ABSTRACT
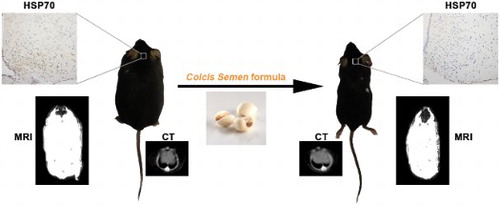
Obesity is a worldwide public health issue, however, efficient strategies to treat obesity without systemic damage is still in serious lack. Herein, we proved that a significant decrease in body weight and abdominal circumference was observed when monosodium glutamate (MSG)-induced obese mice were orally treated with Coicis Semen formula. Consistently, computed tomography and magnetic resonance imaging results showed that both whole-body fat content and subcutaneous fat thickness were lowered, which was further confirmed by hematoxylin–eosin staining. In addition, the weight ratios of adipose tissues and liver to the body weight were reduced, due to the decreased lipid accumulation. The increased triacylglycerol, total cholesterol, and inflammatory cytokines could also be downregulated after the treatment. Furthermore, the neuronal injury marker and inflammatory cytokines in the hypothalamus were obviously reduced after the treatment, revealing that the protective effect of Coicis Semen formula on MSG-induced obesity was achieved by alleviating hypothalamic injury.
GRAPHICAL ABSTRACT
Introduction
According to the World Health Organization (WHO), the prevalence of obesity is increasing worldwide (Roberto et al., Citation2015), and the number of obese people has nearly tripled since 1975 (Abarca-Gómez et al., Citation2017). Obesity is closely related to various metabolic diseases, such as type 2 diabetes, hyperlipidemia, atherosclerosis, even cancer risk (Lauby-Secretan et al., Citation2016; Liu et al., Citation2018). As abdominal obesity is considered to be an important factor for the development of metabolic syndrome (Després & Lemieux, Citation2006), there is an increasing interest in the detailed study of abdominal obese subjects. At the present, drugs are mainly by suppressing appetite, reducing nutrient absorption and increasing energy consumption (Adan, Citation2013), such as orlistat, sibutramine, but some side effects limit their clinical application (Krentz, Fujioka, & Hompesch, Citation2016).
Therefore, an effective strategy to treat abdominal obesity without obvious systemic damage is in urgent need. Natural materials as medicine have been documented for hundreds of years in various traditional systems throughout the world because of their high safety. For this reason, a wide variety of natural products as well as their crude extracts and isolated compounds have been explored for the prevention and treatment of obesity (Shen, Zhang, Dong, Ren, & Chen, Citation2015; Wang et al., Citation2018), including wild ginseng (Yun, Moon, Ko, Im, & Chung, Citation2004), Cosmos caudatus Kunth leaf (Rahman et al., Citation2017), adlay seed (Choi et al., Citation2015; Kim et al., Citation2004). Among which, Coicis Semen, plays an important role in anti-obesity as well as in the prevention of some other ailments. As an herb medicine widely cultivated in China, Thailand, South Korea and India, Coicis Semen contains a large number of nutrients and is usually considered as a healthy food supplement. From the view of ethnopharmacological theory, Coicis Semen can strengthen the functions of spleen and lung, dissipate phlegm, dispel dampness and induce diuresis (Yu, Zhang, Li, Zhao, & Liu, Citation2017). So they are often reported to show activities in reducing serum hyperlipidemia, increasing the total antioxidant capacity and regulating blood glucose levels (Kim et al., Citation2004), which will eventually be beneficial to body weight loss. Hippophae Fructus, which has the ability of invigorating stomach and promoting digestion, was reported to prevent high-fat diet-induced obesity by down-regulation of adipogenic gene expression (Pichiah, Moon, Park, Moon, & Cha, Citation2012). Citri Rubrum Exocarpium was currently used to protect the stomach, reduce food accumulation and eliminate dampness (Zhao et al., Citation2017). Glycyrrhizae Radix et Rhizoma, has been mentioned in many formulas for the anti-inflammatory, immune-regulatory effects (Dastagir & Rizvi, Citation2016), and could reduce toxicity or enhance the effectiveness with other herbs (Wang et al., Citation2013). The combination of the four medicines (Coicis Semen, Hippophae Fructus, Citri Rubrum Exocarpium and Glycyrrhizae Radix et Rhizoma) was expected to enhance the functions of nourishing the stomach, alleviating edema and drying dampness.
Monosodium glutamate (MSG) is a natural constituent of human daily foods, even for infant consumption (Olney & Ho, Citation1970). However, more and more investigations revealed that MSG injection can damage hypothalamic neurons of both neonatal mice (Olney & Ho, Citation1970) and adult mice (Park et al., Citation2000), which eventually leads to obesity. MSG model is usually characterized by severe abdominal obesity (Ma, Zhang, Mou, Fu, & Chen, Citation2018). Hermanussen et al. considered that it was amino acid glutamate that determines the propensity of obesity in German, by intoxicating arcuate nucleus neurons to further disrupt the hypothalamic signaling cascade of leptin action, causing obesity and hyperleptinaemia (Hermanussen & Tresguerres, Citation2003). John W. Olney et al. clearly presented the hypothalamus lesion formation 3 h after a subcutaneous dose of MSG (Olney, Citation1969). The degree of hypothalamus damage exhibited a dose-dependent manner (Olney & Ho, Citation1970). Taking into consideration the medical functions of Coicis Semen, we wondered whether Coicis Semen formula could alleviate hypothalamic injury and further treat MSG-induced obesity in mice.
In this study, we evaluated the effects of Coicis Semen formula on obese mice induced by MSG with imaging methods. It was revealed that administration of Coicis Semen formula could notably decrease the body weight and abdominal circumference, accompanied by the decrease in thickness of subcutaneous adipose tissue and downregulation of whole-body fat distribution manifested by CT and MRI. Anatomy and histopathological tests give the same conclusion. Biochemical indicators in sera after treatment with Coicis Semen were also decreased. Further investigation revealed that the therapeutic effect of Coicis Semen formula on obesity was achieved by alleviating hypothalamic injury caused by MSG. Therefore, Coicis Semen formula has the potential to treat MSG induced-obesity.
Materials and methods
Preparation of Coicis Semen formula
Coicis Semen formula was provided by Anguo juyaotang Pharmaceutical Co., Ltd (China), and the components and content assay were shown in . The raw materials of 4 g Coicis Semen, 2 g Hippophae Fructus, 2 g Citri Rubrum Exocarpium and 1 g Glycyrrhizae Radix et Rhizoma were mixed and extracted by boiling water twice, one with 90 mL water for 1.5 h, another with 72 mL water for 1 h. Then the two extracts were combined and concentrated to obtain the formula.
Table 1. Content assay of Coicis Semen formula.
Animals
The animal experiments were approved by the Institutional Animal Care and Use Committee at Tongji Medical College, Huazhong University of Science and Technology (IACUC Number: 2170). Newborn C57BL/6J male mice were purchased from Laboratory Animal Center of the Academy of Mititary Medical Sciences (quality certification number: SCXK (E) 2017-0094). The mice daily received subcutaneous injection of saline or MSG (3 mg/g body weight, Sinopharm Chemical Reagent Co., Ltd.) from day 2–8 (Olney, Citation1969). The mice were housed at 22 ± 2°C, 55 ± 5% relative humidity, with a 12 h light–dark cycle, and access to food and water freely. After 3 months, the mice were randomly divided into five groups (n = 7): control group (saline injection), model group (MSG injection), F-low group (MSG injection + 150 mg/kg Coicis Semen formula), F-middle group (MSG injection + 300 mg/kg Coicis Semen formula) and positive control group (MSG injection + 15.6 mg/kg orlistat, Pharscin Pharma, China). F-low, F-middle and positive control groups were administrated orally for 2 months (). Control group and model group were administrated an equal volume of saline. Body weight, body length, abdominal circumference and food intake were monitored weekly. The body lengths (anal-to-nasal distance of the mice) were measured to obtain Lee index ([3 square root body weight (g)]/ body length (mm)×10) (Bernardis & Patterson, Citation1968). The body temperature was tested by a rectal probe (BAT-12 Microprobe-Thermometer; Physitemp; USA). Brown adipose tissue (BAT) temperature was measured with an E50 infrared thermal device (FLIR, USA). Two months later, all mice were fasted overnight and sacrificed. Blood samples were collected, centrifuged to obtain sera and stored at −20°C. Then the hypothalamus, liver and adipose tissues were removed, weighed, and rapidly stored at liquid nitrogen.
MRI study
At the end of the experiment, all animals were anesthetized with pentobarbital sodium (1%) and examined on a 3.0T MRI scanner (Magnetom Signa HDxt, GE Medical Systems) using a 3D six-echo IDEAL-T*2-SPGR pulse sequence (Yu et al., Citation2008). The sequence was an investigational research version of the IDEAL software. Imaging parameters were TR = 20 ms, TE spacing = 0.8 ms, first TE = 1.5–1.8 ms, flip angle = 5, receiver bandwidth=±125 kHz, 0.6 mm true(non–zero-interpolated) isotropic spatial resolution, field-of-view (FOV) = 15 cm, fractional phase FOV = 0.75, and an echo train length (ETL, number of echoes per TR) of two with fly-back unipolar readout gradients. For each TE, the number of signal averages (NSA) was set to three to further enhance image signal-to-noise ratio (Hu, Smith, Nayak, Goran, & Nagy, Citation2010). Imaging time for each set of mice was about 20 min. Water, fat, in-phase and out-of-phase data sets were generated online by the IDEAL research software.
CT study
All CT images were acquired using a CT scanner (GE Discovery CT750 HD; GE Healthcare, Milwaukee, WI, USA) in GSI mode. Images with the optimal selected monochromatic level were transferred to an AW 4.5 workstation (GSI viewer 2.00 and GE VolumeShare 4 AW 4.5, GE Healthcare). All animals were anesthetized with pentobarbital sodium (1%), and supinely positioned comfortably in animal holder, aligning its longitudinal axis transverse to the image plane. CT scanning was performed with the following parameters: tube voltage at 80 kVp, automatic mAs, slice thickness at 1.25 mm, spacing at 1.25 mm. One radiographer performed the post-processing. The range of fat was measured from −30 to −190 HU (Blitman et al., Citation2011).
Biochemical analyses
The serum levels of TG, TC, ALT and AST were detected with an automatic biochemical analyzer (Beckman, Germany). The contents of SOD and MDA in liver tissues were assayed based on the manufacturer’s instruction of the kits (Nanjing Jiancheng Bioengineering Institute). The white blood cell and neutrophil levels were tested by Animal blood cell analyzer XT-1800i (Japan).
Oil Red O staining
Liver tissues were fixed in 4% paraformaldehyde and stained with Oil-Red O and counterstained with hematoxylin. The images were taken with a light microscope (Nikon Eclipse TE2000-U, NIKON, Japan).
Histology and immunohistochemistry
Liver tissues and adipose tissues were fixed in paraformaldehyde, embedded in paraffin and sectioned of 5 μm thickness for hematoxylin and eosin staining (HE). TNF-α (60291-1-Ig, 1:1500, Proteintech, China), IL-1β (66737-1-Ig, 1:400, Proteintech, China) and HSP70 (10995-1-AP, 1:300, Proteintech, China) were stained for immunohistochemistry. The pictures were acquired using a light microscope (Nikon Eclipse TE2000-U, NIKON, Japan) and quantified with Image-Pro Plus (Media Cybernetics, USA).
Quantitative real-time PCR analysis
Total RNA of the liver was extracted using the RNAiso Plus reagent (Takara Biotechnology Co., Ltd., China). RNA was quantified by measuring OD values at 260 and 280 nm with nanodrop 2000 spectrophotomer (Thermo Scientific, USA). The cDNA was synthesized in accordance with the PrimeScript RT reagent Kit (Takara Biotechnology Co., Ltd., China) procedure and performed with SYBR Premix Ex TaqTM I and 7500 Real Time PCR System (Applied Biosystems, USA). Sequences of the primers used in the study were listed in . The relative expression levels were normalized to those of β-actin.
Table 2. Primers used for quantitative real-time PCR.
Western blot
Liver tissues were lysed in RIPA Lysis Buffer, centrifuged at 12,000 rpm, 20 min at 4 °C, and quantitated with BCA kit (P0010, Beyotime, China). Then 10 μL protein were electrophoresed on 12% SDS-acrylamide gels. Blots were incubated at 4 °C overnight with primary antibodies (1:1000) against β-actin (#4970, Cell Signaling Technology, USA), TNF-α (60291-1-Ig, Proteintech, China), and IL-1β (66737-1-Ig, Proteintech, China), respectively. Then the blots were washed three times with TBST (0.2% Tween-20 in TBS) and incubated with the secondary antibody (A0216, A0208, 1:10000, Beyotime, China) for 2 h. After washing three times with TBST, the images were obtained with ChemiDoc XRS+ (BIO-RAD, USA).
Statistical analysis
All the data were given as mean ± standard error of mean (SEM). Statistical analysis was conducted by the SPSS 20 software (SPSS Inc, Chicago, USA) and the data were tested to be normal and homogeneous. The statistical differences between the groups were evaluated by one-way ANOVA with Least Significant Difference (LSD) test. P < 0.05 was considered as statistical significance.
Results
Effects of Coicis Semen formula on MSG-induced obesity
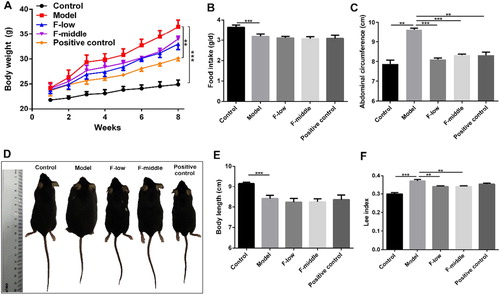
To evaluate the effects of Coicis Semen formula on MSG-induced obesity, we monitored the anthropometrical indexes of mice. Compared with control group, body weight in the model group and the other three treated groups were significantly increased at different degrees. The increasing weight of the three treated groups was not so heavy than the model group ((A)). MSG-treated mice had significantly lower food intake compared with the control group ((B)), indicating the increased body weight was not attributed to food intake. The abdominal circumference of Coicis Semen formula groups and positive control group was remarkably smaller than that of the model group ((C)). Representative pictures of mice in each group were shown in (D). It was observed that the body length of MSG-treated mice was significantly shorter than that of the control group ((E)). Consistent with these results, Lee index, an evaluation index of MSG-induced obesity (Hioki, Yoshida, Kogure, Yoshimoto, & Shimatsu, Citation2010), was dramatically reduced in formula groups compared with that in the model group ((F)). Besides, the rectal temperature and BAT temperature were also remarkably elevated after Coicis Semen formula treatment (Figure S1), which may partly be contributed to the loss of body weight. The results showed that Coicis Semen formula with either low dose (F-low group) or middle dose (F-middle group) could treat MSG-induced obesity with comparable efficacy as positive drug orlistat.
Figure 2. Coicis Semen formula treats MSG-induced obesity. (A) Body weight changes after treatment with formula; (B) Food intake of mice in each group per day; (C) Abdominal circumference of mice; (D) Representative pictures of mice in each group; (E) Body length of mice; (F) Lee index. Results are presented as mean ± SEM. **p < 0.01, ***p <0.001 compare with model group.
Effects of Coicis Semen formula on adipose tissues by imaging
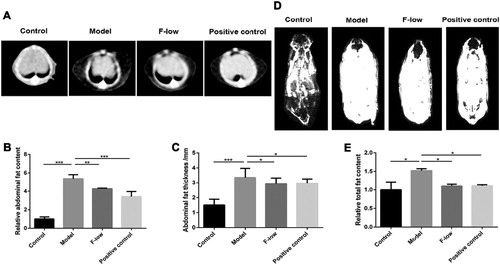
Given the above results regarding the effects of Coicis Semen formula, we selected the F-low group for the following investigation. At the beginning of the treatment with the Coicis Semen formula, the body weight and abdominal circumference of mice was evaluated. As shown in Figure S2, the body weight and abdominal circumference showed no difference in MSG-injected mice, but significantly higher than that in the control group. CT images showed that the subcutaneous adipose tissue in the abdomen of the model group was much thicker than that in the control group ((A)). After low-dose Coicis Semen formula or orlistat treatment, the thickness was obviously reduced ((A)). The quantitative analysis of fat content and thickness in the abdomen were shown in (B and C), which gave similar results. The whole body fat distribution was measured by MRI. Compared with the control group, the total fat content in the model group was relatively high, but decreased in the F-low and orlistat groups ((D and E)). Consistent with related physiological indexes, the results demonstrated that administration of Coicis Semen formula had the effect of improving obesity by imaging study.
Figure 3. Coicis Semen formula decreases fat content in mice. (A) CT imaging of abdomen; (B) Relative abdominal fat content; (C) Thickness of abdominal fat; (D) MRI imaging of whole-body; (E) Relative total fat content of whole body. Results are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p <0.001 compare with model group.
Effects of Coicis Semen formula on lipid accumulation in adipose tissues
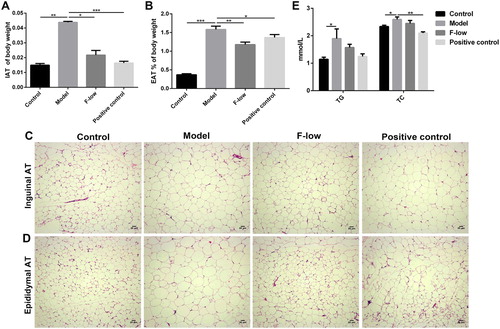
To confirm the imaging results of Coicis Semen formula treating obesity, the adipose tissue of typical fat pad such as inguen and epididymis were removed and weighed. In accordance with the body weight loss, the calculated weight ratio (adipose tissue weight to the relative body weight) demonstrated that Coicis Semen formula and orlistat could reduce this ratio in adipose tissues, respectively ((A and B)). From the corresponding H&E staining results, it can be seen that the size of adipocytes decreased to a certain extent after Coicis Semen formula or orlistat treatment ((C and D)). Besides, the increased triglyceride (TG) and total cholesterol (TC) levels in sera induced by obesity could return to the normal levels after administration of low-dose formula or orlistat ((E)). The results proved that Coicis Semen formula could decrease the fat content in adipose tissues by preventing lipid accumulation.
Figure 4. Coicis Semen formula decreases lipid accumulation in adipose tissues. (A) Ratio of inguinal adipose tissue (IAT) to body weight; (B) Percentage of epididymal adipose tissue (EAT) to body weight; HE staining of IAT (C) and EAT (D) in each group, scale bar: 20 μm. (E) Expression levels of serum TG and TC. Results are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p <0.001 compare with model group.
Effects of Coicis Semen formula on liver toxicity
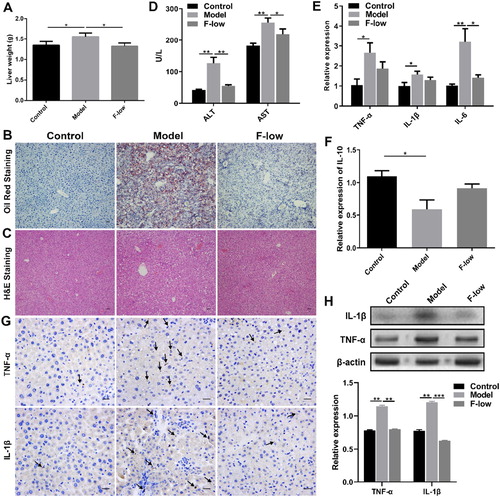
Obesity has been implicated with liver diseases, such as nonalcoholic fatty liver disease and hepatitis (Diehl, Citation2010). We examined whether Coicis Semen formula could decrease liver lipid accumulation or inflammation. Liver weight in the model group was notably higher than that in the F-low group ((A)). Histological analysis results showed more lipid droplets and fat cavitation in the model group, but less in the F-low group ((B and C)). In addition, obesity associated with chronic inflammation was also detected. The alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in sera of Coicis Semen formula treated mice were low ((D)). Compared with the model group, the white blood cell and neutrophil levels were decreased (Figure S3), pro-inflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) expression levels also decreased ((E)), while anti-inflammatory cytokine interleukin-10 (IL-10) level was increased after Coicis Semen formula treatment ((F)). Immunohistochemical staining of liver tissues also confirmed that Coicis Semen formula treatment could decrease the expression of TNF-α and IL-1β ((G)). And western blot results further verified that the expression levels of TNF-α and IL-1β were reduced in the F-low group ((H)). Moreover, we tested the liver activity related factors, the results showed that malonyldialdehyde (MDA) level was reduced, whereas superoxide dismutase (SOD) level was increased after Coicis Semen formula treatment compared to the model group (Figure S4), suggesting that Coicis Semen formula could improve liver inflammation in obesity. Therefore, the results indicated that Coicis Semen formula administration could decrease lipid deposition in the liver and mitigate liver damage induced by obesity.
Figure 5. Coicis Semen formula reduces liver toxicity. (A) Liver weight; (B) Oil Red staining of liver, scale bar: 20 μm; (C) HE staining of liver, scale bar: 20 μm; (D) Expression levels of serum ALT and AST; (E) Liver mRNA levels of TNF-α, IL-1β and IL-6; (F) Liver mRNA levels of IL-10; (G) Immunohistochemical staining of TNF-α and IL-1β in liver, scale bar: 10 μm; (H) Western blot results and quantification of the protein expression in liver. Results are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 compare with model group.
Effects of Coicis Semen formula on hypothalamic injury
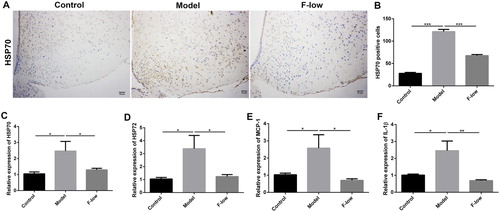
Based on these results, we wondered how Coicis Semen formula treats MSG-induced obesity. As MSG was reported to induce abdominal obesity by hypothalamic lesions, especially the arcuate nucleus injury (Olney & Ho, Citation1970), we speculated whether Coicis Semen formula could alleviate hypothalamic injury. As we expected, heat shock protein 70 (HSP70), a neuronal injury marker (Yang et al., Citation2017), was significantly increased in the arcuate nucleus region of model mice, whereas decreased in the F-low group ((A–C)). HSP72, a stress marker and protects from hypothalamic injury (Thaler et al., Citation2012), was also return back to the normal level after Coicis Semen formula treatment ((D)). Some inflammatory cytokines levels such as IL-1β and monocyte chemoattractant protein-1 (MCP-1) (Yang et al., Citation2017), caused by hypothalamic injury were correspondingly decreased after Coicis Semen formula treatment ((E and F)). These results suggested that Coicis Semen formula could alleviate hypothalamic injury, which was beneficial to treating MSG-induced obesity.
Figure 6. Coicis Semen formula relieves hypothalamic injury. (A) Immunohistochemical staining of HSP70 in hypothalamus, scale bar: 20 μm; (B) Quantification of HSP70 protein expression; Hypothalamic mRNA levels of HSP70 (C), HSP72 (D), IL-1β (E) and MCP-1 (F). Results are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p <0.001 compare with model group.
Discussion
Abdominal obesity, which is the main type of obesity among Chinese people, mainly refers to fat deposition especially in the abdominal cavity, manifesting an increase in abdominal circumference. Abdominal obesity is considered important for the development of metabolic syndrome (Després & Lemieux, Citation2006). Exercise and taking drugs are the two main methods for obese people to lose weight. Mostly, once the exercise stops, it is likely to gain weight again. And drugs often bring in the risk of side effects (Krentz et al., Citation2016). Therefore, several traditional Chinese medicines have been widely studied. The Coicis Semen formula in this study contained Coicis Semen, Hippophae Fructus, Citri Rubrum Exocarpium and Glycyrrhizae Radix et Rhizoma. This formula is hypothesized to have potential treatment for MSG-induced obesity. MSG-injection in neonatal mice can promote hypothalamic lesion in the arcuate nucleus which will induce obesity, specifically showing with a relatively bigger abdominal circumference. So in this study, MSG-induced obesity model is used to evaluate the effects of Coicis Semen formula on weight loss of mice.
When neonatal mice received subcutaneous injection of MSG from day 2 to day 8, they were obviously fatter than control mice without MSG administration after 3-month housing, manifesting with relatively larger abdominal circumference and shorter body length, which was consistent with the study of Olney (Olney, Citation1969). Study also showed that high doses of glutamate reduced growth in piglets (Li et al., Citation2018), suggesting glutamate has an adverse effect on mammalian growth. Once these obese mice were treated with Coicis Semen formula or positive drug orlistat, the increase of body weight was slowed down in the following 8 weeks, but still higher than that in the control group. On the contrary, food intake for MSG-treated mice was significantly lower than the control group, indicating that it is MSG which causes increased body weight not food intake. Compared with the model group, the abdominal circumference of mice in Coicis Semen formula or orlistat treated groups was dramatically decreased, although their body length showed no obvious change. According to the Lee index, we could obtain a conclusion that Coicis Semen formula had great potential for treating MSG-induced obesity even with a better effect than orlistat. It should be noticed that either Coicis Semen formula or orlistat treatment did not change the daily food intake for obese mice, demonstrating that food intake was not the reason for the decrease of abdominal circumference.
Body mass index (BMI) was accepted for measuring obesity in clinic (Machann, Horstmann, Born, Hesse, & Hirsch, Citation2013). However, it only refers to the height factor, and cannot reflect the distribution of fat in the body. The recommendation of abdominal circumference rather than BMI was recognized as important for abdominal obesity (Després & Lemieux, Citation2006). Our result also showed abdominal circumference was prominent in abdominal obese mice, and agreed with the report of waist to height ratio (WHtR) as a better indicator for abdominal obesity (Amato et al., Citation2010), whereas these indexes cannot distinguish between subcutaneous adipose tissue (SCAT) and visceral adipose tissue (VAT). With the rapid development of imaging technology, various methods can accurately evaluate fat content and distribution without invasiveness. In the study, we combined CT and MRI to estimate the thickness and content of abdominal subcutaneous adipose tissue as well as the whole-body adipose distribution. The results of CT imaging in (A) visually reflected that Coicis Semen formula with low dose can significantly decrease the thickness of subcutaneous adipose in the abdomen of MSG-induced obesity mice. After setting and detecting the CT threshold of adipose tissue, the original image was calculated by Image J software, and qusi-quantitative results for the thickness and content of abdomen adipose were obtained. Both of which were diminished after Coicis Semen formula treatment. In addition, the whole body adipose content was also decreased indicated by MR images. Positive drug orlistat had no more effect than Coicis Semen formula. From imaging analysis, we can obtain a conclusion that Coicis Semen formula administration can decrease whole-body adipose content especially in the abdomen region, which is also called visceral fat. The results were in consistent with that from the apparent index monitoring.
These results were further verified. The weight of adipose tissue in two organs (inguen and epididymis) of obese mice was sharply reduced after Coicis Semen formula or orlistat treatment. And the size of adipocytes in both IAT and EAT was obviously decreased, which accounted for the weight loss of IAT and EAT. TC and TG in serum also had a certain degree of reduction. Taken together, Coicis Semen formula could prevent lipid accumulation in adipose tissues to decrease the fat content.
Another important organ of abdominal fat deposition is the liver. It has been shown that non-alcoholic fatty liver (NAFLD) has a great correlation with abdominal obesity (Neuschwander-Tetri, Citation2007). In obesity, adipose tissue is not only specialized in lipids storage, but also secrete pro-inflammatory cytokines, such as TNF-α, IL-6 (Després & Lemieux, Citation2006), and infiltrated macrophages (Weisberg et al., Citation2003; Yudkin, Stehouwer, Emeis, & Coppack, Citation1999). The liver weight, lipid droplet deposition as well as inflammatory cytokines expression for MSG-induced obesity mice were relatively higher than those in the control group. However, these indicators can be downregulated to the normal region by Coicis Semen formula. The decrease in lipid content and shrink in fat cavitation of liver tissue after Coicis Semen formula treatment demonstrated that the lipid accumulation in the liver could be prevented. The main side effect for most diet pills such as orlistat was hepatotoxicity (Douglas, Langham, Bhaskaran, Brauer, & Smeeth, Citation2013). Our results indicated Coicis Semen formula decreased the expression levels of TNF-α and IL-1β in the liver as well as ALT and AST levels in sera, suggesting that Coicis Semen formula could alleviate liver damage induced by MSG, rather than causing additional liver toxicity.
Previous studies have shown that acute neuronal necrosis occurred in the infant mouse brain after subcutaneously injected with MSG (Olney & Ho, Citation1970). And the arcuate nucleus of the hypothalamus is a vulnerable area (Olney, Citation1969). Therefore, MSG injection promotes neuronal necrosis in the arcuate nucleus and further induces gradual increment of body weight over time. As we mentioned above, MSG injection will not increase food intake of mice. Thus, we believe the body weight increase for model mice is due to lower energy expenditure. The rectal temperature and BAT temperature were measured. Compared with control groups, both rectal and BAT temperature of mice in model groups were dramatically decreased, indirectly indicating a lower energy expenditure. After Coicis Semen formula treating, rectal and BAT temperature were obviously elevated. Rectal temperature is corresponding to core body temperature (Meyer, Ootsuka, & Romanovsky, Citation2017), and based on our previous study (Zhao, Zhu, Cong, Yang, & Zhu, Citation2018), we speculated that energy expenditure was higher in Coicis semen formula-treated mice than that of the model mice.
To further clarify the mechanism of Coicis Semen formula on treating MSG-induced obesity, neuronal injury markers (HSP70 and HSP72) and corresponding inflammatory cytokines (IL-1β and MCP-1) in the hypothalamus were tested. HSP70 positive cells in the model group were significantly higher than the control group, whereas reduced in the F-low group. The mRNA levels of HSP70 and HSP72 also returned back to the normal region after Coicis Semen formula treatment, as well as the related inflammatory cytokines levels such as IL-1β and MCP-1. These results suggested that Coicis Semen formula could reduce hypothalamic injury to treat MSG-induced obesity.
Conclusion
This study has been proved that Coicis Semen formula has great potential in treating MSG-induced obesity. Our results showed that treatment with Coicis Semen formula significantly decreased body weight and abdominal circumference of obese mice, accompanied with fat decrease in whole-body especially in waist abdomen, which was noninvasively observed by CT and MRI. Histopathological analysis gave similar results and further revealed that Coicis Semen formula could prevent the accumulation of lipid droplets in inguen and epididymis. As the same in the liver, lipid droplets accumulation in livers were alleviated after Coicis Semen formula treatment. Besides, the expression levels of TNF-α and IL-1β, as well as serum ALT and AST levels were also depressed. Furthermore, treatment with Coicis Semen formula was demonstrated to relieve hypothalamic injury caused by obesity. Therefore, Coicis Semen formula could treat MSG-induced obesity in mice.
Acknowledgments
We are thankful to the General Hospital of Guangzhou Military Command of PLA (Wuhan) and Department of Radiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan). This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 81771978, 81627901 and 81773653) and National Basic Research Program of China (2015CB931802 and 2018YFA0208903).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abarca-Gómez, L., Abdeen, Z. A., Hamid, Z. A., Abu-Rmeileh, N. M., Acosta-Cazares, B., Acuin, C., … Ezzati, M. (2017). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. The Lancet, 390, 2627–2642. doi: 10.1016/S0140-6736(17)32129-3
- Adan, R. A. (2013). Mechanisms underlying current and future anti-obesity drugs. Trends in Neurosciences, 36, 133–140. doi: 10.1016/j.tins.2012.12.001
- Amato, M. C., Giordano, C., Galia, M., Criscimanna, A., Vitabile, S., Midiri, M., & Galluzzo, A. (2010). Visceral adiposity index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care, 33, 920–922. doi: 10.2337/dc09-1825
- Bernardis, L. L., & Patterson, B. D. (1968). Correlation between 'Lee index' and Carcass fat content in weanling and adult female rats with hypothalamic lesions. The Journal of Endocrinology, 40, 527–528. doi: 10.1677/joe.0.0400527
- Blitman, N. M., Baron, L. S., Berkenblit, R. G., Schoenfeld, A. H., Markowitz, M., & Freeman, K. (2011). Feasibility of using single-slice MDCT to evaluate visceral abdominal fat in an urban pediatric population. American Journal of Roentgenology, 197, 482–487. doi: 10.2214/AJR.10.5514
- Choi, E. K., Cho, Y. J., Yang, H. J., Kim, K. S., Lee, I. S., Jang, J. C., … Jang, H. J. (2015). Coix seed extract attenuates the high-fat induced mouse obesity via PPARγ and C/EBPα downregulation. Molecular & Cellular Toxicology, 11, 213–221. doi: 10.1007/s13273-015-0020-8
- Dastagir, G., & Rizvi, M. A. (2016). Review - Glycyrrhiza glabra L. (Liquorice). Pakistan Journal of Pharmaceutical Sciences, 29, 1727–1733.
- Després, J.-P., & Lemieux, I. (2006). Abdominal obesity and metabolic syndrome. Nature, 444, 881–887. doi: 10.1038/nature05488
- Diehl, A. M. (2010). Hepatic complications of obesity. Gastroenterology Clinics of North America, 39, 57–68. doi: 10.1016/j.gtc.2009.12.001
- Douglas, I. J., Langham, J., Bhaskaran, K., Brauer, R., & Smeeth, L. (2013). Orlistat and the risk of acute liver injury: Self controlled case series study in UK clinical Practice Research Datalink. British Medical Journal, 346, f1936. doi: 10.1136/bmj.f1936
- Hermanussen, M., & Tresguerres, J. A. F. (2003). Does high glutamate intake cause obesity? Journal of Pediatric Endocrinology and Metabolism, 16, 965–968.
- Hioki, C., Yoshida, T., Kogure, A., Yoshimoto, K., & Shimatsu, A. (2010). Growth hormone administration controls body composition associated with changes of thermogenesis in obese KK-Ay mice. The Open Endocrinology Journal, 4, 3–8. doi: 10.2174/1874216501004010003
- Hu, H. H., Smith, D. L. Jr, Nayak, K. S., Goran, M. I., & Nagy, T. R. (2010). Identification of brown adipose tissue in mice with fat-water IDEAL-MRI. Journal of Magnetic Resonance Imaging, 31, 1195–1202. doi: 10.1002/jmri.22162
- Kim, S. O., Yun, S. J., Jung, B., Lee, E. H., Hahm, D. H., Shim, I., & Lee, H. J. (2004). Hypolipidemic effects of crude extract of adlay seed (Coix lachrymajobi var. Mayuen) in obesity rat fed high fat diet: Relations of TNF-alpha and leptin mRNA expressions and serum lipid levels. Life Sciences, 75, 1391–1404. doi: 10.1016/j.lfs.2004.03.006
- Krentz, A. J., Fujioka, K., & Hompesch, M. (2016). Evolution of pharmacological obesity treatments: Focus on adverse side-effect profiles. Diabetes, Obesity & Metabolism, 18, 558–570. doi: 10.1111/dom.12657
- Lauby-Secretan, B., Scoccianti, C., Loomis, D., Grosse, Y., Bianchini, F., & Straif, K. (2016). Body fatness and cancer–viewpoint of the IARC working group. The New England Journal of Medicine, 375, 794–798. doi: 10.1056/NEJMsr1606602
- Li, Y., Han, H., Yin, J., Zheng, J., Zhu, X., Li, T., & Yin, Y. (2018). Effects of glutamate and aspartate on growth performance, serum amino acids, and amino acid transporters in piglets. Food and Agricultural Immunology, 29, 675–687. doi: 10.1080/09540105.2018.1437892
- Liu, H., Pei, X., Shi, K., Wang, J., Han, F., & Li, A. (2018). Effects of replacing wheat flour with detoxified ginkgo nut powder on lipid metabolism of obese C57BL/6J male mice. Food and Agricultural Immunology, 29, 39–55. doi: 10.1080/09540105.2017.1358255
- Ma, H., Zhang, G., Mou, C., Fu, X., & Chen, Y. (2018). Peripheral CB1 receptor neutral antagonist, AM6545, ameliorates hypometabolic obesity and improves adipokine secretion in monosodium glutamate induced obese mice. Frontiers in Pharmacology, 9, 156. doi: 10.3389/fphar.2018.00156
- Machann, J., Horstmann, A., Born, M., Hesse, S., & Hirsch, F. W. (2013). Diagnostic imaging in obesity. Best Practice & Research Clinical Endocrinology & Metabolism, 27, 261–277. doi: 10.1016/j.beem.2013.02.003
- Meyer, C. W., Ootsuka, Y., & Romanovsky, A. A. (2017). Body temperature measurements for metabolic phenotyping in mice. Frontiers in Physiology, 8, 520. doi: 10.3389/fphys.2017.00520
- Neuschwander-Tetri, B. A. (2007). Fatty liver and the metabolic syndrome. Current Opinion in Gastroenterology, 23, 193–198. doi: 10.1097/MOG.0b013e32801421a9
- Olney, J. W. (1969). Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science, 164, 719–721. doi: 10.1126/science.164.3880.719
- Olney, J. W., & Ho, O. L. (1970). Brain damage in infant mice following oral intake of glutamate, aspartate or cysteine. Nature, 227, 609–611. doi: 10.1038/227609b0
- Park, C. H., Choi, S. H., Piao, Y., Kim, S., Lee, Y. J., Kim, H. S., … Suh, Y.-H. (2000). Glutamate and aspartate impair memory retention and damage hypothalamic neurons in adult mice. Toxicology Letters, 115, 117–125. doi: 10.1016/S0378-4274(00)00188-0
- Pichiah, P. B., Moon, H. J., Park, J. E., Moon, Y. J., & Cha, Y. S. (2012). Ethanolic extract of seabuckthorn (Hippophae rhamnoides L) prevents high-fat diet-induced obesity in mice through down-regulation of adipogenic and lipogenic gene expression. Nutrition Research (New York, NY), 32, 856–864. doi: 10.1016/j.nutres.2012.09.015
- Rahman, H. A., Sahib, N. G., Saari, N., Abas, F., Ismail, A., Mumtaz, M. W., & Hamid, A. A. (2017). Anti-obesity effect of ethanolic extract from Cosmos caudatus Kunth leaf in lean rats fed a high fat diet. BMC Complementary and Alternative Medicine, 17, 122–138. doi: 10.1186/s12906-017-1640-4
- Roberto, C. A., Swinburn, B., Hawkes, C., Huang, T. T., Costa, S. A., Ashe, M., … Brownell, K. D. (2015). Patchy progress on obesity prevention: Emerging examples, entrenched barriers, and new thinking. Lancet (London, England), 385, 2400–2409. doi: 10.1016/S0140-6736(14)61744-X
- Shen, R.-L., Zhang, W.-L., Dong, J.-L., Ren, G.-X., & Chen, M. (2015). Sorghum resistant starch reduces adiposity in high-fat diet-induced overweight and obese rats via mechanisms involving adipokines and intestinal flora. Food and Agricultural Immunology, 26, 120–130. doi: 10.1080/09540105.2013.876976
- Thaler, J. P., Yi, C. X., Schur, E. A., Guyenet, S. J., Hwang, B. H., Dietrich, M. O., … Schwartz, M. W. (2012). Obesity is associated with hypothalamic injury in rodents and humans. The Journal of Clinical Investigation, 122, 153–162. doi: 10.1172/JCI59660
- Wang, W., Pan, Y., Zhou, H., Wang, L., Chen, X., Song, G., … Li, A. (2018). Ferulic acid suppresses obesity and obesity-related metabolic syndromes in high fat diet-induced obese C57BL/6J mice. Food and Agricultural Immunology, 29, 1116–1125. doi: 10.1080/09540105.2018.1516739
- Wang, X., Zhang, H., Chen, L., Shan, L., Fan, G., & Gao, X. (2013). Liquorice, a unique “guide drug” of traditional Chinese medicine: A review of its role in drug interactions. Journal of Ethnopharmacology, 150, 781–790. doi: 10.1016/j.jep.2013.09.055
- Weisberg, S. P., McCann, D., Desai, M., Rosenbaum, M., Leibel, R. L., & Ferrante, A. W. Jr (2003). Obesity is associated with macrophage accumulation in adipose tissue. The Journal of Clinical Investigation, 112, 1796–1808. doi: 10.1172/JCI200319246
- Yang, J., Kim, C. S., Tu, T. H., Kim, M. S., Goto, T., Kawada, T., … Yun, J. W. (2017). Quercetin protects obesity-induced hypothalamic inflammation by reducing microglia-mediated inflammatory responses via HO-1 induction. Nutrients, 9, 650. doi: 10.3390/nu9070650
- Yu, H., Shimakawa, A., McKenzie, C. A., Brodsky, E., Brittain, J. H., & Reeder, S. B. (2008). Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magnetic Resonance in Medicine, 60, 1122–1134. doi: 10.1002/mrm.21737
- Yu, F., Zhang, J., Li, Y. Z., Zhao, Z. Y., & Liu, C. X. (2017). Research and application of adlay in medicinal field. Chinese Herbal Medicines, 9, 126–133. doi: 10.1016/S1674-6384(17)60086-8
- Yudkin, J. S., Stehouwer, C. D., Emeis, J. J., & Coppack, S. W. (1999). C-reactive protein in healthy subjects: Associations with obesity, insulin resistance, and endothelial dysfunction: A potential role for cytokines originating from adipose tissue? Arteriosclerosis, Thrombosis, and Vascular Biology, 19, 972–978. doi: 10.1161/01.ATV.19.4.972
- Yun, S. N., Moon, S. J., Ko, S. K., Im, B. O., & Chung, S. H. (2004). Wild ginseng prevents the onset of high-fat diet induced hyperglycemia and obesity in ICR mice. Archives of Pharmacal Research, 27, 790–796. doi: 10.1007/BF02980150
- Zhao, Y., Kao, C. P., Liao, C. R., Wu, K. C., Zhou, X., Ho, Y. L., & Chang, Y. S. (2017). Chemical compositions, chromatographic fingerprints and antioxidant activities of Citri Exocarpium Rubrum (Juhong). Chinese Medicine, 12, 6. doi: 10.1186/s13020-017-0127-z
- Zhao, L., Zhu, X., Cong, R., Yang, X., & Zhu, Y. (2018). The protective effects of Danggui-Baizhu-Tang on high-fat diet-induced obesity in mice by activating thermogenesis. Frontiers in Pharmacology, 9, 1019–1029. doi: 10.3389/fphar.2018.01019

