ABSTRACT
Carbofuran is a highly toxic insecticide and has been banned or restricted for pest control in many countries. Being a potent acetylcholinesterase inhibitor, carbofuran and its metabolite 3-hydroxycarbofuran (3-OH-CBF) are required for the analysis of their residues in food. However, an immunochemical method for simultaneous analysis of carbofuran and 3-OH-CBF is still not available. Herein, we report an enzyme-linked immunosorbent assay (ELISA) based on a broad-specific monoclonal antibody (mAb) for simultaneous detection of carbofuran and 3-OH-CBF. The mAb, designated as 2E3, against both carbofuran and 3-OH-CBF was used to develop an indirect competitive ELISA (icELISA) with 50% inhibition concentrations of 0.76 and 0.69 ng/mL, respectively. The developed icELISA was validated by UPLC-MS/MS and is suitable for the rapid and simultaneous detection of carbofuran and 3-OH-CBF in fruits and vegetables.
Introduction
Carbofuran (CBF), a N-methyl carbamate insecticide, has been widely employed to control insects and nematodes in many crops such as rice, maize, and potatoes(Tomlin, Citation1997). As the potent acetylcholinesterase inhibitors, both CBF and one of its key metabolites 3-hydroxy-carbofuran (3-OH-CBF) had posed health hazards to human beings and non-target organisms (Ogada, Citation2014; Otieno, Lalah, Virani, Jondiko, & Schramm, Citation2010). CBF and 3-OH-CBF suppress the activity of acetylcholinesterase, causing overaccumulation of acetylcholine at neuromuscular junctions, leading to grievous symptoms like nausea, vomiting, diarrhoea, and dyspnoea (Goncalves et al., Citation2017; Gupta, Citation1994; Otieno et al., Citation2010). In addition, due to cheap price, high efficiency and long-lasting insecticidal effect, CBF had been reported that illegally added as a hidden ingredient in other types of pesticide preparations (Qiao et al., Citation2015), which tremendously increases the risk of veneration. Even if CBF has been banned or restricted to use in many countries, the worldwide market for CBF has been estimated to be 130 million dollars in 2019, and China is the largest supplier and consumption place of CBF(“Carbofuran Market Insights with Statistics and Growth Prediction 2019–2024,” Citation2019). Maximum residue limits (MRLs) for CBF (sum of CBF and 3-OH-CBF) on wheat, potato, vegetables (without potato), fruits and tea in China (2016) were proposed as 0.05, 0.1, 0.02, 0.02, and 0.05 mg/kg, respectively (NHFPC, Citation2016). While the MRLs in EU (2015) for CBF differs from the Chinese MRLs, such as the EU MRLs on apples, potato, lettuce, and citrus fruit are 0.001, 0.001, 0.002, and 0.01 mg/kg (EFSA, Citation2014). In view of the hazard of CBF and 3-OH-CBF to human beings and non-target organisms, a rapid, sensitive and accurate assay should be developed for simultaneous detection and quantification of both CBF and 3-OH-CBF residues.
Analytical techniques for the determination of CBF have been developed, such as gas chromatography (GC) (Zhang et al., Citation2016), GC-mass spectrometry (GC-MS) (da Silva, Oliveira, Augusti, & Faria, Citation2018), high perform liquid chromatography (HPLC) (Vera-Avila et al., Citation2012), liquid chromatography-mass spectrometry (LC-MS) (Song et al., Citation2018). These methods are reliable and precise but time-consuming, require complicated pretreatment procedures, especially the related instruments are extremely sophisticated and expensive. Immunoassays are simple, cost-effective, sensitive, and selective, which are suitable for fast determination of a large number of samples in the field. Several immunological methods based on antibody–antigen interaction have been applied for the determination of CBF, including enzyme-linked immunosorbent assay (ELISA) (Abad, Moreno, & Montoya, Citation1999; Yao, Liu, Song, Kuang, & Xu, Citation2017), fluorescence immunoassay (Gui, Jin, Sun, Guo, & Zhu, Citation2009; Yang et al., Citation2015), immunosensor (Liu et al., Citation2015; Sun, Zhu, & Wang, Citation2012), chemiluminescent immunoassay (Jin et al., Citation2013), and immunochromatography assay (Guo, Liu, Gui, & Zhu, Citation2009; Zhou et al., Citation2004). As a CBF metabolite, 3-OH-CBF extents poisoning duration after CBF application and presents the equal risk as the parent CBF to human and non-target organisms (Goncalves et al., Citation2017). While among those analytical methods mentioned above, simultaneous determination of CBF and 3-OH-CBF was only achieved by chromatography-based assays (Ogawa et al., Citation2006; Petropoulou, Tsarbopoulos, & Siskos, Citation2006; Silva, Aquino, Dórea, & Navickiene, Citation2008; Soler et al., Citation2007). Compared with instrumentally analytical methods, multianalyte immunoassay based on broad-specific monoclonal antibodies (mAbs) is more efficient and convenient with advantage of detecting two or more target pesticides in one single test (Cliquet, Goddeeris, Okerman, & Cox, Citation2007; Franek, Diblikova, Cernoch, Vass, & Hruska, Citation2006; Guo et al., Citation2009; Jin, Guo, Wang, Wu, & Zhu, Citation2009; Kato et al., Citation2007; Pagkali et al., Citation2018). Due to sharing the similar molecular structures, CBF and 3-OH-CBF possess the potential to be simultaneously detected by ELISA (Jiang, He, Gong, Gao, & Xu, Citation2019).
In the present study, a new hapten based on 3-OH-CBF was synthesized and coupled with keyhole limpet haemocyanin (KLH) as immunogen. After immunization and cell fusion, a hydridoma cell line secreting highly sensitive and broad-specific mAb against CBF and 3-OH-CBF was selected. The obtained mAb was then used to develop icELISA for the simultaneous detection of CBF and 3-OH-CBF in fruits and vegetables.
Materials and methods
Reagents
CBF, 3-OH-CBF, ovalbumin (OVA), KLH, 4-dimethylaminopyridine (DMAP), succinic anhydride, anhydrous N, N-dimethylformamide (DMF), gold nanoparticles (NPs), N-hydroxysuccinimide (NHS), N, N-dicyclohexylcarbodiimide (DCC), complete Freund's adjuvant (CFA), incomplete Freund's adjuvant (IFA), 3, 3′, 5, 5′-tetramethylbenzidine (TMB), dimethyl sulfoxide (DMSO), 8-azaguanine, hypoxanthine-aminopterin-thymidine (HAT), hypoxanthine-thymidine (HT), QuEChERS dispersive SPE tubes were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Dulbecco's Modified Eagle's Media (DMEM) and foetal calf serum were purchased from Gibco BRL (Carlsbad, CA, USA). Goat anti-mouse IgG conjugated with horseradish peroxidase (IgG-HRP) was purchased from Jackson Immunoresearch Laboratories (West Grove, PA, USA).
Hapten synthesis and protein conjugate preparation
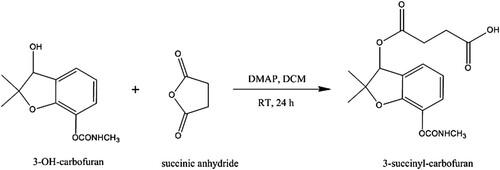
The chemical synthesis procedure of the hapten was shown in . In brief, 3-OH-CBF (23.7 mg, 0.1 mmol) was dissolved in 2 mL dichloromethane (DCM), following by the addition of succinic anhydride (20 mg, 0.2 mmol) and DMAP (12.2 mg, 0.1 mmol). The succinylation reaction was incubated for 24 h with stirring at room temperature (RT). The product was extracted twice with ethyl acetate and purified by silica gel chromatography using a solvent system of DCM: methanol (9: 1). The final hapten (24.2 mg) was obtained and characterized by LC/MS (M + H: 338.2) and 1H NMR (600 MHz, CDCl3) δ 7.20 (d, J = 7.5 Hz, 1H), 7.10 (d, J = 8.0 Hz, 1H), 6.87 (t, J = 7.8 Hz, 1H), 5.95 (s, 1H), 5.08 (d, J = 4.3 Hz, 1H), 3.71 (s, 1H), 2.89 (d, J = 4.9 Hz, 3H), 2.75–2.55 (m, 2H), 1.48 (s, 3H), 1.43 (s, 3H).
The hapten was covalently conjugated to protein carriers with the N-hydroxysuccinimide ester method. Typically, 8 mg hapten was added to 1.1-fold molar excess of NHS and DCC in 0.4 mL DMF. The mixture was stirred overnight at RT and centrifuged to discard the precipitate. Half volume of the supernatant was dropwise added to 9 mg of KLH or 12 mg of OVA in 2 mL coating buffer with stirring (Coating buffer: 14.2 mM Na2CO3 and 35.8 mM NaHCO3, pH 9.6). The reaction solution was magnetically stirred overnight at RT and then dialysed at 4°C against 3 L of PBS (1.5 mM KH2PO4, 8.3 mM Na2HPO4 12H2O and 154 mM NaCl, pH 7.5) for 3 days with 3 times of changes of dialysate each day.
Preparation of broad-specific monoclonal antibody
The protocols of immunization and cell fusion were performed as described earlier (Lan et al., Citation2019). Firstly, 0.5 ml of 1 mg/mL hapten-KLH as immunogen was emulsified with 0.5 mL complete Freund's adjuvant and injected intraperitoneally into five BalB/c mice (0.2 mL for each). Then, injection was subcutaneously performed at two-week intervals using hapten-KLH emulsified with incomplete Freund's adjuvant. The week after the forth immunization, antiserum from each mouse was collected and tested by icELISA. The mouse that showed the best titres and specificity was given a booster immunization with 0.1 mg immunogen in four days before cell fusion. Hybridomas were prepared by fusion with the spleen cells and myeloma cells Sp2/0 at the ratio of 10: 1 using PEG2000. After seven days in culture, the positive cell strains secreting antibodies with the greatest titre and sensitivity were screened by icELISA, and cloned by limiting dilution method. Purposefully, in the process of competition, CBF and 3-OH-CBF were parallelly applied to screen the monoclonal cell lines that recognizing both compounds equally. The monoclonal cells, designated as 2E3, were injected into mice for ascites production. The monoclonal antibody was purified with saturated ammonium sulfate followed by protein A + G column, and freeze-dried for further use after dialysed against PBS for 3 days with 2 times of changes of dialysate each day.
icELISA
The protocol was performed according to a previous report with some modifications (Lan et al., Citation2019; Mukunzi, Suryoprabowo, Song, Liu, & Kuang, Citation2018). Briefly, the microplate was coated with 200 μL hapten-OVA diluted in coating buffer at 37°C for 3 h. The plate was then washed with PBST (0.1% (v/v) Tween-20 diluted in PBS) for 3 times, and blocked by adding non-fat milk/PBS for 30 min. After washed with PBST, 100 μL of the analytes or standard in PBSTG (0.1% (w/v) gelatin in PBST) was added into plate followed by the addition of 100 μL mAb diluted in PBSTG. The mixture was allowed to incubate for 1 h. The plate was washed and further incubated with 200 μL goat anti-mouse IgG-HRP diluted in PBSTG for 30 min. Colour reaction was achieved by loading 200 μL of TMB substrate solution for 20 min and stopped by the addition of 100 μL 2M H2SO4. The absorbance was read at 450 nm with microplate reader. The working range was calculated by fitting 10%-90% B/B0 to logistic equation and the OD of limit of detection (LOD) was defined as B0 −3 × SD (B0).
Cross-reactivity (CR)
The mAb cross-reactivities with structurally similar analytes were identified based on the half-maximum inhibitory concentration (IC50), and calculated in accordance with the following formula: CR = [IC50 (CBF)/IC50 (analyte)] × 100%.
Sample fortification and recovery
Fruit and vegetable samples were purchased from local market and pre-powdered with liquid nitrogen. Total 5 g of samples were weighed, transferred to glass vials, and fortified with different concentrations of CBF or 3-OH-CBF. The samples were then homogenized with 10 mL acetonitrile. The mixtures were ultrasonically extracted for 20 min and centrifuged at 5000 rpm for 10 min. The supernant was then extracted with saturated sodium chloride solution. The organic phase was then applied to QuEChERS dispersive SPE tube, and centrifuged at 12,000 rpm for 5 min. The supernant was transferred to evaporate with nitrogen, dissolved in PBSTG and subjected into icELISA, or dissolved in acetone for UPLC-MS/MS analysis.
Validation by UPLC-MS/MS
The results of icELISA were validated by UPLC-MS/MS according to the previous study (Zhou, Guan, Gao, Lv, & Ge, Citation2018). The UPLC-MS/MS system with Nexera X2 (Shimadzu, Japan) and SCIEX QTRAP 5500 (Applied Biosystems, USA) equipped with an electrospray ionization source in the positive mode was applied. A C18 column (Shim-pack XR-ODS, 100 mm × 2.0 mm, 2.2 μm, Japan) was used for separation. Data acquired were then analysed using Analyst software (AB SCIEX, Foster, CA, USA).
Results and discussion
Synthesis and identification of hapten
The structural characterization makes directly chemical modification of CBF extremely hard. In the previous studies, the phenolic precursor of CBF was employed to induce a carboxylic acid group, however, the obatained hapten cannot induce a broad-specific antibody (Abad et al., Citation1999; Yao et al., Citation2017). The compound 3-OH-CBF shares the main molecular structure with CBF, its hydroxyl group were used to produce a carboxyl-available hapten (). The process of synthesis is mild and easy. The MS and NMR data demonstrated the success of hapten synthesis.
Characterization of the mAb
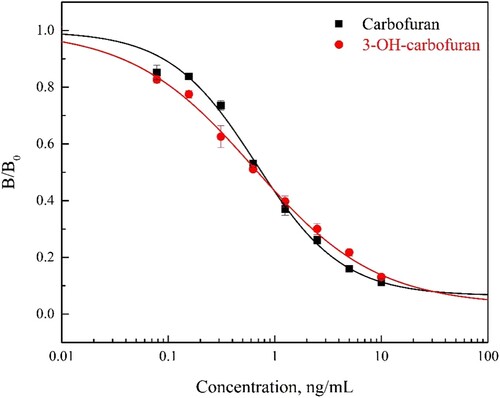
Seven days after cell fusion, icELISA was performed to screen all hybridomas. Hybridoma cells were checked at the same time using 5 ng/mL CBF and 3-OH-CBF, respectively, which gave the possibility that screening out the monoclonal hybridoma cells secreting broad-specific mAbs. As shown in , cell lines showed different sensitivity and inhibition of CBF and 3-OH-CBF. Among them, the hybridoma cell line 2E3 showing the most sensitive and specific inhibition for both compounds was cloned and used to produce ascites. Optimization of icELISA parameters was performed for CBF determination, the combination of 0.5 μg/mL coating antigen and 0.1 μg/mL mAb gave the best result with half-maximum inhibition concentration (IC50) of 0.76 ng/mL, working range of 0.09–13.6 ng/mL and limit of detection (LOD) of 0.03 ng/ml. Meanwhile, the IC50 against 3-OH-CBF was 0.69 ng/μL with working range of 0.04–18.9 ng/mL, and LOD was 0.01 ng/mL (). When compared with the existed mAbs in different immunoassay formats, the mAb we produced showed much higher sensitivity (Abad et al., Citation1999; Jin et al., Citation2013; Jin et al., Citation2009; Moreno et al., Citation2001).
Table 1. Hybridoma cell lines that can recognize both carbofuran and 3-OH-carbofuran.
The specificity of mAb2E3 was determined with different analogs. As shown in , mAb2E3 showed the greatest affinity for both CBF and 3-OH-CBF, and less or no cross-reactivity with other analogs such as carbofuran-phenol, carbosulfan, benfuracarb, bendiocarb, carbaryl. Besides, there is no cross-reactivity between mAb and pesticides with potentially positive addition of CBF including omethoate, isocarbophos, and acephate. In the previous reports, all of the mAbs exhibited simple recognition against CBF with less or no cross-reactivity with 3-OH-CBF (less than 25.2%) (Abad et al., Citation1999; Moreno et al., Citation2001; Yang et al., Citation2015; Yao et al., Citation2017), while mAb2E3 showed the cross-reactivity of 110.1% for 3-OH-CBF, possessed its broad-specific ability of detection for both CBF and 3-OH-CBF.
Table 2. Cross reactivities of carbofuran, 3-OH-carbofuran, and related analytes to the mAb2E3 in the icELISA.
Samples determined by icELISA
Fruit and vegetable samples were fortified with different concentrations of CBF or 3-OH-CBF. Residues were recovered with acetonitrile, evaporated with nitrogen, resolved in PBSTG, and then subjected into icELISA. The recoveries of the icELISA were determined as shown in . The recovery rates varied from 83.3% to 104.8% and 84.7% to 105.9% for CBF and 3-OH-CBF, respectively. The developed icELISA was validated by UPLC-MS/MS which gave a good correlation. For long bean and mango samples, the field trials were carried out using CBF at the concentration of 30 g/L with spraying, while for chives and watermelon samples, the field trails were performed at the concentration of 3 kg/ha with furrow application. Samples were collected at 1, 7, and 14 d after treatment. Other real samples were purchased from local market. As the results are shown in , due to degradation of CBF, the icELISA based on the broad-specific mAb could recognize the residues of both CBF and 3-OH-CBF. Compared with immunoassay reported previously, the developed icELISA could detect more CBF residues (sum of CBF and 3-OH-CBF), which would be more robust and versatile to reduce the residue risk of CBF and 3-OH-CBF.
Table 3. Average recoveries of carbofuran or 3-OH-carbofuran spiked in long bean, chives, mango and watermelon samples.
Table 4. CBF and 3-OH-CBF in real samples determined by icELISA and UPLC-MS/MS.
Conclusion
In the present work, a new hapten derived from 3-OH-CBF was synthesized and coupled with the carrier protein KLH as immunogen. A highly sensitive and broad-specific mAb against CBF and 3-OH-CBF was obtained and used in immunoassays for their synchronous measurement. The results determined by icELISA correlated well with UPLC-MS/MS in spiked or real samples. The developed broad-specific mAb-based icELISA was highly sensitive and could accurately detect both CBF and 3-OH-CBF, which is more suitable for high-throughput determination in fruits and vegetables to reduce the residue risk.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abad, A., Moreno, M. J., & Montoya, A. (1999). Development of monoclonal antibody-based immunoassays to the N-methylcarbamate pesticide carbofuran. Journal of Agricultural and Food Chemistry, 47(6), 2475–2485. doi: 10.1021/jf981184s
- Carbofuran Market Insights with Statistics and Growth Prediction 2019 to 2024. (2019). Retrieved from https://themarketresearchnews.com/2019/04/08/carbofuran-market-insights-with-statistics-and-growth-prediction-2019-to-2024/.
- Cliquet, P., Goddeeris, B., Okerman, L., & Cox, E. (2007). Production of penicillin-specific polyclonal antibodies for a group-specific screening ELISA. Food and Agricultural Immunology, 18(3–4), 237–252. doi: 10.1080/09540100701802908
- da Silva, M. C., Oliveira, M. L. G., Augusti, R., & Faria, A. F. (2018). Simultaneous extraction of pesticides and polycyclic aromatic hydrocarbons in Brazilian cachaça using a modified QuEChERS method followed by gas chromatography coupled to tandem mass spectrometry quantification. Journal of Agricultural and Food Chemistry, 67(1), 399–405. doi: 10.1021/acs.jafc.8b04682
- EFSA. (2014). Reasoned opinion on the review of the existing MRLs for carbofuran, carbosulfan, benfuracarb and furathiocarb and the setting of an import tolerance for carbofuran in cultivated mushrooms. EFSA Journal, 12(2), 3559. doi: 10.2903/j.efsa.2014.3559
- Franek, M., Diblikova, I., Cernoch, I., Vass, M., & Hruska, K. (2006). Broad-specificity immunoassays for sulfonamide detection: Immunochemical strategy for generic antibodies and competitors. Analytical Chemistry, 78(5), 1559–1567. doi: 10.1021/ac0514422
- Goncalves, V. J., Hazarbassanov, N. Q., de Siqueira, A., Florio, J. C., Ciscato, C. H. P., Maiorka, P. C., et al. (2017). Development and validation of carbofuran and 3-hydroxycarbofuran analysis by high-pressure liquid chromatography with diode array detector (HPLC-DAD) for forensic veterinary medicine. Journal Chromatography B, 1065-1066, 8–13. doi: 10.1016/j.jchromb.2017.09.021
- Gui, W., Jin, M., Sun, L., Guo, Y., & Zhu, G. (2009). Residues determination of carbofuran in vegetables based on sensitive time-resolved fluorescence immunoassay. Food and Agricultural Immunology, 20(1), 49–56. doi: 10.1080/09540100802702221
- Guo, Y. R., Liu, S. Y., Gui, W. J., & Zhu, G. N. (2009). Gold immunochromatographic assay for simultaneous detection of carbofuran and triazophos in water samples. Analytical Biochemistry, 389(1), 32–39. doi: 10.1016/j.ab.2009.03.020
- Gupta, R. C. (1994). Carbofuran toxicity. Journal of Toxicology and Environmental Health, 43(4), 383–418. doi: 10.1080/15287399409531931
- Jiang, M., He, J., Gong, J., Gao, H., & Xu, Z. (2019). Development of a quantum dot-labelled biomimetic fluorescence immunoassay for the simultaneous determination of three organophosphorus pesticide residues in agricultural products. Food and Agricultural Immunology, 30(1), 248–261. doi: 10.1080/09540105.2019.1572714
- Jin, R. Y., Guo, Y. R., Wang, C. M., Wu, J. X., & Zhu, G. N. (2009). Development of a bispecific monoclonal antibody to pesticide carbofuran and triazophos using hybrid hybridomas. Journal of Food Science, 74(1), T1–T6. doi: 10.1111/j.1750-3841.2008.01002.x
- Jin, M., Zhu, G., Jin, R., Liu, S., Shao, H., Jin, F., et al. (2013). A sensitive chemiluminescent enzyme immunoassay for carbofuran residue in vegetable, fruit and environmental samples. Food and Agricultural Immunology, 24(3), 345–356. doi: 10.1080/09540105.2012.694096
- Kato, M., Ihara, Y., Nakata, E., Miyazawa, M., Sasaki, M., Kodaira, T., et al. (2007). Development of enrofloxacin ELISA using a monoclonal antibody tolerating an organic solvent with broad cross-reactivity to other newquinolones. Food and Agricultural Immunology, 18(3-4), 179–187. doi: 10.1080/09540100701763365
- Lan, J., Zhao, H., Jin, X., Guan, H., Song, Y., Fan, Y., et al. (2019). Development of a monoclonal antibody-based immunoaffinity chromatography and a sensitive immunoassay for detection of spinosyn A in milk, fruits, and vegetables. Food Control, 95, 196–205. doi: 10.1016/j.foodcont.2018.08.002
- Liu, L., Xu, D., Hu, Y., Liu, S., Wei, H., Zheng, J., et al. (2015). Construction of an impedimetric immunosensor for label-free detecting carbofuran residual in agricultural and environmental samples. Food Control, 53, 72–80. doi: 10.1016/j.foodcont.2015.01.009
- Moreno, M. J., Abad, A., Pelegri, R., Marinez, M. J., Saez, A., Gamon, M., et al. (2001). Validation of a monoclonal enzyme immunoassay for the determination of carbofuran in fruits and vegetables. Journal of Agricultural and Food Chemistry, 49(4), 1713–1719. doi: 10.1021/jf001171q
- Mukunzi, D., Suryoprabowo, S., Song, S., Liu, L., & Kuang, H. (2018). Development of an indirect enzyme-linked immunosorbent assay and lateral-flow test strips for pefloxacin and its analogues in chicken muscle samples. Food and Agricultural Immunology, 29(1), 484–497. doi: 10.1080/09540105.2017.1406460
- National food safety standards. (2016). GB2763-2016 maximum residue limits for pesticides in food and additional 106 national food safety standards on testing method of pesticide residue in foods. Retrieved from http://www.nhc.gov.cn/sps/s7891/201702/ed7b47492d7a42359f839daf3f70eb4b.shtml.
- Ogada, D. L. (2014). The power of poison: Pesticide poisoning of Africa’s wildlife. Annals of the New York Academy of Sciences, 1322(1), 1–20. doi: 10.1111/nyas.12405
- Ogawa, S., Brito, N. M., Silva, M. R. S., Ribeiro, M. L., Leite, L. A., Dórea, H. S., et al. (2006). Determination of carbofuran and 3-hydroxycarbofuran residues in coconut water by solid-phase extraction and liquid chromatography with UV detection. Journal of Liquid Chromatography & Related Technologies, 29(12), 1833–1841. doi: 10.1080/10826070600717064
- Otieno, P. O., Lalah, J. O., Virani, M., Jondiko, I. O., & Schramm, K. W. (2010). Carbofuran and its toxic metabolites provide forensic evidence for Furadan exposure in vultures (Gyps africanus) in Kenya. Bulletin of Environmental Contamination and Toxicology, 84(5), 536–544. doi: 10.1007/s00128-010-9956-5
- Pagkali, V., Petrou, P. S., Makarona, E., Peters, J., Haasnoot, W., Jobst, G., et al. (2018). Simultaneous determination of aflatoxin B1, fumonisin B1 and deoxynivalenol in beer samples with a label-free monolithically integrated optoelectronic biosensor. Journal of Hazardous Materials, 359, 445–453. doi: 10.1016/j.jhazmat.2018.07.080
- Petropoulou, S.-S. E., Tsarbopoulos, A., & Siskos, P. A. (2006). Determination of carbofuran, carbaryl and their main metabolites in plasma samples of agricultural populations using gas chromatography–tandem mass spectrometry. Analytical and Bioanalytical Chemistry, 385(8), 1444–1456. doi: 10.1007/s00216-006-0569-0
- Qiao, C., Huang, Y., Luo, J., Wang, C., Fang, J., & Hanzhong, X. (2015). Determination of 30 hidden ingredients in pesticide products by HPLC-MS/MS. Agrochemicals, 54(5), 340–342.
- Silva, M. G. D., Aquino, A., Dórea, H. S., & Navickiene, S. (2008). Simultaneous determination of eight pesticide residues in coconut using MSPD and GC/MS. Talanta, 76(3), 680–684. doi: 10.1016/j.talanta.2008.04.018
- Soler, C., Hamilton, B., Furey, A., James, K. J., Mañes, J., & Picó, Y. (2007). Liquid chromatography quadrupole time-of-flight mass spectrometry analysis of carbosulfan, carbofuran, 3-hydroxycarbofuran, and other metabolites in food. Analytical Chemistry, 79(4), 1492–1501. doi: 10.1021/ac060709+
- Song, S., Zhu, K., Han, L., Sapozhnikova, Y., Zhang, Z., & Yao, W. (2018). Residue analysis of 60 pesticides in red swamp crayfish using QuEChERS with high-performance liquid chromatography–tandem mass spectrometry. Journal of Agricultural and Food Chemistry, 66(20), 5031–5038. doi: 10.1021/acs.jafc.7b05339
- Sun, X., Zhu, Y., & Wang, X. (2012). Amperometric immunosensor based on deposited gold nanocrystals/4,4′-thiobisbenzenethiol for determination of carbofuran. Food Control, 28(1), 184–191. doi: 10.1016/j.foodcont.2012.04.027
- Tomlin, C. (1997). The Pesticide Manual: A world compendium 11th ed (1011–1013). Farrham: BCPC.
- Vera-Avila, L. E., Márquez-Lira, B. P., Villanueva, M., Covarrubias, R., Zelada, G., & Thibert, V. (2012). Determination of carbofuran in surface water and biological tissue by sol–gel immunoaffinity extraction and on-line preconcentration/HPLC/UV analysis. Talanta, 88, 553–560. doi: 10.1016/j.talanta.2011.11.032
- Yang, J., Zhang, Y., Wang, H., Xu, Z., Eremin, S. A., Shen, Y., et al. (2015). Development of fluorescence polarisation immunoassay for carbofuran in food and environmental water samples. Food and Agricultural Immunology, 26(3), 340–355. doi: 10.1080/09540105.2014.914890
- Yao, L., Liu, L., Song, S., Kuang, H., & Xu, C. (2017). Development of indirect competitive enzyme-linked immunosorbent and immunochromatographic strip assays for carbofuran detection in fruits and vegetables. Food and Agricultural Immunology, 28(4), 639–651. doi: 10.1080/09540105.2017.1309359
- Zhang, C. P., He, H. M., Yu, J. Z., Hu, X. Q., Zhu, Y. H., & Wang, Q. (2016). Residues of carbosulfan and its metabolites carbofuran and 3-hydroxy carbofuran in rice field ecosystem in China. Journal of Environmental Science and Health, Part B, 51(6), 351–357. doi: 10.1080/03601234.2015.1120606
- Zhou, P., Lu, Y., Zhu, J., Hong, J., Li, B., Zhou, J., et al. (2004). Nanocolloidal gold-based immunoassay for the detection of the N-methylcarbamate pesticide carbofuran. Journal of Agricultural and Food Chemistry, 52(14), 4355–4359. doi: 10.1021/jf0499121
- Zhou, Y., Guan, J., Gao, W., Lv, S., & Ge, M. (2018). Quantification and confirmation of fifteen carbamate pesticide residues by multiple reaction monitoring and enhanced product ion scan modes via LC-MS/MS QTRAP system. Molecules, 23(10), 2496. doi: 10.3390/molecules23102496