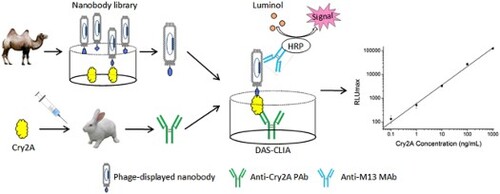
ABSTRACT
In this study, we developed a double antibody sandwich chemiluminescent immunoassay (DAS-CLIA) based on phage-displayed nanobodies for the determination of Cry2A toxin. Nine phage-displayed nanobodies specifically to Cry2A were obtained from a naive nanobody library after four rounds of biopanning. The phage-displayed nanobody P2 with high affinity binding was selected as the detection antibody for the sensitive DAS-CLIA development. The proposed method exhibited high sensitivity with a limit of detection of 0.09 ng/mL, has a wide linear range for Cry2A toxin detection, in the range of 0.1–1000 ng/mL and negligible cross-reactivities to the other Bt toxins. The recoveries of Cry2A toxin spiked in cereal samples (rice, wheat, and corn) ranged from 82.7% to 118%, with a coefficient of variation of less than 10%. This study demonstrated that the DAS-CLIA procedure based on phage-displayed nanobodies may provide an alternative sensitive method for the detection of Bt toxins in agri-products.
Introduction
Dependence on genetically engineered (GM) crops has been rising in recent decades (Demeke & Dobnik, Citation2018; Szekacs et al., Citation2012; Zhang et al., Citation2016). More than 180 million hectares of GM crops were planted worldwide since the first GM tomato FLAVR SAVR was approved for commercialization in 1994 (Fraiture et al., Citation2015; ISAAA, Citation2017; Razavi et al., Citation2017). Cry toxins (e.g. Cry1A, Cry2A, and Cry3B) are produced by Bacillus thuringiensis (Bt) during the sporulation phase, and are major components of GM crops by now (Guimaraes et al., Citation2008; Xu, Zhang et al., Citation2016). Due to the high specific activity against lepidopterans, Cry toxins are widely applied in GM crops for pest control (Lu, Gu, Liu, Lin, & Yu, Citation2017; Soberon, Gill, & Bravo, Citation2009; Xu et al., Citation2016). However, GM crops have not gained universal acceptance because of concerns about the potential risk to the environment and human health (Andreassen et al., Citation2015; Dong et al., Citation2016; Gassmann et al., Citation2014; Haslberger, Citation2000). For this and other reasons, it is necessary to conduct surveillance and monitoring by using on-site, rapid and reliable methods for food material identification, quantification, and information collection.
Currently, numerous methods have been applied for the detection of Cry toxins based on exogenous DNA or expressed proteins (Gao, Wen, Wu, Fu, & Wu, Citation2017; Guertler, Paul, Albrecht, & Meyer, Citation2009; Kamle, Kumar, & Bhatnagar, Citation2011; Xu et al., Citation2017; Walschus, Witt, & Wittmann, Citation2002). Both polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (ELISA) are commonly used. PCR methods are highly sensitive and precise for qualitative identification; however, they cannot quantify the amount of expressed Cry proteins. ELISA methods have the advantages of simplicity, high-throughput and low-cost, which are suitable for qualification and quantification of transgenic proteins expressed in GM crops (Clark, Phillips, & Coats, Citation2005; Lipton et al., Citation2010). Since the first ELISA was reported for the toxic proteins of B. thuringiensis (Wie, Andrews, Hammock, Faust, & Bulla, Citation1982), immunoassay has been the mainstay of analytical procedures for the protein toxins. Cheung and Hammock (Citation1988) stated immunoassays being essential tools to monitor production, efficacy, residue analysis and other critical analytical problems both for the bacterial toxins and engineered toxins. Over the last three decades immunochemical technologies have complemented PCR and become the mainstay for analysis of insecticidal proteins from B. thuringiensis (Albright, Hellmich, & Coats, Citation2016). Recent literature also indicates that the Cry toxins are common targets driving improvement in antibody and ELISA technology. Double antibody sandwich ELISA (DAS-ELISA) is the preferable immunoassay format used for Cry toxins analysis, commonly based on polyclonal antibodies (PAbs) and monoclonal antibodies (MAbs) or single-chain variable fragments (ScFvs) (Dong et al., Citation2017; Dong, Bo, Zhang, Feng, & Liu, Citation2018; Zhang et al., Citation2014; Zhu et al., Citation2011). Dong et al. (Citation2017) developed a DAS-ELISA based on PAb and MAb for Cry1Ab toxin detection with a LOD was 0.47 ng/mL. Zhang et al. (Citation2014) selected an anti-Cry1Ab ScFv from a phage displayed ScFv library and applied to DAS-ELISA for detection of Cry1Ab, with LOD of 0.008 μg/mL. However, the preparation of traditional monoclonal antibody need a complicated procedure, and the single-chain antibody usually exhibited a lower affinity.
Nanobodies provide an alternative reagent for the development of ELISA for Cry toxins, the smallest functional antigen-binding unit, are derived from heavy-chain antibodies of camelid (De Genst et al., Citation2006; Hamers-Casterman et al., Citation1993). Compared with conventional antibodies, nanobodies have high stability, high affinity, the advantages of easy to produce, and can recognize inaccessible epitopes (Shu et al., Citation2019; Zhu et al., Citation2015). Moreover, phage-displayed nanobodies can provide an advantage over the soluble nanobodies being used as reporter elements for immunoassays development. For example, M13 phage particles contain approximately 2700 copies of pVIII capsid protein per phage. This can be used for signal amplification and modification (Goldman et al., Citation2009; Peltomaa, Lopez-Perolio, Benito-Pena, Barderas, & Moreno-Bondi, Citation2016). So, phage-displayed nanobodies could be valuable reagents for immunoassay development.
In this study, specific phage-displayed nanobodies against Cry2A were isolated from a naive nanobody library. Using these phage-displayed nanobodies as the detection antibody, a sensitive double antibody sandwich chemiluminescent immunoassay (DAS-CLIA) was established for the detection of Cry2A toxin. Method validation has been conducted with spiked cereal samples. The proposed phage-displayed nanobodies based DAS-CLIA can provide an alternative approach for the determination of Bt Cry toxins offering a number of advantages including an unlimited supply of uniform reagent.
Materials and methods
Materials and reagents
Bt toxins (Cry2A, Cry1Ab, Cry1F, Cry3B, and VIP3A) were purchased from You Long Bio. Co. Ltd. (Shanghai, China). HRP-anti-M13 monoclonal antibody was purchased from GE Healthcare (Piscataway, NJ, USA). TMB substrate and bovine serum albumin (BSA) were purchased from Sigma (St. Louis, MO, USA). Chemiluminescent substrate was purchased from Thermo Fisher Scientific (Waltham, MA, USA). The naive phage-displayed nanobody library had been constructed previously (Qiu et al., Citation2018). Purified anti-Cry2A PAb was produced in our laboratory as described by Zhu et al. (Citation2011). The 96-well microplates were purchased from Costar (Cambridge, MA, USA).
Biopanning of anti-Cry2A phage-displayed nanobodies
Biopanning of phage-displayed nanobodies specifically for Cry2A toxin was carried out as described by Kim et al. (Citation2012) with slight modification. Cry2A toxin was diluted with 0.01 M PBS to 100 μg/mL and incubated in microplate overnight at 4°C. 300 μL of 3% BSA diluted in PBS was added into wells for 2 h incubation at 37°C. After washing three times with 0.1% PBST (Tween-20 v/v), 100 μL of phage-displayed nanobody library (2 × 1011 pfu) was added and incubated at 37°C for 1 h. Then, unbound phages were discarded by 10 times washing, the specific phages were eluted using 100 μL of Glycine–HCl (0.2 M, pH 2.2) with gentle shaking for 10 min and neutralized with Tris–HCl (1 M, pH 9.0). Thereafter, eluted phages were titered and infected with E.coli TG1 cells for phage amplification. For the next three rounds, the added phage libraries remained the same (2 × 1011 pfu), whereas the coating concentration of Cry2A toxin was reduced to 75, 50, and 25 μg/mL, respectively.
Characterization of phage-displayed nanobodies against Cry2A
Forty-eight individual nanobody clones from the last round of elution were randomly picked and amplified according to previously report (Tu et al., Citation2012). After centrifugation and purification, nanobody phages were collected and suspended with PBS.
Phage-ELISA was carried out for the identification of phage-displayed nanobodies binding Cry2A. Briefly, 100 μL of Cry2A toxin (5 μg/mL) was added onto 96-wells microplate and incubated at 4°C overnight. After washing three times with PBST, 300 μL of 5% skim milk in PBS was added and incubated at 37°C for 2 h. Then, 100 μL of each phage-displayed nanobodies were putted into the wells for 1 h at 37°C. Unbound phages were washed with PBST, 100 μL of a 1:5000 dilution of HRP-anti-M13 monoclonal antibody was added for 1 h incubation at 37°C. Thereafter, 100 μL of TMB substrate solution was added at 37°C for 10 min. Finally, the reaction was stopped with 50 μL per well of 2 M H2SO4, and the OD450 values were measured with a microplate reader (Thermo, USA). Positive/negative >3.0 was considered that positive phage clones and DNA were identified by colony PCR. Then the positive phages were sequenced by Invitrogen Biotechnology Co., Ltd. (Shanghai, China).
Phage-displayed nanobodies based DAS-CLIA
A double antibody sandwich chemiluminescent immunoassay for the determination of Cry2A toxin was established using a phage-displayed nanobody. In brief, 100 μL of anti-Cry2A PAbs were coated onto 96-well black microplate at 4°C overnight. The coated microplate was blocked with 5% BSA in 0.1% PBST at 37°C for 2 h, 100 μL of Cry2A toxin at different concentrations (from 0.1–1000 ng/mL) or samples were added for 1 h incubation at 37°C. After five times washing with 0.1% PBST, 100 μL of phage-displayed nanobodies against Cry2A were added onto washed wells and incubated for 1 h. To detect the bound phage-displayed nanobodies, 100 μL of HRP-anti-M13 monoclonal antibody (1:5000 dilution) was added for 1 h incubation at 37°C. Then, 100 μL per well of the chemiluminescent substrate was added at room temperature for 3 min. Finally, chemiluminescence intensity was measured by a luminescence reader (Berthold, Germany). To optimize the reaction conditions of DAS-CLIA, different concentrations of anti-Cry2A PAbs and phage-displayed nanobodies were evaluated by a checkerboard assay in advance.
Cross-reactivity
Bt toxins Cry1Ab, Cry1F, Cry3B and VIP3A were applied to DAS-CLIA. Briefly, 100 μL of anti-Cry2A PAbs (0.4 μg/mL) were coated onto microtiter plates overnight. After blocking, 1000 ng/mL of Cry2A toxin and other Bt toxins were added to the coated plates and incubated for 1 h respectively. Thereafter, 100 μL of phage-displayed nanobodies were added into the wells. Following steps were undertaken as described above. All analyses are reported as mean ± standard deviation from triplicate measurements.
Assessment of the DAS-CLIA by spiked samples
The resulting DAS-CLIA method was assessed by detecting Cry2A toxin in cereal samples (rice, wheat, and corn). Blank cereal samples were spiked with Cry2A toxin at four different concentrations (5, 50, 500, and 5000 μg/kg). Cry2A toxin extraction from cereal samples was performed as following procedure. In brief, the ground cereal samples (1.0 g) were added to 1 mL of extraction buffer (10 mM PBS, pH 7.4, containing 0.1% BSA and 0.05% Tween-20) and extracted by shaking for 1 h at 37 °C. After the mixture was centrifugated at 10,000 g for 20 min, the supernatants were collected and analyzed by DAS-CLIA assay after diluted 10-fold by PBS.
Results and discussion
Selection of anti-Cry2A phage-displayed nanobodies
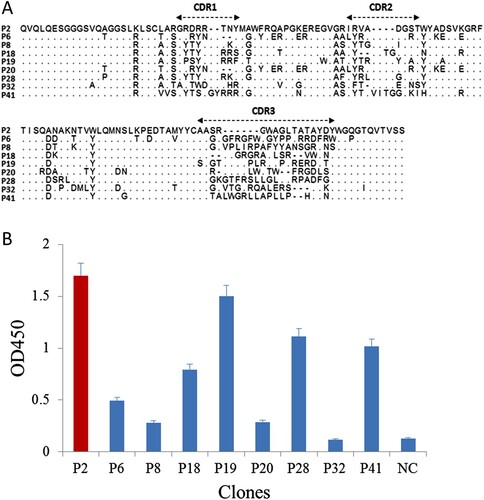
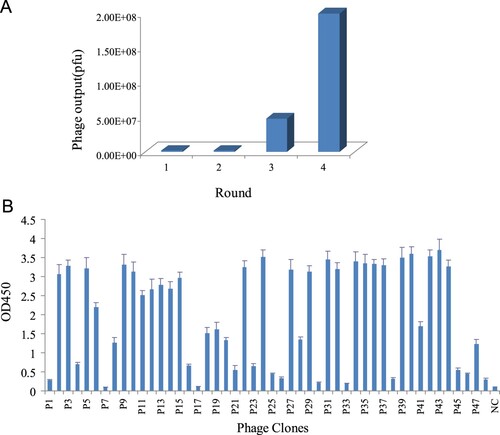
Anti-Cry2A nanobodies were isolated from a nanobody library constructed previously with a capacity of 1.7 × 108 cfu. To obtain the specific phage clones by biopanning, washing times were gradually increased. In addition, the concentration of Tween-20 was increased up to 0.25% in the third and fourth round. After biopanning, the eluted phages increased from an initial 1.2 × 106 pfu to 2.0 × 108 pfu in the fourth round of panning, which means effective enrichment of positive phages binding to target Cry2A toxin ((a)). Totally 48 phage clones from the last biopanning were randomly picked and analyzed by phage-ELISA. As shown in (b), 40 phage clones were identified to be specific for Cry2A recognition, with the OD450 values of P/N >3.0. Then the coding DNA of the 40 positive clones was sequenced.
Figure 1. (a) Number of phage output in each round of panning. (b) Identification the positive clones binding to Cry2A toxin by phage-ELISA. NC, negative control.
As shown in (a), the 40 positive phage clones were actually nine unique sequences. The framework regions were conserved among the nine sequences, and the CDR1 and CDR2 regions were similar, while the amino acids in the CDR3 region exhibited great diversity. Interestingly, P2 and P19 have the consensus sequences of “WA-L” in the middle of the CDR3 region, and they showed similar binding activity in ELISA, indicating the structure of amino acid sequences “WA-L” may play an important role in Cry2A toxin recognition.
Phage-displayed nanobodies based DAS-CLIA
Nine phage-displayed nanobodies were paired with anti-Cry2A PAb to develop a sandwich ELISA for Cry2A toxin. As shown in (b), six phage-displayed nanobodies (P2, P6, P18, P19, P28 and P41) can form pairs with anti-Cry2A PAb. Among them, the nanobody P2 exhibited the highest binding activity, and was hence selected for further development of the DAS-CLIA.
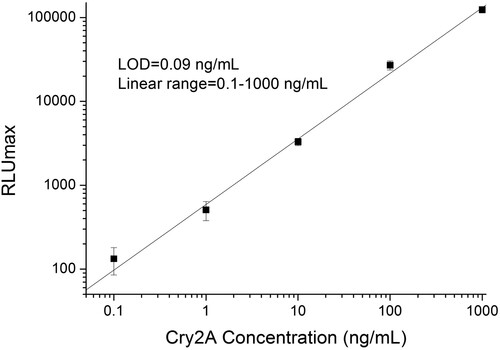
To improve the performance of the immunoassay, different concentrations of anti-Cry2A PAb (0.04, 0.4, and 4 μg/mL) and phage-displayed nanobody P2 (2.5 × 107, 2.5 × 108, and 2.5 × 109 pfu/mL) were optimized in advance. As shown in , considering the signal-to-noise ratio and LOD of the curve, as well as low consumption of antibodies, the optimal concentrations of the anti-Cry2A PAb and phage-displayed nanobody P2 were determined to be 0.4 μg/mL and 2.5 × 109 pfu/mL, respectively. In the optimal conditions, the linear range of DAS-CLIA was 0.1–1000 ng/mL, the limit of detection (LOD) was 0.09 ng/mL ().
Figure 3. Optimization of (a) capture antibody (anti-Cry2A PAb) and (b) detection antibody (phage-displayed nanobody P2) concentrations.
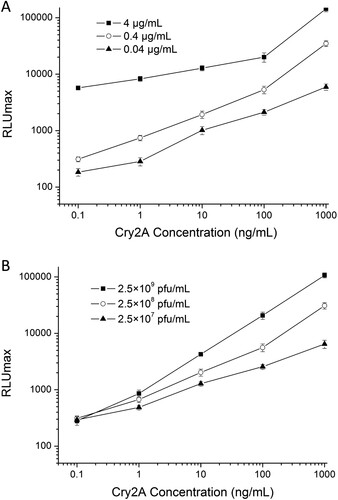
Figure 4. Standard curve of phage-displayed nanobody based DAS-CLIA for Cry2A toxin analysis under the optimized conditions.
Cry toxins, such as Cry1A, Cry1F and Cry2A, were the most prevalent insecticidal crystal proteins applied in GM crops. Transgenic crops expressing Cry1 and Cry2 toxins are being commercially planted in many countries. Although resistance was surprisingly slow to develop considering the high selection pressure of first generation Bt crops, insect-resistance is becoming a increasingly serious problem. Subsequently, second or third generation GM crops which introduce two or more Bt toxins have been explored. These crops broaden the insecticidal spectrum. Cry2A toxin is a promising candidate for management of insect-resistance because its receptor protein and insecticidal mechanism are different from the widely used Cry1 toxins. To date, however, most of the studies focused on the analysis of Cry1 toxins, and few have been targeted to Cry2A. Li, Yan, et al. (Citation2014) developed a LAMP assay for the determination of Cry2Ab, the limit of detection was five copies of haploid genomic DNA. Kamle et al. (Citation2011) established a MAb based DAS-ELISA for the analysis of Cry2Ab, with a LOD of 1 pg/g. Our study is the first report of developing a phage-displayed nanobody based DAS-ELISA for the detection of Cry2A toxin.
Cross-reactivity
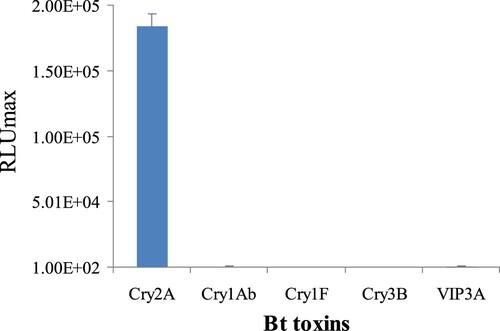
The cross-reactivity of the DAS-CLIA reported here was determined against Cry1Ab, Cry1F, Cry3B, and VIP3A toxins and the results are shown in . There were no positive RLUmax values obtained with the nanobody P2 to these other Bt toxins at concentrations of 1000 ng/mL. The results showed that the established DAS-CLIA was highly specific to Cry2A toxin and had no cross reaction with Cry1Ab, Cry1F, Cry3B, and VIP3A.
Assay validation
Matrix effects can interfere with reactions between antibodies and antigens, thus affecting the sensitivity and accuracy of the immunoassay. The matrix effects of cereal samples were evaluated by dilution with extract buffer. After a 10-fold dilution of extracts, no significant difference in OD450 values was observed between sample extracts and extract buffer, indicating that matrix effects were negligible.
The accuracy and reproducibility of the developed DAS-CLIA was assessed by spiking blank cereal samples with a series of different concentrations of Cry2A (5, 50, 500, and 5000 μg/kg). The results were shown in , the average recoveries of Cry2A from rice sample ranged from 82.7% to 106% with a coefficient of variation (CV) of 4.4%–9.3%. Recoveries of the wheat sample ranged from 88.5% to 118% with a CV of 5.3%–8.9%. Meanwhile, recoveries of corn sample were 85.3%–110% with CV less than 8.2%. There are no Cry2A modified GM crops in the Chinese market so far, real sample testing would be more practical and realistic for the method application. However, our results suggested that good recoveries have been achieved using phage-displayed nanobody based DAS-CLIA for Cry2A toxin detection developed here. These phage-displayed nanobodies provided an alternative reagent for Cry2A toxin detection.
Table 1. Recovery analysis of Cry2A in rice, wheat, and corn samples by DAS-CLIA.
The conventional DAS-ELISA requires a high specificity and affinity monoclonal antibody. However, the production of monoclonal antibodies is complicated and time-consuming. In recent years, with the development of antibody engineering technology, genetically engineered antibodies (such as ScFvs, nanobodies) are being employed for the detection of Bt toxin. Nanobodies derived from camelid heavy-chain antibodies, are the smallest intact antigen binding fragments with high affinity, stability, solubility and contain a long CDR3 loop, which have access to the clefts and cavities of target proteins. With these unique features, nanobody-based approaches are becoming promising tools for diagnostic applications (Li et al., Citation2014).
When compared with the traditional antibodies or soluble nanobodies, phage-displayed nanobodies have the advantages of ease of production, reproducibility and low-cost. Besides, M13 filamentous phages, which have ∼2700 copies of the 5 kD major pVIII coat protein, can provide great signal amplification when using reporter-conjugated anti-M13 pVIII antibodies. Various studies have shown that M13 phages are valuable reporter elements for use in ELISAs (Goldman, Anderson, Bernstein, & Swain, Citation2010). In addition, for increasing sensitivity, chemiluminescence has been employed to combine phage-displayed nanobodies, HRP-conjugated anti-M13 pVIII antibodies and chemiluminescent substrates. In further studies, phages could be directly conjugated to a fluorescent dye or other label to improve the detection sensitivity and shorten the procedure. Meanwhile, the affinity of nanobody P2 could be improved by site-directed mutagenesis of key amino acids in CDR3.
Conclusion
We successfully obtained several nanobodies specific to Cry2A from a naive nanobody library though biopanning. The phage-displayed nanobody P2, which exhibited high affinity binding, was further used for the DAS-CLIA development. The established assay showed a wide detection range of 0.1–1000 ng/mL and a low limit detection of 0.09 ng/mL. The average recoveries of cereal samples were 82.7%–118%, with a CV at 4.4%–9.3% and the matrix effects seemed minimal. These results indicate that a DAS-CLIA using phage-displayed nanobodies could be a promising method for the detection of Cry toxins and other proteins.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Albright, V. C., 3rd, Hellmich, R. L., & Coats, J. R. (2016). A review of cry protein detection with enzyme-linked immunosorbent assays. Journal of Agricultural and Food Chemistry, 64, 2175–2189. doi: 10.1021/acs.jafc.5b03766
- Andreassen, M., Rocca, E., Bohn, T., Wikmark, O. G., van den Berg, J., Lovik, M., & Nygaard, U. C. (2015). Humoral and cellular immune responses in mice after airway administration of Bacillus thuringiensis Cry1Ab and MON810 cry1Ab-transgenic maize. Food and Agricultural Immunology, 26, 521–537. doi: 10.1080/09540105.2014.988128
- Cheung, P. Y. K., & Hammock, B. D. (1988). Monitoring Bacillus thuringiensis in the environment with enzyme-linked immunosorbant assay. In P. A. Hedin, J. J. Menn, & R. M. Hollingworth (Eds.), Biotechnology for Crop Protection (pp. 359–372). Washington, DC: American Chemical Society.
- Clark, B. W., Phillips, T. A., & Coats, J. R. (2005). Environmental fate and effects of Bacillus thuringiensis (Bt) proteins from transgenic crops: A review. Journal of Agricultural and Food Chemistry, 53, 4643–4653. doi: 10.1021/jf040442k
- De Genst, E., Silence, K., Decanniere, K., Conrath, K., Loris, R., Kinne, J., … Wyns, L. (2006). Molecular basis for the preferential cleft recognition by dromedary heavy-chain antibodies. Proceedings of the National Academy of Sciences, 103, 4586–4591. doi: 10.1073/pnas.0505379103
- Demeke, T., & Dobnik, D. (2018). Critical assessment of digital PCR for the detection and quantification of genetically modified organisms. Analytical and Bioanalytical Chemistry, 410, 4039–4050. doi: 10.1007/s00216-018-1010-1
- Dong, S., Bo, Z. Y., Zhang, C. Z., Feng, J. G., & Liu, X. J. (2018). Screening for single-chain variable fragment antibodies against multiple Cry1 toxins from an immunized mouse phage display antibody library. Applied Microbiology and Biotechnology, 102, 3363–3374. doi: 10.1007/s00253-018-8797-8
- Dong, S., Zhang, X., Liu, Y., Zhang, C., Xie, Y., Zhong, J., … Liu, X. (2017). Establishment of a sandwich enzyme-linked immunosorbent assay for specific detection of Bacillus thuringiensis (Bt) Cry1Ab toxin utilizing a monoclonal antibody produced with a novel hapten designed with molecular model. Analytical and Bioanalytical Chemistry, 409, 1985–1994. doi: 10.1007/s00216-016-0146-0
- Dong, S., Zhang, C., Zhang, X., Liu, Y., Zhong, J., Xie, Y., … Liu, X. (2016). Production and characterization of monoclonal antibody broadly recognizing Cry1 toxins by use of designed polypeptide as hapten. Analytical Chemistry, 88, 7023–7032. doi: 10.1021/acs.analchem.6b00429
- Fraiture, M. A., Herman, P., Taverniers, I., De Loose, M., Deforce, D., & Roosens, N. H. (2015). Current and new approaches in GMO detection: Challenges and solutions. Biomed Research International, 2015, 1–22. doi:10.1155/2015/392872.
- Gao, H., Wen, L., Wu, Y., Fu, Z., & Wu, G. (2017). An ultrasensitive label-free electrochemiluminescent immunosensor for measuring Cry1Ab level and genetically modified crops content. Biosensors and Bioelectronics, 97, 122–127. doi: 10.1016/j.bios.2017.04.033
- Gassmann, A. J., Petzold-Maxwell, J. L., Clifton, E. H., Dunbar, M. W., Hoffmann, A. M., Ingber, D. A., & Keweshan, R. S. (2014). Field-evolved resistance by western corn rootworm to multiple Bacillus thuringiensis toxins in transgenic maize. Proceedings of the National Academy of Sciences, 111, 5141–5146. doi: 10.1073/pnas.1317179111
- Goldman, E. R., Anderson, G. P., Bernstein, R. D., & Swain, M. D. (2010). Amplification of immunoassay using phage-displayed single domain antibodies. Journal of Immunological Methods, 352, 182–185. doi: 10.1016/j.jim.2009.10.014
- Goldman, E. R., Liu, J. L., Bernstein, R. D., Swain, M. D., Mitchell, S. Q., & Anderson, G. P. (2009). Ricin detection using phage displayed single domain antibodies. Sensors, 9, 542–555. doi: 10.3390/s90100542
- Guertler, P., Paul, V., Albrecht, C., & Meyer, H. H. (2009). Sensitive and highly specific quantitative real-time PCR and ELISA for recording a potential transfer of novel DNA and Cry1Ab protein from feed into bovine milk. Analytical and Bioanalytical Chemistry, 393, 1629–1638. doi: 10.1007/s00216-009-2667-2
- Guimaraes, V. D., Drumare, M. F., Ah-Leung, S., Lereclus, D., Bernard, H., Creminon, C., … Adel-Patient, K. (2008). Comparative study of the adjuvanticity of Bacillus thuringiensis Cry1Ab protein and cholera toxin on allergic sensitisation and elicitation to peanut. Food and Agricultural Immunology, 19, 325–337. doi: 10.1080/09540100802495651
- Hamers-Casterman, C., Atarhouch, T., Muyldermans, S., Robinson, G., Hamers, C., Songa, E. B., … Hamers, R. (1993). Naturally occurring antibodies devoid of light chains. Nature, 363, 446–448. doi: 10.1038/363446a0
- Haslberger, A. G. (2000). Genetic Technologies: Monitoring and Labeling for genetically modified products. Science, 287, 431–432. doi: 10.1126/science.287.5452.431
- ISAAA. (2017). Global status of commercialized Biotech/GM crops: 2017. ISAAA. http://isaaa.org/resources/publications/briefs/53/default.asp.
- Kamle, S., Kumar, A., & Bhatnagar, R. K. (2011). Development of multiplex and construct specific PCR assay for detection of cry2Ab transgene in genetically modified crops and product. GM Crops, 2, 74–81. doi: 10.4161/gmcr.2.1.16017
- Kamle, S., Ojha, A., & Kumar, A. (2011). Development of an enzyme linked immunosorbant assay for the detection of Cry2Ab protein in transgenic plants. GM Crops, 2, 118–125. doi: 10.4161/gmcr.2.2.16191
- Kim, H. J., McCoy, M. R., Majkova, Z., Dechant, J. E., Gee, S. J., Tabares-da Rosa, S., … Hammock, B. D. (2012). Isolation of alpaca anti-hapten heavy chain single domain antibodies for development of sensitive immunoassay. Analytical Chemistry, 84, 1165–1171. doi: 10.1021/ac2030255
- Li, F., Yan, W., Long, L., Qi, X., Li, C., & Zhang, S. (2014). Development and application of loop-mediated isothermal amplification assays for rapid visual detection of cry2Ab and cry3A genes in genetically-modified crops. International Journal of Molecular Sciences, 15, 15109–15121. doi: 10.3390/ijms150915109
- Li, M., Zhu, M., Zhang, C. Z., Liu, X. J., & Wan, Y. K. (2014). Uniform orientation of biotinylated nanobody as an affinity binder for detection of Bacillus thuringiensis (Bt) Cry1Ac toxin. Toxins, 6, 3208–3222. doi: 10.3390/toxins6123208
- Lipton, C. R., Dautlick, J. X., Grothaus, G. D., Hunst, P. L., Magin, K. M., Mihaliak, C. A., … Stave, J. W. (2010). Guidelines for the validation and use of immunoassays for determination of introduced proteins in biotechnology enhanced crops and derived food ingredients. Food and Agricultural Immunology, 12, 153–164. doi: 10.1080/095401000404094
- Lu, K. Y., Gu, Y. Q., Liu, X. P., Lin, Y., & Yu, X. Q. (2017). Possible insecticidal mechanisms mediated by immune-response-related Cry-binding proteins in the midgut juice of Plutella xylostella and Spodoptera exigua. Journal of Agricultural and Food Chemistry, 65, 2048–2055. doi: 10.1021/acs.jafc.6b05769
- Peltomaa, R., Lopez-Perolio, I., Benito-Pena, E., Barderas, R., & Moreno-Bondi, M. C. (2016). Application of bacteriophages in sensor development. Analytical and Bioanalytical Chemistry, 408, 1805–1828. doi: 10.1007/s00216-015-9087-2
- Qiu, Y. L., Li, P., Dong, S., Zhang, X. S., Yang, Q. R., Wang, Y. L., … Liu, X. J. (2018). Phage-mediated competitive chemiluminescent immunoassay for detecting Cry1Ab toxin by using an anti-idiotypic camel nanobody. Journal of Agricultural and Food Chemistry, 66, 950–956. doi: 10.1021/acs.jafc.7b04923
- Razavi, A., Malhotra, I., Ghosh, A., Pusztai-Carey, M., Marks, J., & King, C. (2017). Antibodies as epidemiological markers of genetically modified crop exposure: Detection of Cry1Ab-specific IgG. Food and Agricultural Immunology, 28, 779–788. doi: 10.1080/09540105.2017.1313200
- Shu, M., Xu, Y., Dong, J., Zhong, C., Hammock, B. D., Wang, W., & Wu, G. (2019). Development of a noncompetitive idiometric nanobodies phage immumoassay for the determination of fumonisin B1. Food and Agricultural Immunology, 30, 510–521. doi: 10.1080/09540105.2019.1604637
- Soberon, M., Gill, S. S., & Bravo, A. (2009). Signaling versus punching hole: How do Bacillus thuringiensis toxins kill insect midgut cells? Cellular and Molecular Life Sciences, 66, 1337–1349. doi: 10.1007/s00018-008-8330-9
- Szekacs, A., Weiss, G., Quist, D., Takacs, E., Darvas, B., Meier, M., … Hilbeck, A. (2012). Inter-laboratory comparison of Cry1Ab toxin quantification in MON 810 maize by enzyme-immunoassay. Food and Agricultural Immunology, 23, 99–121. doi: 10.1080/09540105.2011.604773
- Tu, Z., Xu, Y., He, Q., Fu, J., Liu, X., & Tao, Y. (2012). Isolation and characterisation of deoxynivalenol affinity binders from a phage display library based on single-domain camelid heavy chain antibodies (VHHs). Food and Agricultural Immunology, 23, 123–131. doi: 10.1080/09540105.2011.606560
- Walschus, U. W. E., Witt, S., & Wittmann, C. (2002). Development of monoclonal antibodies against Cry1Ab protein from Bacillus thuringiensis and their application in an ELISA for detection of transgenic Bt-maize. Food and Agricultural Immunology, 14, 231–240. doi: 10.1080/0954010021000096382a
- Wie, S. I., Andrews, R. E., Jr., Hammock, B. D., Faust, R. M., & Bulla, L. A., Jr. (1982). Enzyme-linked immunosorbent assays for detection and quantitation of the entomocidal parasporal crystalline protein of Bacillus thuringiensis subspp. Kurstaki and israelensis. Applied and Environmental Microbiology, 43, 891–894.
- Xu, L., Pan, Z. Z., Zhang, J., Liu, B., Zhu, Y. J., & Chen, Q. X. (2016). Proteolytic activation of Bacillus thuringiensis Cry2Ab through a belt-and-braces approach. Journal of Agricultural and Food Chemistry, 64, 7195–7200. doi: 10.1021/acs.jafc.6b03111
- Xu, C. X., Zhang, X., Liu, X. Q., Liu, Y., Hu, X. D., Zhong, J. F., … Liu, X. J. (2016). Selection and application of broad-specificity human domain antibody for simultaneous detection of Bt Cry toxins. Analytical Biochemistry, 512, 70–77. doi: 10.1016/j.ab.2016.08.012
- Xu, C., Zhang, C., Zhong, J., Hu, H., Luo, S., Liu, X., … Liu, X. (2017). Construction of an immunized rabbit phage display library for selecting high activity against Bacillus thuringiensis Cry1F toxin single-chain antibodies. Journal of Agricultural and Food Chemistry, 65, 6016–6022. doi: 10.1021/acs.jafc.7b01985
- Zhang, X., Xu, C. X., Zhang, C. Z., Liu, Y., Xie, Y. J., & Liu, X. J. (2014). Established a new double antibodies sandwich enzyme-linked immunosorbent assay for detecting Bacillus thuringiensis (Bt) Cry1Ab toxin based single-chain variable fragments from a naive mouse phage displayed library. Toxicon, 81, 13–22. doi: 10.1016/j.toxicon.2014.01.010
- Zhang, Y. W., Zhang, W., Liu, Y., Wang, J. H., Wang, G. Y., & Liu, Y. J. (2016). Development of monoclonal antibody-based sensitive ELISA for the determination of Cry1Ie protein in transgenic plant. Analytical and Bioanalytical Chemistry, 408, 8231–8239. doi: 10.1007/s00216-016-9938-5
- Zhu, X. L., Chen, L. L., Shen, P., Jia, J. W., Zhang, D. B., & Yang, L. T. (2011). High sensitive detection of Cry1Ab protein using a quantum dot-based fluorescence-linked immunosorbent assay. Journal of Agricultural and Food Chemistry, 59, 2184–2218. doi: 10.1021/jf104140t
- Zhu, M., Li, M., Li, G. H., Zhou, Z. K., Liu, H., Lei, H. T., … Wan, Y. K. (2015). Nanobody-based electrochemical immunoassay for Bacillus thuringiensis Cry1Ab toxin by detecting the enzymatic formation of polyaniline. Microchimica Acta, 182, 2451–2459. doi: 10.1007/s00604-015-1602-9