line was the cut-off value, and the results were directly observed using the naked eye (Liu, Chu, & Yu, Citation2016). When the concentration of the BPS standard was 2.5 ng/mL, the T line disappeared completely. Therefore, the cut-off value for PBS was 2.5 ng/mL. Equally, as shown in (b), the milk samples were spiked with BPS (0, 0.25, 0.5, 1, 2.5 and 5 ng/mL) and the cut-off value seen by the naked eye in milk sample was 5 ng/mL.
Figure 7. Image of bisphenol S detection by immunochromatographic strip in PBS (a) and milk sample (b). (a), 1–6 corresponds to concentration of bisphenol S spiked in PBS buffer solution were 0, 0.1, 0.25, 0.5, 1 and 2.5 ng/mL. (b), 1–6 corresponds to concentration of bisphenol S standard spiked in milk samples were 0, 0.25, 0.5, 1, 2.5 and 5 ng/mL.
Conclusion
The molecular structure of BPS was modified and conjugated with a carrier protein KLH. Monoclonal antibodies were obtained by immunization, antiserum detection and cell fusion. Under the optimized ic-ELISA analysis condition, the IC50 value of the BPS mAb was 0228 ng/mL and the linear range was 0.064–0.810 ng/mL. The BPS mAb did not cross-react with other bisphenol structural analogues. The recovery rate of BPS in milk samples was in the range of 89%–95% and the coefficient of variation ranged from 1.0% to 6.0%. The cutoff value of the immunochromatographic strip assay method in milk samples was 5 ng/mL. Our findings confirm therefore, that the ic-ELISA method and immunochromatographic strip assay method established in this study are feasible for the detection of BPS in milk samples.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aung, M. T., Ferguson, K. K., Cantonwine, D. E., McElrath, T. F., & Meeker, J. D. (2019). Preterm birth in relation to the bisphenol A replacement, bisphenol S, and other phenols and parabens. Environmental Research, 169, 131–138. doi: 10.1016/j.envres.2018.10.037
- Eladak, S., Grisin, T., Moison, D., Guerquin, M. J., N’Tumba-Byn, T., Pozzi-Gaudin, S., … Habert, R. (2015). A new chapter in the bisphenol A story: Bisphenol S and bisphenol F are not safe alternatives to this compound. Fertility and Sterility, 103(1), 11–21. doi: 10.1016/j.fertnstert.2014.11.005
- Giacomo, R., Francesco, B., Eleonora, C., Margherita, F., Stefania, A., & Lucia, G. (2018). Bisphenol A and bisphenol S release in milk under household conditions from baby bottles marketed in Italy. Journal of Environmental Science and Health. Part. B, Pesticides, Food Contaminants, and Agricultural Wastes, 53(2), 116–120. doi: 10.1080/03601234.2017.1388662
- Grandin, F., Picard-Hagen, N., Gayrard, V., Puel, S., Viguié, C., Toutain, P.-L., … Lacroix, M. Z. (2017). Development of an on-line solid phase extraction ultra-high-performance liquid chromatography technique coupled to tandem mass spectrometry for quantification of bisphenol S and bisphenol S glucuronide: Applicability to toxicokinetic investigations. Journal of Chromatography A, 1526, 39–46. doi: 10.1016/j.chroma.2017.10.020
- Guo, L., Xu, L., Song, S., Liu, L., & Kuang, H. (2018). Development of an immunochromatographic strip for the rapid detection of maduramicin in chicken and egg samples. Food and Agricultural Immunology, 29(1), 458–469. doi: 10.1080/09540105.2017.1401045
- Kong, D., Liu, L., Song, S., Kuang, H., & Xu, C. (2017). Development of sensitive, rapid, and Effective immunoassays for the detection of Vitamin B12 in Fortified Food and Nutritional Supplements. Food Analytical Methods, 10(1), 10–18. doi: 10.1007/s12161-016-0543-1
- Kong, D., Liu, L., Song, S., Zheng, Q., Wu, X., & Kuang, H. (2017). Rapid detection of tenuazonic acid in cereal and fruit juice using a lateral-flow immunochromatographic assay strip. Food and Agricultural Immunology, 28(6), 1293–1303. doi: 10.1080/09540105.2017.1337085
- Kong, D., Wu, X., Li, Y., Liu, L., Song, S., Zheng, Q., … Xu, C. (2019). Ultrasensitive and eco-friendly immunoassays based monoclonal antibody for detection of deoxynivalenol in cereal and feed samples. Food Chemistry, 270, 130–137. doi: 10.1016/j.foodchem.2018.07.075
- Liang, X., Ni, H., Beier, R. C., Dong, Y., Li, J., Luo, X., … Wang, Z. (2014). Highly Broad-specific and sensitive enzyme-linked immunosorbent assay for screening Sulfonamides: Assay optimization and Application to milk samples. Food Analytical Methods, 7(10), 1992–2002. doi: 10.1007/s12161-014-9845-3
- Liu, B.-H., Chu, K. C., & Yu, F.-Y. (2016). Novel monoclonal antibody-based sensitive enzyme-linked immunosorbent assay and rapid immunochromatographic strip for detecting aflatoxin M1 in milk. Food Control, 66, 1–7. doi: 10.1016/j.foodcont.2016.01.036
- Liu, L., Peng, J., Xie, Z., Song, S., Kuang, H., & Xu, C. (2017). Development of an icELISA and immunochromatographic assay for methyl-3-Quinoxaline-2-Carboxylic acid Residues in Fish. Food Analytical Methods, 10(9), 3128–3136. doi: 10.1007/s12161-017-0888-0
- Liu, L., Xu, L., Suryoprabowo, S., Song, S., & Kuang, H. (2018). Rapid detection of tulathromycin in pure milk and honey with an immunochromatographic test strip. Food and Agricultural Immunology, 29(1), 1–11. doi: 10.1080/09540105.2017.1341400
- Loureiro, V., Quirós, R.-B. D., & Sendón, R. (2018). Determination of bisphenol S in food and drink take away packaging by LC-MS/MS. International Journal of Environmental Analytical Chemistry, 98(15), 1413–1422. doi: 10.1080/03067319.2018.1545902
- Qiu, W., Zhan, H., Hu, J., Zhang, T., Xu, H., Wong, M., … Zheng, C. (2019). The occurrence, potential toxicity, and toxicity mechanism of bisphenol S, a substitute of bisphenol A: A critical review of recent progress. Ecotoxicology and Environmental Safety, 173, 192–202. doi: 10.1016/j.ecoenv.2019.01.114
- Regueiro, J., & Wenzl, T. (2015). Determination of bisphenols in beverages by mixed-mode solid-phase extraction and liquid chromatography coupled to tandem mass spectrometry. Journal of Chromatography A, 1422, 230–238. doi: 10.1016/j.chroma.2015.10.046
- Shan, W., Yao, K., Wen, K., & Shao, B. (2019). Development of low matrix effects method for the analysis of bisphenol A and bisphenol S in aquatic products by immunoaffinity purification. Journal of Chromatography B, 1109, 19–24. doi: 10.1016/j.jchromb.2019.01.012
- Shengdong, P., Qian, H., Xiaohong, C., Li, W., Qiaoli, Q., & Micong, J. (2017). Rapid determination of four phenolic environmental estrogen residues in cooking oil by ultra-performance liquid chromatography-high resolution mass spectrometry coupled with solid-phase extraction. Se pu=Chinese Journal of Chromatography, 35(9), 980–986.
- Simoneau, C., Valzacchi, S., Morkunas, V., & Eede, L. V. D. (2011). Comparison of migration from polyethersulphone and polycarbonate baby bottles. Food Additives & Contaminants: Part A, 28(12), 1763–1768.
- Siracusa, J. S., Yin, L., Measel, E., Liang, S., & Yu, X. (2018). Effects of bisphenol A and its analogs on reproductive health: A mini review. Reproductive Toxicology, 79, 96–123. doi: 10.1016/j.reprotox.2018.06.005
- Ullah, H., Jahan, S., Ain, Q. U., Shaheen, G., & Ahsan, N. (2016). Effect of bisphenol S exposure on male reproductive system of rats: A histological and biochemical study. Chemosphere, 152, 383–391. doi: 10.1016/j.chemosphere.2016.02.125
- Ullah, A., Pirzada, M., Jahan, S., Ullah, H., Shaheen, G., Rehman, H., … Butt, M. A. (2018). Bisphenol A and its analogs bisphenol B, bisphenol F, and bisphenol S: Comparative in vitro and in vivo studies on the sperms and testicular tissues of rats. Chemosphere, 209, 508–516. doi: 10.1016/j.chemosphere.2018.06.089
- Vázquez Loureiro, P., Rodríguez-Bernaldo De Quirós, A., & Sendón, R. (2018). Determination of bisphenol S in food and drink take away packaging by LC-MS/MS. International Journal of Environmental Analytical Chemistry, 98(15), 1413–1422. doi: 10.1080/03067319.2018.1545902
- Viñas, P., Campillo, N., Martínez-Castillo, N., & Hernández-Córdoba, M. (2010). Comparison of two derivatization-based methods for solid-phase microextraction–gas chromatography–mass spectrometric determination of bisphenol A, bisphenol S and biphenol migrated from food cans. Analytical and Bioanalytical Chemistry, 397(1), 115–125. doi: 10.1007/s00216-010-3464-7
- Wang, B., Gu, J., Chen, B., Xu, C., Zheng, H., Peng, Z., … Hu, Z. (2018). Development of an enzyme-linked immunosorbent assay and gold-labelled immunochromatographic strip assay for the detection of Ancient Wool. Journal of Analytical Methods in Chemistry, 2018, 1–9.
- Wang, Y., Wang, Z., Jiang, H., Xia, X., Shen, J., & Ding, S. (2013). Development of a monoclonal antibody-based enzyme-linked immunosorbent assay for the analysis of Diclazuril in Chicken Tissues. Food Analytical Methods, 6(6), 1685–1692. doi: 10.1007/s12161-013-9582-z
- Wang, Z., Xie, Z., Cui, G., Liu, L., Song, S., Kuang, H., & Xu, C. (2017). Development of an indirect competitive enzyme-linked immunosorbent assay and immunochromatographic assay for hydrocortisone residues in milk. Food and Agricultural Immunology, 28(3), 476–488. doi: 10.1080/09540105.2017.1297779
- Wang, S., Xu, B., Zhang, Y., & He, J. X. (2008). Development of enzyme-linked immunosorbent assay (ELISA) for the detection of neomycin residues in pig muscle, chicken muscle, egg, fish, milk and kidney. Meat Science, 82(1), 53–58. doi: 10.1016/j.meatsci.2008.12.003
- Wang, Z., Zheng, Q., Guo, L., Suryoprabowo, S., Liu, L., & Kuang, H. (2017). Preparation of an anti-dexamethasone monoclonal antibody and its use in development of a colloidal gold immunoassay. Food and Agricultural Immunology, 28(6), 958–968. doi: 10.1080/09540105.2017.1320360
- Wang, Q., Zhu, L., Chen, M., Ma, X., Wang, X., & Xia, J. (2017). Simultaneously determination of bisphenol A and its alternatives in sediment by ultrasound-assisted and solid phase extractions followed by derivatization using GC-MS. Chemosphere, 169, 709–715. doi: 10.1016/j.chemosphere.2016.11.095
- Xie, P., Zhao, C., Zhao, H., Chen, X., & Cai, Z. (2018). Determination of bisphenol A and bisphenol S in sacked mouse foods by liquid chromatography-tandem mass spectrometry. International Journal of Mass Spectrometry, 434, 17–22. doi: 10.1016/j.ijms.2018.08.011
- Xiong, L., Yan, P., Chu, M., Gao, Y.-Q., Li, W.-H., & Yang, X.-L. (2018). A rapid and simple HPLC–FLD screening method with QuEChERS as the sample treatment for the simultaneous monitoring of nine bisphenols in milk. Food Chemistry, 244, 371–377. doi: 10.1016/j.foodchem.2017.10.030
- Xu, N., Pan, L., Yu, C., Wei, X., & Wang, Y. (2017). Goldmag-based enzyme-linked immunosorbent assay for determination of α-lactalbumin in milk. Food and Agricultural Immunology, 28(6), 1–15.
- Yamada, H., Satoh, R.-I., Yamashita, T., Kambegawa, A., & Iwata, M. (1997). Development of a time-Resolved Fluoroimmunoassay (TR-FIA) for Testosterone: Measurement of serum Testosterone concentrations after Testosterone treatment in the Rainbow Trout (Oncorhynchus mykiss). General and Comparative Endocrinology, 106(2), 181–188. doi: 10.1006/gcen.1996.6861
- Zhao, L., Li, J., Li, Y., Wang, T., Jin, X., Wang, K., … Zhou, F. (2017). Preparation of monoclonal antibody and development of an indirect competitive enzyme-linked immunosorbent assay for ornidazole detection. Food Chemistry, 229, 439–444. doi: 10.1016/j.foodchem.2017.02.100