ABSTRACT
Lotus root (Nelumbo nucifera) is a popular food in Japan and southeast Asia. It is reported that lotus root includes dietary fiber, vitamin C, and abundant polyphenolic compounds. In Chinese medicine, lotus powder is also utilized in anti-tussive or anti-inflammatory drugs. In this study, we focused on the effects of lotus root powder on allergy symptoms in mice. First, we assessed nasal allergy symptoms in 2,4-diisocyanic acid toluene (TDI)-sensitized nasal allergy model mice. We found that lotus root powder alleviated nasal allergy symptoms in the mice. In addition, we estimated serum inflammatory parameters such as serum total IgE, histamine, and leukotriene B4. Lotus root powder intake significantly decreased serum total IgE and leukotriene B4 compared with standard food intake. We suggest that lotus root powder intake may alleviate allergic symptoms.
1. Introduction
The rhizome of lotus root (Nelumbo nucifera), an aquatic plant, is a very popular food in Japan and southeast Asia (Sridhar & Bhat, Citation2007; Wang, Yen, Liang, & Wu, Citation2003). There are some local brands of lotus root on the Japanese market. In Yamaguchi prefecture, an indigenous brand of lotus root is called “Iwakuni-renkon,” which is white, soft, and sticky. Iwakuni-renkon’s morphology is unique in that its root has nine holes (water-conducting tissue), whereas common lotus root has eight holes, although there are individual differences.
The lotus root includes abundant nutrients such as dietary fiber and vitamin C. Some studies have shown that lotus root contains abundant polyphenolic compounds and possesses several beneficial health properties, including hypoglycemic, anti-inflammatory, anti-epileptic, antioxidant activities and therapeutic application in memory disorders (Huang et al., Citation2011; Khan, Rajput, & Assad, Citation2019; Mukherjee, Mukherjee, Maji, Rai, & Heinrich, Citation2009; Mukherjee, Saha, Pal, & Saha, Citation1997; Rajput, Khan, & Assad, Citation2017; Yan, Wang, & Peng, Citation2009). Furthermore, in Chinese medicine, lotus root is utilized as an anti-tussive and anti-inflammatory drug. Thus, we expected that intake of lotus root could lead to the improvement of allergy symptoms. In this study, we examined the influence of lotus root powder intake on allergy onset using 2,4-diisocyanic acid toluene (TDI)-sensitized nasal allergy model mice (Dearman, Basketter, & Kimber, Citation1996).
2. Materials and methods
2.1. Animals and housing conditions
Four-week-old female BALB/c mice were reared at 22 ± 2°C with 50 ± 10% humidity under a 12-hour light/dark cycle (lights on at 08:00 and off at 20:00). After the mice were acclimated for one week, we divided the mice into a nonsensitization group and a sensitization group. The animals in the nonsensitization group were fed standard food, while those in the sensitization group were further classified into three groups of 5 mice each that were fed as follows for 4 weeks: standard food (based on AIN-93G), standard food containing 5% lotus root powder, and standard food containing 10% lotus root powder. The lotus root powder was processed and provided by Hironaka Food Ltd. (Yamaguchi, Japan).
2.2. Experimental procedure
2.2.1. Schedule of sensitization
After the 4-week feeding period, we applied 2 μL of 5% 2,4-diisocyanic acid toluene (TDI) into a nostril of each animal under anesthesia in the sensitization group for 5 consecutive days. As a control, we applied ethyl acetate, a solvent of TDI solution, into a nostril of each mouse in the nonsensitization group. One week after the first sensitization, we carried out re-sensitization for 5 days in a row using the same method described above. One week after the re-sensitization, we applied the final drop of 5 μL of 2.5% TDI into each animal’s nostril under nonanesthetic conditions.
2.2.2. Visual assessment of allergic symptoms
We evaluated the severity of allergic symptoms for 10 min immediately after the final sensitization. As visual characteristics of nasal allergic symptoms, both reddish tinges and hair loss were observed on the nose. The severity of the nasal allergies was scored on a 4-point scale from 0 (no symptoms) to 3 (very severe symptoms) by observing the appearance of each mouse.
2.2.3. Measurement of serum inflammatory parameters
One week after the final sensitization, the mice were sacrificed under anesthesia with pentobarbital (Sumitomo Dainippon Pharma Co., Ltd., Osaka, Japan) and their trunk blood was collected to evaluate the levels of inflammatory parameters such as serum total IgE, histamine, and leukotriene B4 (LTB4). The concentrations of serum total IgE, histamine, and LTB4 were analyzed using three respective ELISA kits: the Mouse IgE ELISA Quantitation Kit (Bethyl Laboratories, Montgomery, TX, USA), the Histamine ELISA Kit (Neogen, Lansing, MI, USA), and the Leukotriene B4 EIA Kit (Oxford Biomedical Research, Rochester Hills, MI, USA). The concentrations were measured according to the respective manufacturers’ instructions.
2.2.4. Measurement of splenic inflammatory parameters
One week after the final sensitization, the mice were sacrificed under anesthesia with pentobarbital (Sumitomo Dainippon Pharma Co., Ltd., Osaka, Japan) and their spleens were collected. The spleens were weighed, and the ratios of splenic inflammatory parameters, such as CD45R+ cells (B-cells) of splenic lymphocytes, a CD3+ cell (mature T-cell), a CD4+ cell (helper T-cell), and a CD8+ cell (suppressor T-cell), were measured. Spleen cells were divided into two test tubes. The cells in one tube were fixed and stained with FITC-conjugated anti-mouse CD4 mAb and PE-conjugated anti-mouse CD8 mAb. The cells in the other tube were fixed and stained with FITC-conjugated anti-mouse CD45R mAb and PE-conjugated anti-mouse CD3 mAb. The samples were analyzed in a flow cytometer (FACS Calibur, Becton-Dickinson, Franklin Lakes, NJ, USA), and the data were processed by CELL Quest software (Becton-Dickinson).
In addition, we measured concanavalin A (Con A)-stimulated interleukin-4 (IL-4) production. Briefly, spleen cells derived from sensitized or nonsensitized mice were cultivated for 48 h at 37°C, 5% CO2. IL-4 in the supernatant was measured by ANALYZA Immunoassay System mouse IL-4 (Bio-Techne, Minneapolis, MN, USA).
2.3. Statistical analysis
All data were analyzed using one-way ANOVA and were subjected to a post-hoc Fisher’s PLSD test. The significance threshold was set at P < 0.05. All analyses were performed with StatView (version 5, SAS Institute, Cary, NC, USA).
3. Results and discussion
3.1. Physical conditions
We tested anti-allergic or anti-inflammatory activity using TDI-sensitized nasal allergy model mice. Intranasal application of TDI induced nasal allergic symptoms. Before TDI treatment, mice were given the standard diet or the standard diet supplemented with either 5% or 10% lotus root powder for 4 weeks. TDI treatment was performed intermittently for 4 weeks. During the whole experimental period including before and after intermittent TDI treatment, food intake and body weight gain were quite similar among all groups (data not shown). We inferred from these results that lotus root powder does not affect the food intake or growth of animals.
3.2. Allergic symptoms
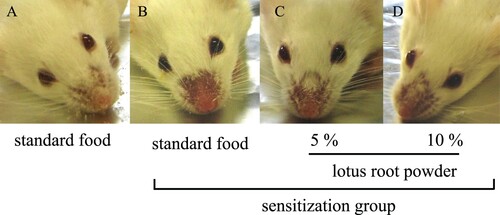
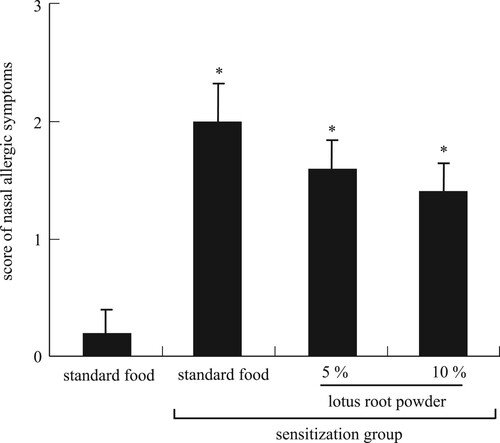
We assessed the state of TDI-sensitized nasal allergy symptoms. In TDI-sensitized mice, the hair loss and redness around the nose were prominent, indicating the occurrence of severe allergic reactions. Meanwhile, these allergic symptoms were clearly alleviated by lotus root intake in a dose-dependent manner (A–D). The severity scores of allergic symptoms were significantly (P < 0.05) increased in the TDI-sensitized mice than in the nonsensitized mice (). These scores also tended to decrease by lotus root intake in a dose-dependent manner. These results show that lotus root powder alleviates nasal allergic symptoms.
3.3. Serum inflammatory parameters
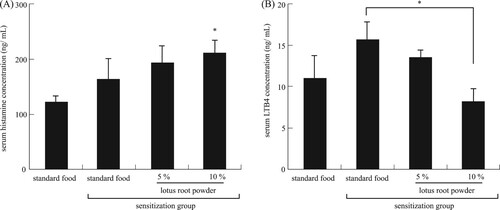
We investigated serum inflammatory parameters. The concentration of serum histamine, which is released from activated mast cells having an allergic response, differed significantly (P < 0.05) between the non-TDI-sensitized mice and the TDI-sensitized mice fed the 10% lotus root powder diet (A). Elevated levels of LTB4 have been reported in people suffering from various allergic diseases, and these levels have been related to disease activity and response to treatment (Ohnishi, Miyahara, & Gelfand, Citation2008). In this study, the concentration of serum LTB4 was significantly (P < 0.05) decreased in the TDI-sensitized 10% lotus root powder diet group compared with the TDI-sensitized standard diet group (B). It is reported that not only histamine but also leukotriene are parameters indicated allergy response. In allergy response, chemical mediators such as histamine and leukotrienes are released from mast cells. The chemical mediators cause sneezing, watery rhinorrhea and nasal blockage as an early phase reaction. In addition, Leukotrienes, produced by inflammatory cells, cause nasal blockage as a late phase reaction, seen at 6–10 h after antigen exposure (Miyake & Karasuyama, Citation2017; Okubo et al., Citation2017; Wedi, Novacovic, Koerner, & Kapp, Citation1999). The Other studies such these reports indicated that histamine release and leukotriene production are regulated differentially. Although lotus powder induce histamine release from mast cells by different regulation, it is suggest that lotus powder may strongly inhibit LTB4 production in inflammatory cells. The results suggested that the alleviation of allergic symptoms by lotus root powder intake.
Figure 3. Serum inflammatory parameters. (A) Serum histamine concentration, *significant difference compared to nonsensitization group (P < 0.05). (B) Serum LTB4 concentration, *significant difference between standard food sensitization group and 10% lotus root powder sensitization group (P < 0.05). Each value is the mean ± SEM (n = 5/group).
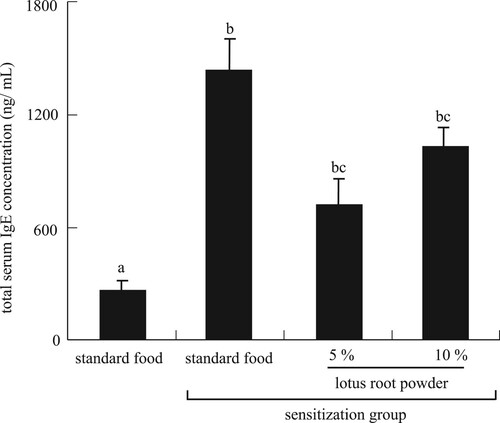
IgE, an antibody produced by the body’s immune system, is associated with allergic responses. The total IgE test is used to diagnose allergic diseases such as atopic disease (Čelakovská & Bukač, Citation2016). In this study, the total serum IgE concentration was significantly increased by TDI sensitization and significantly decreased in the TDI-sensitized 5% and 10% lotus root powder diet groups ().
3.4. Anti-allergic inflammatory parameters
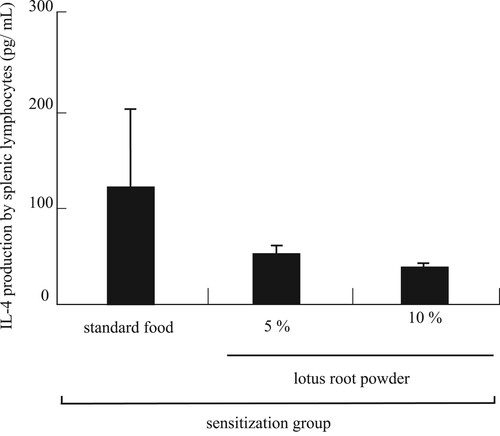
IL-4 was produced by a splenic lymphocyte and leads to the differentiation of B-cells into plasma cells (antibody-producing cells). The serum IL-4 level was no significant difference. However, it was tended to dose-dependent decrease in the TDI-sensitized lotus root powder diet groups compared to the TDI-sensitized standard diet group (). These results suggested that the decrease in total IgE level was caused by the decrease in IL-4, which in turn was produced by a splenic lymphocyte.
Figure 5. IL-4 production by splenic lymphocytes. There is no significant difference. Each value is the mean ± SEM (n = 5/group).
It is known that CD4+ cells (helper T-cells) and CD8+ cells (suppressor T-cells) can contribute to anti-allergic inflammation. It is well established that CD4+ T-cells are required during sensitization (Corry et al., Citation1998; Koya et al., Citation2007, Citation2009). Our experiments revealed a significant (P < 0.05) increase in the CD4+ cell population in the TDI-sensitized 10% lotus root powder diet group compared with the TDI-sensitized standard diet group (A). The CD8+ cell population differed significantly (P < 0.05) between the non-TDI-sensitized group and the 10% lotus root powder diet TDI-sensitized group (B). Whereas CD4+ cells have been demonstrated to have a significant role in the development of allergic diseases, the role of CD8+ cells is not clear. Some researchers have failed to find any functional or numerical differences in T-lymphocyte subpopulations in patients with allergic asthma (Schuyler, Gerblich, & Urda, Citation1985) while others have reported decreased numbers of CD8+ T-cells (Jensen, Cramers, & Thestrup-Pedersen, Citation1981; Kus, Tse, Vedal, & Chan-Yeung, Citation1985; Strannegard & Strannegard, Citation1978) or no changed in allergic contact dermatitis (Usuda, Fujii, & Nonogaki, Citation2016). Other groups have found imbalances in the T-cell subpopulations in patients with atopic disease (Canonica, Mingari, Melioli, Colombatti, & Moretta, Citation1979; Zhang et al., Citation2017). Moreover, CD4+ T-cells/CD8+ T-cells was no significant difference between the groups (). Based on these results, we hypothesized this mechanism of alleviation nasal allergy by lotus root powder. The lotus root powder could be related to the suppression of IL-4, IgE, and LTB4 production ().
Figure 6. Ratio of (A) CD4+, *significant difference between standard food sensitization group and 10% lotus root powder sensitization group (P < 0.05), (B) CD8+ to splenic lymphocytes, *significant difference compared to nonsensitization group (P < 0.05). Each value is the mean ± SEM (n = 5/group).
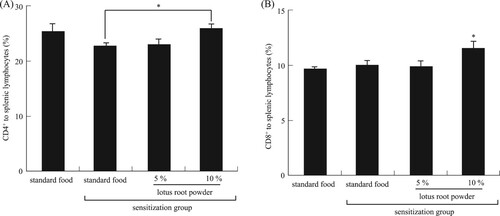
Figure 7. CD4+/CD8+, no significant difference between sensitization group. Each value is the mean ± SEM (n = 4 or 5/group).
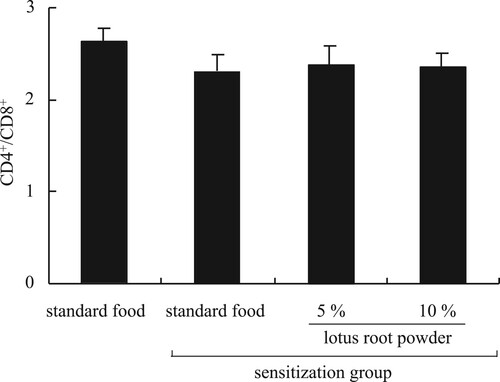
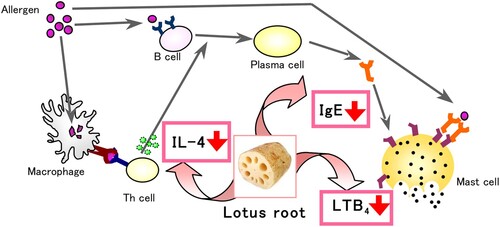
Figure 8. The mechanism of alleviation nasal allergy by lotus root powder. The lotus root powder could be related to the suppression of IL-4, IgE, and LTB4 production.
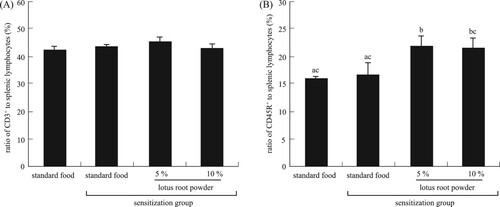
We further measured some factors relating to allergy. The weight of the spleen and the number of splenic lymphocytes did not differ among the groups (data not shown). The CD3+ cell (mature T-cell) ratio did not differ significantly among the groups (A), whereas the CD45R+ cell (B-cell) ratio increased significantly (P < 0.05) in the TDI-sensitized 5% and 10% lotus root powder diet group than in the non-TDI-sensitized and TDI-sensitized standard diet groups (B).
4. Conclusion
Our study revealed that TDI-sensitized nasal allergic symptoms in mice, such as hair loss and redness around the nose, were prominently alleviated by the intake of lotus root powder. The results suggested that the alleviation of allergic symptoms by lotus root powder intake could be related to the suppression of IL-4, IgE, and LTB4 production. These results suggest that lotus root can be utilized to treat allergic symptoms such as hay fever or food allergy.
Acknowledgments
We thank Dr. Satoru Moriguchi for his helpful advice on this experiment. We thank Ms. Junka Oguchi for her technical support of our experimental analysis. We also would like to thank Dr. Wataru Mizunoya and Dr. Shoko Sawano for their valuable and constructive writing assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Canonica, G. W., Mingari, M. C., Melioli, G., Colombatti, M., & Moretta, L. (1979). Imbalances of T cell subpopulations in patients with atopic diseases and effect of specific immunotherapy. The Journal of Immunology, 123(6), 2669–2672.
- Čelakovská, J., & Bukač, J. (2016). Eosinophils in patients suffering from atopic dermatitis and the relation to the occurrence of food allergy and other atopic diseases. Food and Agricultural Immunology, 27(5), 700–710. doi: 10.1080/09540105.2016.1148669
- Corry, D. B., Grünig, G., Hadeiba, H., Kurup, V. P., Warnock, M. L., Sheppard, D., & Locksley, R. M. (1998). Requirements for allergen-induced airway hyperreactivity in T and B cell-deficient mice. Molecular Medicine, 4(5), 344–355. doi: 10.1007/BF03401741
- Dearman, R., Basketter, D., & Kimber, I. (1996). Characterization of chemical allergens as a function of divergent cytokine secretion profiles induced in mice. Toxicology and Applied Pharmacology, 138(2), 308–316. doi: 10.1006/taap.1996.0129
- Huang, B., He, J., Ban, X., Zeng, H., Yao, X., & Wang, Y. (2011). Antioxidant activity of bovine and porcine meat treated with extracts from edible lotus (Nelumbo nucifera) rhizome knot and leaf. Meat Science, 87(1), 46–53. doi: 10.1016/j.meatsci.2010.09.001
- Jensen, J., Cramers, M., & Thestrup-Pedersen, K. (1981). Subpopulations of T lymphocytes and non-specific suppressor cell activity in patients with atopic dermatitis. Clinical and Experimental Immunology, 45(1), 118.
- Khan, R. A., Rajput, M. A., & Assad, T. (2019). Effect of Nelumbo nucifera fruit on scopolamine induced memory deficits and motor coordination. Metabolic Brain Disease, 34(1), 87–92. doi: 10.1007/s11011-018-0324-1
- Koya, T., Matsuda, H., Matsubara, S., Miyahara, N., Dakhama, A., Takeda, K., & Gelfand, E. W. (2009). Differential effects of dendritic cell transfer on airway hyperresponsiveness and inflammation. American Journal of Respiratory Cell and Molecular Biology, 41(3), 271–280. doi: 10.1165/rcmb.2008-0256OC
- Koya, T., Miyahara, N., Takeda, K., Matsubara, S., Matsuda, H., Swasey, C., … Gelfand, E. W. (2007). CD8+ t cell-mediated airway hyperresponsiveness and inflammation is dependent on CD4+ IL-4+ T cells. The Journal of Immunology, 179(5), 2787–2796. doi: 10.4049/jimmunol.179.5.2787
- Kus, J., Tse, K. S., Vedal, S., & Chan-Yeung, M. (1985). Lymphocyte sub-populations in patients with allergic and non-allergic asthma. Clinical & Experimental Allergy, 15(6), 523–529. doi: 10.1111/j.1365-2222.1985.tb02305.x
- Miyake, K., & Karasuyama, H. (2017). Emerging roles of basophils in allergic inflammation. Allergology International, 66, 382–391. doi: 10.1016/j.alit.2017.04.007
- Mukherjee, P. K., Mukherjee, D., Maji, A. K., Rai, S., & Heinrich, M. (2009). The sacred lotus (Nelumbo nucifera)–phytochemical and therapeutic profile. Journal of Pharmacy and Pharmacology, 61(4), 407–422. doi: 10.1211/jpp.61.04.0001
- Mukherjee, P. K., Saha, K., Pal, M., & Saha, B. (1997). Effect of Nelumbo nucifera rhizome extract on blood sugar level in rats. Journal of Ethnopharmacology, 58(3), 207–213. doi: 10.1016/S0378-8741(97)00107-4
- Ohnishi, H., Miyahara, N., & Gelfand, E. W. (2008). The role of leukotriene B4 in allergic diseases. Allergology International, 57(4), 291–298. doi: 10.2332/allergolint.08-RAI-0019
- Okubo, K., Kurono, Y., Ichimura, K., Enomoto, T., Okamoto, Y., Kawauchi, H., … Masuyama, K. (2017). Japanese guidelines for allergic rhinitis 2017. Allergology International, 66, 205–219. doi: 10.1016/j.alit.2016.11.001
- Rajput, M. A., Khan, R. A., & Assad, T. (2017). Anti-epileptic activity of Nelumbo nucifera fruit. Metabolic Brain Disease, 32(6), 1883–1887. doi: 10.1007/s11011-017-0064-7
- Schuyler, M., Gerblich, A., & Urda, G. (1985). Atopic asthma: T lymphocyte subpopulations. Clinical & Experimental Allergy, 15(2), 131–138. doi: 10.1111/j.1365-2222.1985.tb02264.x
- Sridhar, K., & Bhat, R. (2007). Lotus-A potential nutraceutical source. Journal of Agricultural Technology, 3(1), 143–155.
- Strannegard, Ö, & Strannegard, I. L. (1978). T lymphocyte numbers and function in human IgE-mediated allergy. Immunological Reviews, 41(1), 149–170. doi: 10.1111/j.1600-065X.1978.tb01463.x
- Usuda, H., Fujii, H., & Nonogaki, T. (2016). Sasa veitchii extracts suppress 2,4- dinitrofluorobenzene-induced contact hypersensitivity in mice. Food and Agricultural Immunology, 27(4), 523–534. doi: 10.1080/09540105.2015.1129597
- Wang, L., Yen, J.-H., Liang, H.-L., & Wu, M.-J. (2003). Antioxidant effect of methanol extracts from lotus plumule and blossom (Nelumbo nucifera Gertn.). Journal of Food and Drug Analysis, 11(1), 60–66.
- Wedi, B., Novacovic, V., Koerner, M., & Kapp, A. (1999). Chronic urticaria serum induces histamine release, leukotriene production, and basophil CD63 surface expression-Inhibitory effects of anti-inflammatory drugs. Journal of Allergy and Clinical Immunology, 105(3), 552–560. doi: 10.1067/mai.2000.104939
- Yan, S.-L., Wang, Q.-Z., & Peng, G.-H. (2009). Determination of catechin in lotus rhizomes by high-performance liquid chromatography. International Journal of Food Sciences and Nutrition, 60(5), 432–438. doi: 10.1080/09637480701780062
- Zhang, Z., Zheng, W., Xie, H., Chai, R., Wang, J., Zhang, H., & He, S. (2017). Up-regulated expression of substance P in CD8+ T cells and NK1R on monocytes of atopic dermatitis. Journal of Translational Medicine, 15(1), 93. doi: 10.1186/s12967-017-1196-6