ABSTRACT
In this study, a heterologous competitive indirect enzyme-linked immunosorbent assay (ciELISA) for the determination of estriol (E3) in milk was developed. Rabbit polyclonal antibody specific for E3 was raised by introducing a carboxyl spacer arm from bromoacetic acid of E3 as immunizing hapten. A heterologous ciELISA using a coating hapten containing a carboxyl spacer arm from 6-ketoestriol of E3. The assay exhibited the required sensitivity for detection of E3 with limit of detection of 0.07 ng mL−1 and a working range from 0.41 ng mL−1 to 15.78 ng mL−1. Good recoveries were obtained ranging from 83.2% to 125.8% for E3 spiked milk samples. A satisfactory correlation (R2 = 0.994) between the results of ciELISA analysis and that of LC–MS/MS analysis indicated good accuracy and reproducibility of the proposed method, which was ideally suited as a rapid screening tool for routine E3 monitoring.
Introduction
Estriol ((16α,17β)-estra-1,3,5(10)-triene-3,16,17-triol) is one of the essential steroid estrogens that exhibits a critical impact on maintaining female second features and protein assimilation (Cincotto et al., Citation2015; Lin & Li, Citation2006). Applied on clinical medicine, estriol reacted on controlling estrus synchronisation, promoting livestock growth and improving feed conversion rate (Wang et al., Citation2007). Recently, estriol was illegal applied to livestock and aquaculture with playing an important role on fast fattening, urging big and improving livestock milk production rate. It has also been illegally added to dairy products and animal health foods in the elderly. The long-term intake of estrogen hormone residues in food can cause precocious puberty, sexual abnormalities, cancer and other diseases, and increase the risk of certain diseases (Hu, Wu, Yi, & Cui, Citation2002). Driven by economic interests, in China, hormone residues and excessive phenomenon was still very common (Hu et al., Citation2002; Lin & Li, Citation2006). Milk and milk powder, as babies and children long-term intake of food, including hormones such as estriol residue can directly lead to precocious puberty (Cleemann & Holm, Citation2011). Estriol additional intake during pregnancy can affect fetal health of pregnant women and judgment, while embryonic cell proliferation and differentiation (Kim et al., Citation2013). The United States identified estriol as drinking water contaminants. Japan predetermined remaining amount of estriol may not exceed 0.01 mg g−1 in beef. People's Republic of China Ministry of Agriculture, No. 176 (2002) document banned the addition of estriol and other estrogen drugs in animal foods, while estrogen residues should not be detectable in animal foods.
There are extracted with ethyl acetate, thin layer chromatography, high performance liquid chromatography (Gorga, Insa, Petrovic, & Barceló, Citation2014; Piwowarska, Radowicki, & Pachecka, Citation2010), gas chromatography analysis-mass spectrometry (Andrási, Helenkár, Vasanits-Zsigrai, Záray, & Molnár-Perl, Citation2011; Lu, Wu, Stoffella, & Chris Wilson, Citation2012), electroanalytical determination (Cincotto et al., Citation2015; Fu et al., Citation2016; Santos, Braga, Vieira, & Spinelli, Citation2010), enzyme-linked immunosorbent assay (ELISA) (Bai et al., Citation2017), fluoroimmunoassay (Tang, Zhao, Wu, Zhou, & Li, Citation2013), and immunochromatographic assay (Mukunzi et al., Citation2016; Wang et al., Citation2017) for estriol and its analogs analysis. Above all of instrument assays, chromatography spectroscopy was generally used to detect estriol in urine of pregnant women and drug. However, Chromatography spectroscopy, with high testing costs, complex sample pretreatment, time-consuming, tedious, professional operators, could not be simultaneously detected for samples with high throughput, limiting its application in food detection. With respect to the instrument method, ELISA has high sensitivity and specificity, high throughput for trace detection of estriol.
In the work, a ciELISA for the determination of E3 in milks was developed based on a highly specific antibody against the E3. The developed ciELISA exhibited the high sensitivity and specificity for detection of E3 for milk samples. Thus, the ciELISA described in this study was ideally suited as a rapid screening tool for routine E3 monitoring in large numbers of milk samples.
Materials and methods
Animals and reagents
Estriol, estradiol, progesterone, ethinylestradiol, oestrone, 3,3’,5,5'-tetramethylbenzidine (TMB), complete and incomplete Freund’s adjuvants, bovine serum albumin (BSA), ovalbumin (OVA), and peroxidase-labelled goat anti-rabbit IgG (secondary antibody) were obtained from Sigma-Aldrich (St. Louis, MO, USA). N-hydrosuccinimide (NHS), dicyclohexylcarbodiimide (DCC) and triethylamine were obtained from Aladdin Chemical Technology Co., Ltd. (Shanghai, China). N,N-Dimethylformamide (DMF), acetonitrile (ACN), Tween-20, ethanol, methanol (MeOH) were obtained from Damao Chemical Reagent Co., Ltd. (Tianjin, China). New Zealand white rabbits were raised at the Guangdong Medical Experimental Animal Centre. 96-well polystyrene micro-plates were obtained from Shenzhen Jinchanhua Industrial Co. Ltd. (Shenzhen, China). All other reagents were of analytical reagent grade or higher purity.
Instruments
ELISA Plates were washed in a Multiskan MK2 microplate washer (Thermo Scientific, Hudson, NH, USA). Absorbance was measured at a wavelength of 450 nm using a Multiskan MK3 microplate reader (Thermo Scientific). Ultraviolet spectrometry (UV) was recorded on a UV-3010 spectrophotometer (Hitachi, Tokyo, Japan). LC-MS/MS analysis was carried out by using the 1200 series LC system (Agilent Technologies) equipped with the Agilent 6410 Triple Quad LC-MS System (Agilent Technologies). Nuclear magnetic resonance (NMR) spectra were achieved with either a DRX-400 or DRX-600 NMR spectrometer (Bruker, Rheinstetten, Germany).
Buffers and solutions
The following were the buffers and solutions used in this study: (1) 0.05 mol L−1 carbonate buffer (CB, pH 9.6) was used for coating antibody on plates; (2) PBST (0.01 mol L−1 phosphate-buffered saline (PBS) with Tween-20 (0.05%), pH 7.4) was used for washing plates, secondary antibody dilution and was the working buffer used in the non-optimised ciELISA; (3) 2 mol L−1 H2SO4 was used for the stop reagent; and (4) E3 stock standard solution (1 mg mL −1 E3 in methanol).
Synthesis and verification of estriol hapten
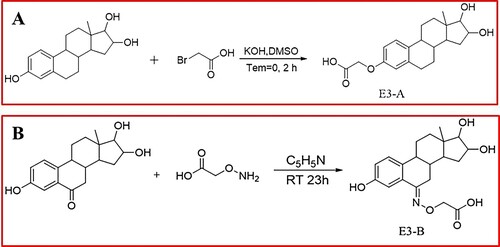
Estriol hapten A (E3-A) was synthesised by introduction of spacer arms containing a carboxyl group originated from bromoacetic acid by substitution of H on the benzene ring’s hydroxyl group (Wang, Guo, Li, & Chang, Citation2002). Estriol hapten B (E3-B) was synthesised by introduction of spacer arms containing a carboxyl group on the carbonyl group of 6-ketoestriol. The following is a detailed description of the synthetic procedure and characterisation of these compounds ().
Estriol (1.4 g, 4.8 mmol) was dissolved into DMF (20 mL) and KOH (0.7 g, 0.0125 mol L−1) was added with stirring for 5 mins. Bromoacetic acid (1.3 g, 9.6 mmol) was added into the reaction solution with stirring for 3 h at 25°C. The mixture was added 50 mL ice water and extracted with ethyl acetate 3 times for recovering the unreacted estriol. The aqueous solution was acidified with 2 mol L−1 HCl to pH 3.0 and generated a white precipitate and then filtered. The filtrate was evaporated under vacuum, and the residue was washed with acidic water (0°C) to obtain white powder.
The crude product was purified on a silica gel column, using MeOH / CHCl3 (1:10) as eluent and dried to afford E3-A (Figure S1A). ESI analysis (positive ion) m/z 346.4 [M + H]+;1H NMR (600 MHz, DMSO-d6) δ 7.30 (s, 1H), 7.22 (s, 1H), 7.13 (s, 1H), 6.49 (s, 1H), 5.09 (s, 2H), 4.92 (s, 1H), 4.85 (s, 3H), 4.77 (d, J = 7.1 Hz, 1H), 4.71 (s, 1H), 4.66 (d, J = 10.0 Hz, 2H), 4.60 (d, J = 13.9 Hz, 2H), 4.50 (s, 1H), 4.26 (d, J = 10.5 Hz, 4H), 4.13–4.06 (m, 2H), 4.04 (s, 6H), 4.00 (s, 1H), 3.90 (s, 4H), 3.68 (d, J = 6.6 Hz, 2H), 3.62 (s, 2H), 3.21 (s, 2H), 3.15 (s, 4H), 2.89 (s, 1H), 2.72 (s, 1H), 1.90 (s, 8H), 1.22 (s, 2H), 0.65 (d, J = 2.5 Hz, 1H).
6-ketoestriol (1.0 g, 3.3 mmol) was placed into round-bottom flask (100 mL) and then added 30 mL azabenzene, carboxymethoxylamine hemihydrochloride (0.21 g, 1.2 mmol) with stirring in turn. The mixture was stirred at 25°C for 23 h, and then was concentrated by rotoevaporation under vacuum. The crude product was purified on a silica gel column, using MeOH / CHCl3 (1:8) as eluent and dried to yield E3-B (Figure S1B). ESI analysis (positive ion) m/z 375.4 [M + H]+;1H NMR (600 MHz, DMSO-d6) δ 10.07 (s, 1H), 7.24 (d, J = 2.7 Hz, 1H), 7.03 (d, J = 8.5 Hz, 1H), 6.67 (dd, J = 8.5, 2.7 Hz, 1H), 4.83 (s, 2H), 4.34–4.25 (m, 2H), 3.87–3.81 (m, 1H), 3.28 (d, J = 5.5 Hz, 1H), 2.91 (dd, J = 18.0, 4.5 Hz, 1H), 2.16 (dq, J = 13.3, 3.6 Hz, 1H), 2.02 (td, J = 11.2, 4.6 Hz, 1H), 1.86–1.73 (m, 2H), 1.70–1.60 (m, 1H), 1.45–1.29 (m, 3H), 1.20 (td, J = 12.9, 3.8 Hz, 1H), 1.05 (ddd, J = 16.0, 12.7, 7.6 Hz, 1H), 0.60 (s, 3H).
Synthesis of immunogen and coating antigen
Haptens (E3-A and E3-B) all had carboxyl group and inexistent amino group, so they were conjugated to BSA with active ester and used as the immunogen and they were conjugated to OVA and used for the coating antigen according to previous work, with modifications (Luo et al., Citation2014). Briefly, a hapten (0.1 mmol) was dissolved in the DMF (1 mL), then 0.13 mmol L−1 DDC and 0.13 mmol L−1 NHS were added and stirred for 12 h to active the carboxy group of hapten. Then carrier protein (10 mg) were dissolved in the conjugation buffer (0.01 mol L−1 PBS, pH 7.0, 2 mL). The above-mentioned resulting solution was added into the protein solution dropwise, and held at 4°C overnight, and then the conjugation mixture was dialysed against 0.01 mol L−1 PBS (4 × 5 L) at 4°C for 72 h, and finally stored at −20°C until used. Before the production was stored, the reactions were all occurring under stirring. UV-Vis spectral data was used to confirm the structures of final conjugates and the hapten density was estimated by the TNBS method.
Production of polyclonal antibody
Four New Zealand white rabbits were housed and maintained at the Guangdong Medical Laboratory Animal Center. All animal experiments were performed in compliance with the protective and administrative laws for laboratory animals of China and conducted with the approval of the Institutional Authority for Laboratory Animal Care, South China Agricultural University, Guangzhou, China. The rabbits (10-wk-old, 1.5–2.0 kg) were equally divided into two groups for the two immunogens, and each were intradermally and intramuscularly immunised with 1 mL of an emulsion (1:1) containing 0.5 mg mL−1 of an immunogen in PBS and complete Freund’s adjuvant. Afterwards, four booster immunizations using the same amount of immunogen emulsified in incomplete Freund’s adjuvant were given monthly. Ten days after the fourth booster injection, the rabbits were exsanguinated, and their blood was collected. The obtained antiserum was stored at −20°C until purified by saturated ammonium sulfate precipitation (Page & Thorpe, Citation2002). Serum was collected from the marginal ear vein from each rabbit prior to the first immunisation and used as the negative controls.
Antibody screening and characterization
A ciELISA was used to evaluate the antiserums for binding ability to the analytes (estriol). Briefly, the coating antigens (1 mg L−1, 100 μL well−1) in carbonate buffer were added to 96-well polystyrene ELISA plates and incubated at 4°C overnight, and then the wells were washed twice with PBST solution prior to adding 5% skimmed milk in PBST1 (120 μL well−1) to block the uncoated sites for 3 h at 37°C and dried at 37 °C for 1 h. Estriol standards or other competitors in PBS (50 μL) and the diluted antiserum (50 μL) in PBST were added to each well and incubated at 37°C for 40 min, and then the wells were washed five times with PBST1. The secondary antibody diluted 1:5000 in PBST (100 μL well−1) was then added to the wells and incubated for 40 min at 37°C. The wells were washed again for five times with PBST before the TMB solution was added to the wells (100 μL well−1) and incubated for 10 min. Finally, 50 μL of 2 mol L−1 H2SO4 was added to terminate the reaction and the optical density was measured at 450 nm.
Optimisation of ELISA conditions
E3-A-BSA was chosen as the optimal immunogen and E3-B-OVA was used for the optimal coating antigen, according to previous work of effect of antiserum. To further improve performance of the ELISA, optimisation was performed of the physicochemical parameters considered to have significant effects on immunoassay performance, such as antibody dilution/coating concentration, and working buffer (concentration of Tween 20, ionic strength and pH). For each condition, estriol was used as the competitor analyte to construct inhibition curves (n = 3). The maximum value of the inhibition curve (Amax) and competitor analyte concentration leading to a 50% decrease in Amax (IC50) were calculated from these curves. The optimal conditions were confirmed by evaluating the ratio of Amax to IC50. The higher the value of Amax/IC50 equates to better assay performance under such conditions.
Preparation of milk samples
Milk samples were purchased from a local supermarket in Guangzhou, China. The milk samples were subjected to a series of pretreatment before analyzing by ciELISA and LC-MS/MS, three different methods as follows: (1) the samples were directly diluted to different concentrations with the aid of PBS; (2) Centrifugation was accomplished by two steps with different rotational speeds and then diluted; (3) the samples were purified by zinc acetate and potassium ferrocyanide to remove the vast majority of protein and then diluted by PBS. The supernatant was analyzed by ciELISA and LC-MS/MS. All prepared samples were filtered through a 0.22 μm membrane before LC-MS/MS analysis.
Estriol assay
Each prepared sample was split and subjected to estriol analysis by both ciELISA and LC–MS/MS methods.
ciELISA: A calibration curve was constructed as follow, 50 μL of the E3 solution was used as competitor in the ciELISA, then the calibration curve was obtained by plotting the percent binding of antibodies in the wells A450 against the logarithm of the E3 concentration. For sample analysis, 50 μL of the prepared samples, as described above, were used as competitor in the ciELISA, and the determined A450 was used to calculate the E3 concentration from the calibration curve.
LC–MS/MS: The results from the ciELISA were confirmed by the LC-MS/MS method (completed by our laboratory, Guangzhou, China). A 5 μL portion of the prepared samples was injected into the LC system (Agilent 1200–6410) equipped with an Agilent ZORBAX SB-C18 column (4.6 mm × 150 mm, 5 μm) and separated at a 1 mL min−1 flow rate (mobile phase A: acetonitrile; mobile phase B: H2O). Analytes were determined by an Agilent 6410 Triple Quad mass spectrometer (Agilent Technologies, Lexington, MA, USA) using ESI-MS/MS in the positive ion mode. The MS parameters were as follows: capillary voltage at 4.0 kV; desolvation temperature at 350°C; desolvation gas (N2), flow rate at 10 L min−1; ion spray voltage at 4000 V; The analytes were identified by parent/daughter ions as well as peak retention times in comparison to the standards (Figure S2). A calibration curve for E3 analysis by LC–MS/MS was constructed as follows: Serial concentrations (125–4000 ng mL−1) of E3 standard solutions in PBS were prepared from the E3 stock solution (Figure S3). The treated solutions (10 μL) were injected into the instrument and analyzed. The responses of the mass spectrometer were plotted against the concentration of E3, which resulted in the calibration curve.
Results and discussion
Hapten synthesis and antibody production
In this work, we designed two haptens with different blinding sites based on estriol (). E3-A (or E3-B) cannot be used directly as antigens due to its low molecule weight and non-immunogenicity; therefore, it must be conjugated to carrier protein as immunogens and coating antigens. Synthesis of E3-A-BSA and E3-A-OVA was based on the reaction between the carboxyl group of FA and the amino of carrier protein. The UV-vis absorption spectra are shown in Figure S4 (see Supplemental Information). The synthesised conjugates demonstrated qualitative differences between the carrier protein and conjugate in the UV-vis spectra, suggesting successful hapten conjugation to the carrier protein. Similarly, E3-B-BSA and E3-B-OVA were linked to carrier protein successfully. After immunisation, the antibody from four rabbits are obtained and shown in Table S1. The results showed that the two haptens coupling with BSA could have immunoreaction in vivo of animal. But the specificities of antibodies are not consistent. In consideration of the spatial structures of E3-A and A were superimposition, the antibodies generated from artificial antigen (E3-A-BSA) could recognise E3 with high specificity, and coating antigen (E3-A-OVA) and estriol are competed equally for the same antibodies in the ELISA system. Meanwhile, the antibodies generated from artificial antigen (E3-B-BSA) could recognise E3 but with low specificity, and coating antigen (E3-B-OVA) is more ascendant than estriol that competing for the same antibodies in the ELISA system. The result indicated that the latter antibodies have low inhibition ratio of this ELISA system because that antibodies tend to combine coating antigen and with weak properties to recognise estriol.
The most popular strategy for hapten design to produce antibodies against extriol was using estriol-6-(O-carboxymethyl) oxime, which retains three functional hydroxyl groups of estriol (Freeman & Johnso,Citation1999; Otsuki et al., Citation1979; Wang, Du, Lin, & Zhuang, Citation2006). However, in this work, we used the similar strategy for hapten design, but the obtained antibody seemed to be unsatisfactory (Table S1). Therefore, we used another strategy, by introducing a carboxyl group spacer from C3-OH of estriol (Bai et al., Citation2017). The results indicated that by using this antigen (E3-A-BSA) as immunogen, and E3-B-OVA as heterologous coating antigen, the obtained antibody showed better sensitivity in ELISA in comparison with literatures. Moreover, the antibody generated from E3-B-BSA was expected to higher specificity since it retains all three hydroxyl groups of estriol, which were considered as the important binding sites of antibody. However, the results indicated that antibody generated from E3-A-BSA showing higher specificity. This might due to the introducing of spacer at C3-OH still exhibiting the oxygen atom, which did not change the characteristics of hydroxyl group. Compared to hapten E3-B, hapten E3-A seemed able to hold the intact structure of estriol preferably.
Optimisation of the ciELISA
Effects of the physicochemical parameters on ELISA performance are summarised in Figure S5 (see Supporting information). To determine the coating concentration and antibody dilution, a preliminary checkerboard titration was employed to screen several combinations of coating concentration and antibody dilution, while keeping the Amax value at about 1.0 (Table S2). As shown in Table S2, the highest sensitivity was achieved when the coating concentration and antibody dilution was at 125 ng mL−1 and 1:256000, respectively. For the working buffer, As shown in Figure S5, PBS with different PO43– concentrations (10–100 mmol L−1), pH values (4.4–9.4), Tween-20 concentrations (0–0.08%), and the time of competitive binding between primary antibody and target from the sample (20–60 mins) were evaluated. The results indicated that a PO43– concentration of 20 mmol L−1, a pH value of 6.4, a Tween-20 concentration of 0% as well as 30 min of competitive binding between primary antibody and target from the sample were the most suitable for improving assay sensitivity.
Sensitivity and specificity of the ciELISA
The calibration curve for estriol constructed under the optimal conditions is shown in . The assay demonstrated an IC50 of 2.55 ng mL−1 and a limit of detection (LOD) of 0.07 ng mL−1 (the E3 concentration which inhibits 10% of the binding of the antibody, IC10) for estriol, whereas the quantification linear range was 0.41–15.78 ng mL−1 (y = −0.187 x + 0.407). The assay specificity, cross-reactivity (CR) of related compounds including some estrogen and progestational hormone commonly found in foods, was examined, and the results are summarised in . Since estradiol has a high degree of similarity to E3 and may be coexisted in foods at high concentration levels, a small CR may interfere with the analysis of E3 and would be unacceptable. However, no CR was observed to all the related compounds tested, including estradiol. Although only a hydroxyl group on C16 site is different between estradiol and estriol, the antibody generated from estriol could not recognise estradiol. The phenomenon showed that recognition of antibody and antigen does not rely on individual group or sectional structure but whole molecule. Thus, this result demonstrated the excellent specificity of the developed ciELISA to E3, which probably results from the high specificity of the antibody.
Figure 2. (A) Calibration curve and (B) ciELISA calibration curve in the linear range for histamine (n = 3).
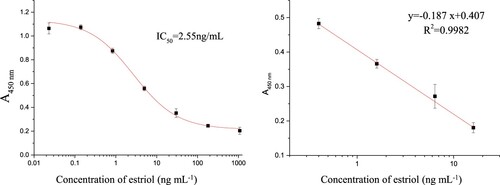
Table 1. Cross-reactivity of related compounds in ciELISA under optimised conditions
Detection of estriol in spiked milks
Sample pretreatment is the process of sample cleanup and matrix effect elimination and plays a significant part in development of the analytical procedure. To study the matrix effect, a matrix-matched inhibition curve for E3 was constructed by diluting the stock solution of E3 with blank sample extracts. Milk containing E3 at a level below the LOD of LC-MS/MS was considered a blank sample. The matrix effect was assessed by comparing the matrix-matched inhibition curves with standard inhibitory curves. As shown in , it was found that the matrix-matched inhibition curve of centrifugation was similar to the solvent inhibition curve after a 5-fold dilution of supernatant, indicating the effectiveness of the sample preparation method; while a 20-fold dilution of milk was required to minimise the matrix effect without any auxiliary process; and a 10-fold dilution of supernatant was treated after the purification of zinc acetate and potassium ferrocyanide. As shown in , three pretreatments of centrifugalising method, zinc acetate -potassium ferrocyanide method, and dilution method with different results on recoveries (ranging from 88.9% to 111.2%, 83.2% to 125.8%, and 83.2% to 110.1%) and CVs (3.2% to 16.2%, 1.1% to 3.1%, and 4.2% to 9.4%). Compared with dilution of sample directly, centrifugation and precipitation by zinc acetate and potassium ferrocyanide decrease the dilution times of sample and can improve the sensitivity and with lower limit of detection. However, since the assay showed high sensitivity for E3, the direct dilution pretreatment might be preferred.
Figure 3. Analysis of matrix effect by different sample pretreatment: (A) centrifugalising method; (B) zinc acetate -potassium ferrocyanide method; and (C) dilution method.
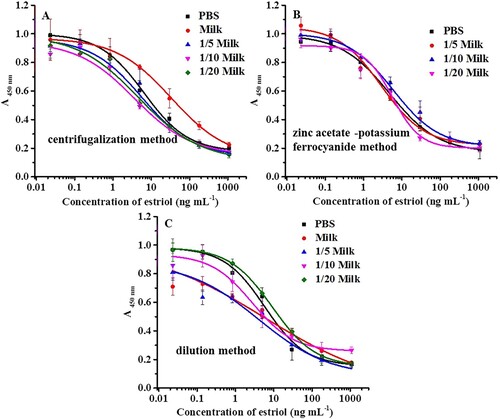
Table 2. Results of the determination of E3 in spiked milk samples with different pretreatment method under optimised conditions (n = 3).
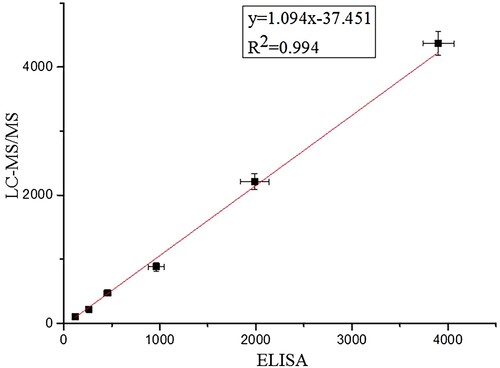
To confirm the reliability of the developed ciELISA, spiked food samples were subjected to E3 analysis by LC–MS/MS. Average recoveries obtained by LC–MS/MS ranged from 79.9% to 110.6% with CVs between 4.8% and 10.8% (See Table S3 in Supporting information). The results of the ciELISA was in good agreement with that of LC–MS/MS with a correlation coefficient (R2) of 0.994 (), revealing good reliability and accuracy for this ciELISA.
Conclusion
In summary, a ciELISA for the determination of E3 in milks was developed based on a highly specific antibody against the E3. The assay exhibited the required sensitivity for detection of E3 with limit of detection (LOD) of 0.07 ng mL−1 with a working range from 0.41 ng mL−1 to 15.78 ng mL−1. Different recoveries (ranging from 88.9% to 111.2% for centrifugalising method; 83.2% to 125.8% for zinc acetate -potassium ferrocyanide method; and 83.2% to 110.1% for dilution method) from E3-spiked milk samples. A satisfactory correlation (R2 = 0.994) between the results of ciELISA analysis and that of LC–MS/MS analysis were observed. Thus, the ciELISA described in this study was ideally suited as a rapid screening tool for routine E3 monitoring in large numbers of milk samples.
Supplemental Material
Download ()Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Andrási, N., Helenkár, A., Vasanits-Zsigrai, A., Záray, G., & Molnár-Perl, I. (2011). The role of the acquisition methods in the analysis of natural and synthetic steroids and cholic acids by gas chromatography–mass spectrometry. Journal of Chromatography A, 1218, 8264–8272. doi.org/10.1016/j.chroma.2011.09.006
- Bai, Y., Hu, J., Liu, S., Zhang, W., Zhang, J., He, J., … Wang, Z. (2017). Production of antibodies and development of an enzyme-linked immunosorbent assay for 17β-estradiol in milk. Food and Agricultural Immunology, 28(6), 1519–1529. doi: 10.1080/09540105.2017.1350833
- Cincotto, F. H., Canevari, T. C., Machado, S. A. S., Sánchez, A., Barrio, M. A. R., Villalonga, R., & Pingarrón, J. M. (2015). Reduced graphene oxide-Sb2O5 hybrid nanomaterial for the design of a laccase-based amperometric biosensor for estriol. Electrochimica Acta, 174, 332–339. doi.org/10.1016/j.electacta.2015.06.013
- Cleemann, L., & Holm, K. (2011). Perifer pubertas praecox som bivirkning i forbindelse med østrogenbehandling af labial adhærens hos en syvårig pige. Ugeskrift for Laeger, 173, 1435–1436. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21586251
- Freeman, J. V., & Johnson, G. M. (1999). Synthesis of 6alpha-functionalized estriol haptens and protein conjugates thereof. US 5902888. Official Gazette of the United States Patent and Trademark Office Patents, 1222, 2.
- Fu, H. J., Wang, Y., Dong, X. X., Liu, Y. X., Chen, Z. J., Shen, Y. D., … Xu, Z. L. (2016). Application of nickel cobalt oxide nanoflakes for electrochemical sensing of estriol in milk. RSC Advances, 6, 65588–65593. doi.org/10.1039/C6RA09186E
- Gorga, M., Insa, S., Petrovic, M., & Barceló, D. (2014). Analysis of endocrine disrupters and related compounds in sediments and sewage sludge using on-line turbulent flow chromatography-liquid chromatography-tandem mass spectrometry. Journal of Chromatography A, 1352, 29–37. doi.org/10.1016/j.chroma.2014.05.028
- Hu, S., Wu, K., Yi, H., & Cui, D. (2002). Voltammetric behavior and determination of estrogens at Nafion-modified glassy carbon electrode in the presence of cetyltrimethylammonium bromide. Analytica Chimica Acta, 464, 209–216. doi.org/10.1016/S0003-2670(02)00496-8
- Kim, H., Kim, Y. Y., Ku, S. Y., Kim, S. H., Choi, Y. M., & Moon, S. Y. (2013). The effect of estrogen compounds on human embryoid bodies. Reproductive Sciences, 20, 661–669. doi.org/10.1177/1933719112462630
- Lin, X., & Li, Y. (2006). A sensitive determination of estrogens with a Pt nano-clusters/multi-walled carbon nanotubes modified glassy carbon electrode. Biosensors & Bioelectronics, 22, 253–259. doi.org/10.1016/j.bios.2006.01.005
- Lu, J., Wu, J., Stoffella, P. J., & Chris Wilson, P. (2012). Isotope dilution-gas chromatography/mass spectrometry method for the analysis of alkylphenols, bisphenol A, and estrogens in food crops. Journal of Chromatography A, 1258, 128–135. doi.org/10.1016/j.chroma.2012.08.033
- Luo, L., Xu, Z. L., Yang, J. Y., Xiao, Z. L., Li, Y. J., Beier, R. C., … Shen, Y. D. (2014). Synthesis of novel haptens and development of an enzyme-linked immunosorbent assay for quantification of histamine in foods. Journal of Agricultural and Food Chemistry, 62, 12299–12308. doi.org/10.1021/jf504689x
- Mukunzi, M., Tochi, B. N., Isanga, J., Liu, L., Kuang, H., & Xu, C. (2016). Development of an immunochromatographic assay for hexestrol and diethylstilbestrol residues in milk. Food and Agricultural Immunology, 27(6), 855–869. doi: 10.1080/09540105.2016.1183601
- Otsuki, Y., Yamaji, K., Tanizawa, O., Fujita, M., Kurachi, K., Ishibashi, K., & Miyai, K. (1979). An enzyme-immunoassay for estriol. Endocrinologia Japonica, 26(6), 687–691. doi: 10.1507/endocrj1954.26.687
- Page, M., & Thorpe, R. (2002). Purification of IgG by precipitation with sodium sulfate or ammonium sulfate. The Protein Protocols Handbook, 983–984. doi.org/10.1385/1-59259-169-8:983
- Piwowarska, J., Radowicki, S., & Pachecka, J. (2010). Simultaneous determination of eight estrogens and their metabolites in serum using liquid chromatography with electrochemical detection. Talanta, 81, 275–280. doi.org/10.1016/j.talanta.2009.11.069
- Santos, K. D., Braga, O. C., Vieira, I. C., & Spinelli, A. (2010). Electroanalytical determination of estriol hormone using a boron-doped diamond electrode. Talanta, 80, 1999–2006. doi.org/10.1016/j.talanta.2009.10.058
- Tang, Y., Zhao, S., Wu, Y., Zhou, J., & Li, M. (2013). A direct competitive inhibition time-resolved fluoroimmunoassay for the detection of unconjugated estriol in serum of pregnant women. Analytical Methods, 5, 4068–4073. doi.org/10.1039/C3AY40446C
- Wang, S., Du, L., Lin, S., & Zhuang, H. (2006). Flow injection chemiluminescence for the determination of estriol via a noncompetitive enzyme immunoassay. Microchimica Acta, 155, 421–426. doi: 10.1007/s00604-006-0568-z
- Wang, Y. C., Guo, Z. Q., Li, Y. Z., & Chang, W. B. (2002). Production and characterization of anti-estrone monoclonal antibody. Biomedical and Environmental Sciences, 15, 103–112. doi: 10.1080/00222340601067129
- Wang, S., Wang, Y., Huang, W., Fang, G., Duan, Z., Qiao, H., & Zhang, Y. (2007). Development of a solid-phase extraction enzyme-linked immunosorbent assay method with a new sorbent of multiwall carbon nanotube for the determination of estrone in water. Analytical Letters, 40, 2338–2350. doi: 10.1080/00032710701577559
- Wang, Z., Xie, Z., Cui, G., Liu, L., Song, S., Kuang, H., & Xu, C. (2017). Development of an indirect competitive enzyme-linked immunosorbent assay and immunochromatographic assay for hydrocortisone residues in milk. Food and Agricultural Immunology, 28(3), 476–488. doi: 10.1080/09540105.2017.1297779